Background: The signal module comprising Dok-3 and Grb2 controls differential BCR signal intensity.
Results: Dok-3/Grb2 translocate to BCR microsignalosomes and inhibit Lyn-dependent activation of the BCR transducer kinase Syk.
Conclusion: Dok-3/Grb2 change the balance of activatory and inhibitory Lyn functions toward BCR signal inhibition.
Significance: Learning how adapter proteins translocate to and change signal processes in BCR microsignalosomes is important to understand the regulation of antigen-induced B cell activation.
Keywords: Calcium Signaling, Immunology, in Vivo Imaging, Signal Transduction, Src, B Cell Antigen Receptor, Dok-3, Grb2, Lyn, Syk
Abstract
Recruitment of the growth factor receptor-bound protein 2 (Grb2) by the plasma membrane-associated adapter protein downstream of kinase 3 (Dok-3) attenuates signals transduced by the B cell antigen receptor (BCR). Here we describe molecular details of Dok-3/Grb2 signal integration and function, showing that the Lyn-dependent activation of the BCR transducer kinase Syk is attenuated by Dok-3/Grb2 in a site-specific manner. This process is associated with the SH3 domain-dependent translocation of Dok-3/Grb2 complexes into BCR microsignalosomes and augmented phosphorylation of the inhibitory Lyn target SH2 domain-containing inositol 5′ phosphatase. Hence, our findings imply that Dok-3/Grb2 modulates the balance between activatory and inhibitory Lyn functions with the aim to adjust BCR signaling efficiency.
Introduction
B cell development and function is strictly dependent on signals that are transduced by the B cell antigen receptor (BCR)7 consisting of a transmembrane immunoglobulin component and an Igα/Igβ heterodimer (1, 2). Antigen binding to the BCR induces the formation of microclusters containing several BCR molecules (3) that provide a structural basis for the spatial organization of the BCR signaling cascade into discrete microsignalosomes (4). Although the details of BCR microcluster generation as well as the exact composition of BCR microsignalosomes are still under investigation, it is well accepted that proteins need to be recruited into microsignalosomes to participate in BCR-proximal signaling.
A crucial step in BCR signal initiation is the recruitment of the spleen tyrosine kinase (Syk) by phosphorylated immunoreceptor tyrosine-based activation motifs within the cytosolic parts of Igα/Igβ, which provide binding sites for the two N-terminal Src homology 2 (SH2) domains in Syk. By this process Syk is allosterically activated (5, 6) and phosphorylates the adapter protein SH2 domain-containing leukocyte protein of 65 kDa (SLP-65) (7–9), which allows for multiprotein complex formation with Bruton's tyrosine kinase (Btk) and phospholipase C-γ2 (PLC-γ2). Correct plasma membrane localization of this Ca2+ initiation complex requires the Cbl-interacting protein of 85 kDa (10) and is crucial for PLC-γ2-mediated production of the second messengers diacylglycerol and inositol-1,4,5-trisphosphate (11–14). Although diacylglycerol mediates activation of the NF-κB and MAPK pathways, inositol-1,4,5-trisphosphate induces mobilization of Ca2+ ions from the endoplasmic reticulum and subsequent influx through Ca2+ channels in the plasma membrane (15, 16).
The lck/yes-related novel protein tyrosine kinase (Lyn) plays an important role in BCR signal initiation, which is evident from studies in Lyn-deficient mice and DT40 B cells. There, BCR-induced Ca2+ mobilization is compromised, and Syk recruitment into microsignalosomes is abolished (4, 17). However, Lyn-deficient mice establish a hypersensitive and autoimmune phenotype, and B cells from lyn−/− mice exhibit stronger proliferative responses upon BCR engagement, proving that Lyn exerts positive and negative functions in BCR signaling (18–20). We recently discovered a Lyn-dependent inhibitory process that involves the adapter protein downstream of kinase 3 (Dok-3). Dok-3 is permanently localized at the plasma membrane because of its pleckstrin homology (PH) domain (21). After BCR engagement, Dok-3 is phosphorylated by Lyn and provides a docking site for the SH2 domain of the growth factor receptor-bound protein 2 (Grb2), leading to increased Dok-3 phosphorylation and the oligomerization of Dok-3·Grb2 complexes. These complexes attenuate PLC-γ2 activation, which results in decreased inositol-1,4,5-trisphosphate production and Ca2+ mobilization (21). Consistently, Grb2- or Dok-3-deficient DT40 B cells as well as grb2−/− or dok-3−/− primary B cells mount enhanced BCR-induced Ca2+ responses (21–25), which is associated with augmented T cell-independent immune responses and elevated serum IgM titers in Dok-3-deficient mice (22). Hence, Dok-3 appears to adjust differential BCR signals and contribute to divergent cellular responses like proliferation and differentiation or anergy and apoptosis (16, 26).
We have now elucidated the details of Dok-3/Grb2-mediated BCR signal attenuation by combining genetic, biochemical, and life cell imaging techniques as well as phosphoproteomics. Here we show that Lyn-dependent phosphorylation of Syk is decreased by Dok-3/Grb2 in a site-specific manner, which results in decreased kinase activity of Syk. Grb2-binding to Dok-3 is required for translocation into BCR microsignalosomes and for efficient signal inhibition, which is associated with increased phosphorylation of the SH2 domain-containing inositol 5′ phosphatase (SHIP). Hence, Dok-3/Grb2 appears to modulate the balance of activatory and inhibitory Lyn functions, resulting in diminished efficiency of Ca2+-mobilizing enzymes.
EXPERIMENTAL PROCEDURES
Cell Culture, Antibodies, and Reagent
DT40 cells were cultured in RPMI 1640 medium containing 10% FCS, 3 mm l-glutamine, 2 mm pyruvate, 50 μm β-mercaptoethanol, and antibiotics. Cell culture reagents were purchased from Invitrogen. Anti-chicken Dok-3 serum was generated by immunizing rabbits with GST fusion proteins of the Dok-3 amino acids 332–409. Antibodies against GFP, HA (3F10, Roche), phosphotyrosine (4G10, Millipore), Syk (N19, Santa Cruz Biotechnology), pLyn 507, pSrc 416 (Cell Signaling Technology), pZap70 (Tyr-319)/pSyk (Tyr-352) (BD Biosciences), and GST and actin (Sigma) were used for Western blot analysis (1:1000) and immunopurifications (0.2–1 μg). Affinity purifications using pITAM peptides and GST fusion proteins have been described in Ref. 27) and Ref. 28, respectively. DT40 cells were stimulated with 2 μg/ml mouse anti-chicken IgM clone M1 or M4 (Southern Biotechnology) and prepared for biochemical experiments as described previously (21). For quantification of Western blot data we used ImageJ software. The Syk inhibitor Bay 61-3606 (Merck, Darmstadt, Germany) was used in a final concentration of 500 nm for 1 h.
Expression Constructs and Generation of Genetic DT40 Variants
The targeting vectors pDok-3-bleo and pDok-3-gpt were constructed to insert bleomycine and mycophenolic acid resistance cassettes into intron 1 of dok-3 alleles of lyn−/− DT40 cells. The resistance cassettes were flanked by 1.8 kb and 2.9 kb at the 5′ and 3′ sites, respectively, as described in Ref. 21. To generate dok-3−/−/syk−/− DT40 cells, we took Dok-3-deficient DT40 cells and utilized a targeting construct by which exons 1 and 2 of Syk were replaced by a bleomycine resistance cassette. For this purpose, genomic syk fragments were amplified from DT40 genomic DNA using primers 5′-tgcgaaccttgcctcatctcagtggataca-3′ and 5′-gcctggacagctaagtactgtcctatcg-3′ (left arm) and 5′-ggctacatgctgactgccttgcttg-3′ and 5′-tgtgctggtggtaatgtactg-3′ (right arm), respectively. Targeting vectors were introduced by electroporation at 550 V, 25 μF. For selection, bleocin (Merck) was used at 100 μg/ml and mycophenolic acid (Sigma Aldrich) at 15 μg/ml. Clones were screened by PCR and immunoblot analysis. The plasmids pApuroII OneSTrEP Syk, pMSCV citSyk, pApuroII HA-Dok-3, and pMSCVpuro Dok-3 GFP have been described in Ref. 21. The chimeric protein tSH2-Dok-3 contains amino acids 114–426 of chicken Dok-3 and amino acids 1–277 of human Syk. Dok-3-cSH3 encompasses the 322 N-terminal amino acids of chicken Dok-3 and amino acids 151–217 of chicken Grb2. Constructs coding for chimeric proteins were generated by overlap extension PCR as described in Ref. 23. Point mutations leading to indicated amino acid exchanges were generated by site-directed mutagenesis using the QuikChange protocol (Stratagene). Plasmids encoding Dok-3-cerulean and Grb2-citrine were established as in Ref. 21 after ligating cerulean or citrine cDNAs into the AgeI and BsrGI sites of pEGFP-N1 (BD Biosciences). All cDNAs were ligated into the expression vectors pMSCV (BD Biosciences) or pApuroII (29) and transfected by retroviral gene transfer or electroporation as described in Ref. 21. The transfected cells were selected with puromycin (Invivogen) (1 mg/ml) or bleocin (Merck) (70 μg/ml).
Ca2+ Monitoring and Förster Resonance Energy Transfer Analysis
Cytosolic Ca2+ concentration was measured in Indo-1AM-loaded cells as described previously (21). FRET between cerulean and citrine was analyzed on a LSRII flow cytometer (BD Biosciences) equipped with a violet laser (405 nm) for excitation. Emitted cerulean and citrine fluorescence was monitored contemporaneously using 450/50-nm and 550/25-nm band pass filters, respectively, and FRET was determined by ratioing citrine and cerulean signal intensities. All cytometry data were processed by FlowJo (Tristar).
Confocal Laser Scanning and Total Internal Reflection Fluorescence Microscopy
For life cell confocal LSM, cells were resuspended in Krebs Ringer solution composed of 10 mm HEPES (pH 7.0), 140 mm NaCl, 4 mm KCl, 1 mm MgCl2, 1 mm CaCl2, and 10 mm glucose at a concentration of 106 cells/ml and seeded onto Lab-TekTM chambered coverglasses. For stimulation, we used mouse anti-chicken IgM (M4) at 3 μg/ml. Cells were analyzed on a Leica SP2 system, and images were processed by Adobe Photoshop CS. Total internal reflection fluorescence microscopy was performed as described (4). For colocalization analysis, Imaris software was used. Briefly, images were cropped to the size of the cell, and both fluorescence channels were background-subtracted. For quantification, the original Mander's coefficient for the Alexa Fluor 633 channel was calculated using the Imaris colocalization module (30).
Intracellular Staining of DT40 Cells
For intracellular staining of phosphorylated Syk, 106 DT40 cells were starved for 30 min at 37 °C before they were stimulated with 2 μg/ml M4 antibody. After fixation with PBS, 2% paraformaldehyde cells were permeabilized with PBS containing 1% BSA, 0.1% saponine (Roth), and 0.09% NaN3 for 30 min at room temperature. This buffer was also used to dilute Alexa Fluor 633-conjugated anti-pSyk Tyr-352 antibodies (BD Biosciences), and cells were incubated for 30 min at room temperature before they were washed and analyzed with a LSRII cytometer (BD Biosciences).
Affinity Purifications after Stable Isotope Labeling with Amino Acids in Cell Culture and Mass Spectrometric Analysis
Mass spectrometric identification and quantification of phospho-acceptor sites as well as metabolic labeling of DT40 cells via stable isotope labeling with amino acids in cell culture was done as described (31). To examine the impact of Dok-3/Grb2 on BCR-induced Syk phosphorylation, syk−/−/dok-3−/− DT40 cells expressing OneSTrEP-tagged human Syk together with Dok-3 HA were cultured in heavy stable isotope labeling with amino acids in cell culture medium containing 2D4,12C6,14N2-Lys; 13C6,14N4-Arg, whereas cells expressing OneSTrEP Syk together with Dok-3 Y331F HA served as negative control and were cultured in light medium containing 12C6,14N2-Lys; 12C6,14N4-Arg. For affinity purifications, 2 × 108 cells were BCR-stimulated for 3 min, lysed, and incubated with 200 μl of STrEP-Tactin Superflow matrix (IBA BioTAGnology) for 1 h at 4 °C. For each approach, 500 μl of desthiobiotin buffer (IBA BioTagnology) was used to elute purified proteins at room temperature. Eluates were pooled in a 1:1 ratio, concentrated in ultrafiltration spin columns (Sartorius), and proteins were separated on a 4–12% NuPAGE Bis-Tris gel (Invitrogen). After Coomassie Brilliant Blue staining, the gel slice containing Syk was excised and digested. MS parameter settings and data analysis were done as described earlier (31, 32).
In Vitro Kinase Assays
To determine Lyn and Syk kinase activity in vitro, we used signal transduction and gastric precursor (Tyr-87) peptides (Cell Signaling Technology), respectively. LynHA or Syk were purified by immunopurification as described above. Beads were resuspended in kinase buffer containing 60 mm HEPES (pH7.5), 5 mm MgCL2, 5 mm MnCL2, 3 μm Na3VO4, and 1.25 mm DTT. 1.5 μm biotinylated substrate peptide and 20 μm ATP were added, incubated for 15 min at room temperature, and the reaction was stopped by adding EDTA to an end concentration of 25 mm. Peptides were immobilized on streptavidin-coated 96-well plates (Millipore), and phosphorylation efficiency was determined by ELISA using anti-pTyr (1 μg/ml) and horseradish peroxidase-conjugated anti-mouse IgG (1:1000) antibodies.
RESULTS
Dok-3/Grb2 Attenuates BCR-induced Syk Activation
A possible regulation of Syk by the Dok-3/Grb2 signal module was analyzed using DT40 B cell variants that either lack Syk or Dok-3 or expressed HA-tagged Dok-3. Cells were left untreated or stimulated through their BCR, and lysates were subjected to affinity purification with immobilized doubly phosphorylated immunoreceptor tyrosine-based activation motif peptides of Igα, which specifically bind tandem SH2 domains of Syk. Anti-phosphotyrosine immunoblotting of the obtained proteins and subsequent densitometric quantification of the signals (Fig. 1A, left and right panels, respectively) showed that the presence of Dok-3 markedly inhibited BCR-induced overall phosphorylation of Syk. To examine the specificity of the inhibitory effect in more detail, we quantified the phosphorylation of individual Syk tyrosine residues using stable isotope labeling with amino acids in cell culture followed by liquid chromatography-coupled tandem mass spectrometry (for details, see Refs. 32, 33). Briefly, syk−/−/dok-3−/− DT40 B cells were generated and transfected with a plasmid encoding OneSTrEP-tagged Syk. Next we expressed in these cells either HA-tagged wild-type Dok-3 or a dysfunctional variant harboring a Tyr to Phe exchange in amino acid position 331 (Dok-3(YF)) that abolished SH2-mediated recruitment of Grb2 to phosphorylated Dok-3 (21). Resulting cell batches were metabolically labeled and stimulated by anti-BCR antibodies. Affinity-purified Syk was in-gel-digested with trypsin, and the enriched phosphopeptides were identified and quantified by mass spectrometry (Fig. 1B). We identified five of the known phosphotyrosine residues at amino acid positions 352, 525, 526, 629, and 630. Fig. 1C shows that the latter four tyrosine residues were equally well phosphorylated upon BCR ligation in the presence of wild-type Dok-3. In marked contrast, phosphorylation of tyrosine 352 was reduced by ∼60% in Dok-3-expressing cells compared with control cells. The strongly reduced phosphorylation of Tyr-352 was confirmed by immunoblot and flow cytometry analyses using phosphosite-specific antibodies to phospho-Tyr-352 (Fig. 1, D and E, respectively). No differences in the phosphorylation efficiencies of serine/threonine phospho-acceptor sites were detected (data not shown). To confirm the Dok-3/Grb2 function in a human B cell line, we transduced DG75 B cells with either control virus or virus encoding GFP fusion proteins of Dok-3 or Dok-3(YF). Consistent with our data obtained from DT40 cells (21) Dok-3(YF) acts dominantly negative over endogenous Dok-3 (Fig. 1F), and the increased Ca2+ response is associated with a stronger phosphorylation of Syk Tyr-352 (G).
FIGURE 1.
Dok-3/Grb2 attenuates the BCR-induced activation of Syk. A, Dok-3-deficient and HA-Dok-3-reconstituted DT40 cells as well as syk−/− DT40 cells were left untreated (-) or stimulated for 3 min via their BCR (+). Syk was affinity-purified (AP) from cleared cellular lysates (CCL) using a doubly phosphorylated immunoreceptor tyrosine-based activation motif (pITAM) peptide. Samples were analyzed by anti-phosphotyrosine (α-pTyr, upper panel) or anti-Syk (α-Syk, lower panel) immunoblotting. The ratio of phospho-Syk (pSyk) to Syk signal intensities was calculated and plotted (right panel). The statistical significance of four independent experiments was determined using Student's t test. **, p < 0.01). B, schematic work flow of mass spectrometric analysis of Syk phosphotyrosine residues. Briefly, Syk/Dok-3-double-deficient DT40 cells were transfected with constructs encoding STrEP-tagged Syk together with either Dok-3(YF) as a control or Dok-3 and incubated in media containing “light” isotopes (12C6,14N2-Lys; 12C6,14N4-Arg) or “heavy” isotopes (2D4,12C6,14N2-Lys; 13C6,14N4-Arg), respectively. Cells were stimulated for 3 min via their BCR, and Syk was purified with a Streptactin matrix. Samples were pooled 1:1, separated by PAGE, and the gel band referring to Syk was digested and analyzed by LC-MS/MS. C, the abundance of phosphopeptides encompassing the indicated phosphotyrosine residues was determined, and heavy/light ratios ± S.D. were calculated and depicted as a bar plot. DT40 wild-type and transfectants described in A were treated and lysed as above and subjected to Western blot analysis using phosphospecific anti-Syk Tyr-352 and anti-Syk antibodies (D, upper and lower panel, respectively). Signal quantification of n = 10 experiments was calculated as described in A. *, p < 0.05; **, p > 0.01. E, alternatively, cells were stimulated via their BCR, intracellularly stained with Alexa Fluor 633-conjugated anti-Syk pTyr-352 antibodies, and analyzed by flow cytometry. F, DG75 human B cells were either transduced with control virus or virus encoding for GFP fusion proteins of either Dok-3 or Dok-3(YF). Cells were loaded with Indo-I, and Ca2+ flux was analyzed by flow cytometry. As indicated, cells were stimulated after 30 s with anti-IgM antibodies. G, DG75 transductants were exposed to anti-Syk pTyr-352 Western blot analysis as described in D, and signals from n = 8 experiments were quantified. **, p < 0.01).
Phospho-Tyr-352 has been reported to be a major and highly dynamic phosphorylation site that critically contributes to BCR-induced Syk activation (5, 31). Hence, we investigated whether the Dok-3/Grb2-mediated inhibition of phospho-Tyr-352 affected Syk kinase activity. Therefore, we coexpressed citrine-tagged Syk with either HA Dok-3 or HA Dok-3(YF) in syk−/−/dok-3−/− DT40 B cells. Cells were stimulated through their BCR, and purified Syk was subjected to an in vitro kinase assay that revealed that the Dok-3-mediated inhibition of Tyr-352 phosphorylation was associated with significantly reduced kinase activity of Syk (Fig. 2A). This finding was confirmed by monitoring the in vivo phosphorylation of the proximal Syk substrate, e.g. the adaptor protein SLP65 using phosphosite-specific antibodies that recognize phospho-Tyr-178 and phospho-Tyr-189 of SLP65 (Fig. 2B). Collectively, these experiments revealed that the negative signaling element Dok-3 can inhibit BCR-induced B cell activation at the level of Syk activation.
FIGURE 2.
Dok-3/Grb2 attenuates Syk activity in activated B cells. A, Syk/Dok-3-double-deficient DT40 cells expressing citrine-tagged Syk together with either wild-type HA Dok-3 or HA Dok-3(YF) were stimulated for 3 min via their BCR, lysed, and subjected to anti-GFP immunopurification. To examine the Syk kinase activity in vitro, purified proteins were incubated with biotinylated phosphoacceptor peptides. The efficiency of phosphorylation was determined by anti-phosphotyrosine ELISA. Shown is the mean ± S.D. of n = 3 independent experiments, and statistical significance was determined using Student's t test. **, p < 0.01. B, Dok-3-deficient and DT40 cells that have been reconstituted with HA Dok-3 were transfected with a construct encoding citrine-tagged human SLP-65 and treated as described above. Cellular lysates were subjected to anti-GFP immunopurification (IP), and Western blot analyses were developed with phosphospecific antibodies detecting either of the PLC-γ2 binding sites at positions 178 and 189 (upper and center panel, respectively). As loading control, the same samples were reprobed with anti-SLP65 antibodies. Signal quantification was calculated from n = 3 experiments as described above. *, p < 0.05).
Dok-3/Grb2 Inhibits Lyn-dependent Syk Phosphorylation
Activation of Syk involves autophosphorylation as well as phosphorylation by Lyn (6). To test which of these processes is controlled by Dok-3, we inhibited Syk activity using the well established Bay 61-3606 (34), which blunted BCR-induced Ca2+ mobilization in human Syk-expressing DT40 B cells (Fig. 3A), in DT40 B cell transfectants that expressed citrine-tagged human Syk together with either wild-type Dok-3 or the Tyr-to-Phe variant. Immunoblotting with anti-phospho-Syk Tyr-352 and densitometric signal quantification showed that wild-type Dok-3 inhibited phosphorylation of this Syk residue regardless of the inhibition of Syk itself (Fig. 3B). These data suggest an impact of Dok-3 on Lyn-dependent Syk phosphorylation at Tyr-352. However, Lyn kinase activity was not affected by Dok-3, as monitored by an ELISA-based in vitro kinase assay using Lyn that was purified from DT40 B cells expressing wild-type or Tyr-to-Phe mutant Dok-3 (Fig. 3C). The role of Lyn in Dok-3-mediated signal inhibition was further investigated using a chimeric protein comprising the N-terminal 322 amino acids of Tyr-to-Phe-mutated Dok-3 and the C-terminal SH3 domain of Grb2 (Dok-3-cSH3, Fig. 3D) that is able to inhibit BCR-induced Ca2+ mobilization similar to wild-type Dok-3 (E, gray and red lines) but independent of its phosphorylation and concomitant recruitment of Grb2. As control we used a Dok-3 chimera that harbored a dysfunctional Grb2 SH3 domain because of an exchange of tryptophan 193 to lysine (W193K). The Dok-3-cSH3 chimera inhibited BCR-induced Ca2+ mobilization as well as phosphorylation of Syk at tyrosine 352 in Lyn-positive but not in Lyn-negative DT40 B cell transfectants (Fig. 3, E and F, respectively). These data further confirm that Dok-3 attenuates Lyn-dependent activation of Syk but not Syk autophosphorylation.
FIGURE 3.
Dok-3/Grb2 negatively regulates Lyn-dependent Syk phosphorylation. A, Syk-deficient DT40 cells and cells reconstituted with human Syk were subjected to Ca2+ flux analysis as described above. Cells corresponding to the red line were treated with the Syk inhibitor Bay 61-3606 prior to measurement. B, the transfectants described in Fig. 2A were either left untreated or stimulated via their BCR. Cells used for lanes 3, 6, and 9 were treated with Bay 61-3606 prior to stimulation. Quantification of 10 independent experiments was done as described above. *, p < 0.05; **, p < 0.01. C, Lyn/Dok-3-double-deficient DT40 cells expressing HA-tagged Lyn together with either Dok-3-GFP or Dok-3(YF)-GFP were stimulated for 3 min via their BCR, lysed, and subjected to anti-HA immunopurification. Purified Lyn was subjected to an in vitro kinase assay that was performed and analyzed as described above. Plotted are the mean values ± S.D. of n = 3 experiments. Chimeric proteins encompassing the first 322 amino acids of Dok-3 including the PH and phosphotyrosine-binding (PTB) domains and the cSH3 domain of Grb2 (Dok-3(Y/F)-cSH3) or a dysfunctional cSH3 variant (Dok-3(Y/F)-cSH3(W193K)) and lacking tyrosine-based phosphoacceptor sites as depicted in D were expressed together with Lyn HA (E, left panel, gray and black lines) or control vector (right panel, gray and black lines) in Lyn/Dok-3-double-deficient DT40 cells. As control served DT40 wild-type cells and lyn−/−/dok-3−/− cells expressing Lyn HA together with Dok-3 (E, left panel, green and red lines, respectively). Ca2+ flux analysis was performed as described above. F, transfectants used in C and syk−/− DT40 cells were stimulated for 5 min via their BCR, intracellularly stained with anti-pSyk Y352 antibodies, and analyzed as described above.
Grb2 Targets Dok-3 into BCR Microsignalosomes
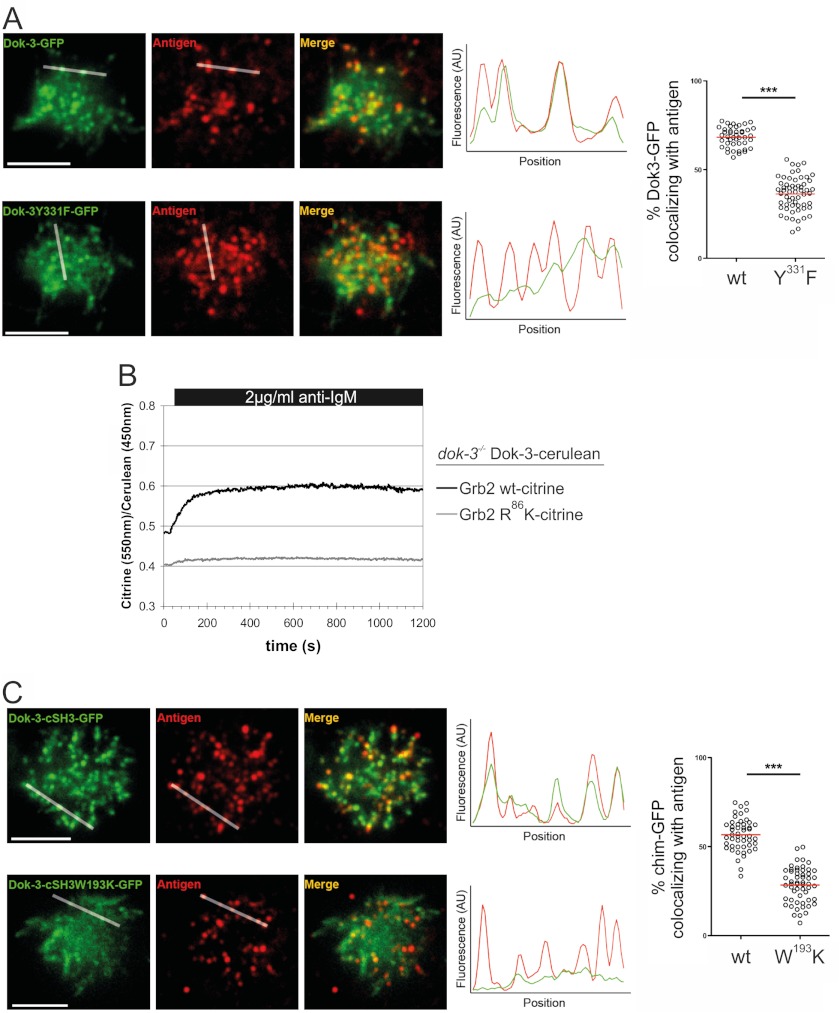
To interfere with Syk activation, the Dok-3/Grb2 module requires access to sites of BCR microclusters that provide a structural platform for the activation of proximal BCR effector proteins (4). The entry of Dok-3 into these BCR microsignalosomes was investigated using dok3−/− DT40 B cells expressing GFP-tagged versions of wild-type Dok-3 or the dysfunctional Dok-3 Tyr-to-Phe variant. Cells were settled onto lipid bilayers that contained anti-BCR antibodies coupled to Alexa Fluor 633-conjugated streptavidin. Upon contact with these lipid bilayers, B cells undergo spreading and gathering of BCR microclusters in a single cluster for antigen internalization (35). We monitored the localization of Dok-3 and BCR microclusters in fully spread cells by two-color total internal reflection fluorescence microscopy and found that BCR/Dok-3 colocalization was critically dependent on the intact Grb2 binding sites of Dok-3 (Fig. 4A). This result suggests that Grb2 provides a localization signal that directs Dok-3 to sites of BCR microclusters. This conclusion was further supported by flow cytometric monitoring of FRET in DT40 B cells that coexpress cerulean-tagged Dok-3 together with either citrine-tagged wild-type Grb2 or mutant Grb2 that harbors an Arg-to-Lys amino acid exchange in its SH2 domain and, therefore, cannot bind phosphorylated Dok-3. Fig. 4B shows that BCR engagement induced a rapid and sustained increase of FRET in cells expressing wild-type Grb2 but not in cells expressing the Grb2 variant. Moreover, the time course of the Dok-3/Grb2 association matches the kinetic of colocalization of Dok-3 with BCR microclusters. To investigate the molecular requirements of the translocation process, we used the chimeric Dok-3 protein that accommodates either the wild-type C-terminal SH3 domain of Grb2 or its dysfunctional counterpart (W193K) described above. The colocalization of the two Dok-3 chimeras with BCR microclusters was visualized by total internal reflection fluorescence microscopy (Fig. 4C), which revealed that the colocalization of the proteins requires a functional C-terminal SH3 domain of Grb2. Collectively, the data showed that the subcellular targeting of Dok-3 into BCR microclusters where Syk activation occurs requires SH2-mediated complex formation with Grb2 and a C-terminal SH3 domain of Grb2 to more directly link up with components of the microclusters.
FIGURE 4.
The Grb2 cSH3 domain mediates the translocation of Dok-3·Grb2 complexes into BCR microsignalosomes. A, Dok-3-deficient DT40 cells expressing GFP fusion proteins of Dok-3 (upper panel) and Dok-3 Y331F (lower panel) were settled onto lipid bilayers containing Alexa Fluor 633-conjugated anti-chicken IgM antibodies (M1). GFP and Alexa Fluor 633 fluorescence intensities were monitored by total internal reflection fluorescence microscopy, and representative images of spread cells are shown. Colocalization of fluorescences was measured and plotted along the indicated lines, AU, arbitrary units, and the dot plots (right panel) show the quantification of the localization of GFP fusion protein-containing microclusters to antigen-containing microclusters. Statistical significance was determined using Student's t test. ***, p < 0.0001. Scale bars = 5 μm. B, life cell analysis of BCR-induced Dok-3/Grb2 association by flow cytometric monitoring of FRET. Dok-3-deficient DT40 cells expressing Dok-3-cerulean together with citrine fusion proteins of either wild-type Grb2 (black line) or a non-Dok-3-binding variant (Grb2 R86K, gray line). Samples were excited by a violet laser (405 nm), and fluorescence was detected through 450/50-nm and 525/25-nm band pass filters. The relative FRET efficiency was calculated by ratioing citrine and cerulean signal intensities and plotted over time. Cells were stimulated by anti-chicken IgM after 30 s. C, Dok-3-deficient DT40 cells were transfected with constructs encoding Dok-3-cSH3 chimeric proteins encompassing the first 322 amino acids of Dok-3 and the cSH3 domain of Grb2 (amino acids 151–217) or the dysfunctional variant. Colocalization of Dok-3-cSH3 (upper panel) and Dok-3-cSH3(W193K) (lower panel) with BCR microclusters was analyzed as described in A.
Grb2 Facilitates Targeting to as well as Signal Inhibition in BCR Microsignalosomes
The data described above together with our previously published results (21) suggest a dual role of Grb2 for signal inhibition in association with Dok-3 in that Grb2 is required for the correct subcellular localization as well as the execution of signal inhibition. To directly test this conclusion, we replaced the PH domain of Dok-3 with the tandem SH2 domains of Syk that directly link the tSH2-Dok-3 fusion protein to the phosphorylated BCR. Importantly, and as shown by confocal laser scanning microscopy, the BCR-induced membrane translocation of the chimera was independent of the Grb2 docking sites in Dok-3 and, hence, complex formation with Grb2 (Fig. 5A). Likewise, the BCR-induced overall phosphorylation of Dok-3 (Fig. 5B, lanes 1 and 2) was recapitulated with the SH2 domain-containing Dok-3 fusion proteins independently of their ability to bind Grb2 (lanes 3 and 4 and 7 and 8). In contrast, inactivation of the Grb2 binding sites in wild-type Dok-3 strongly compromised stimulation-dependent overall phosphorylation of the protein (Fig. 5B, lanes 5 and 6). Despite its association with the BCR and its inducible tyrosine phosphorylation, the tSH2-Dok-3 chimera that lacked binding to Grb2 because of the Tyr-to-Phe exchange does not inhibit BCR-induced Ca2+ mobilization (Fig. 5C). These data are consistent with a dual role of Grb2, e.g. in conferring precise subcellular localization and subsequent inhibition of early BCR signaling events.
FIGURE 5.
Dok-3-associated Grb2 is required for negative regulation in BCR microsignalosomes. A, Dok-3-deficient cells expressing chimeric proteins where the tandem SH2 domains of human Syk (amino acids 1–277) replace the PH domain of Dok-3-GFP or Dok-3(YF)-GFP (tSH2-Dok-3 and tSH2-Dok-3(YF), respectively) were subjected to confocal LSM analysis. PTB, phosphotyrosine-binding domain. Depicted are representative images before (left panel) and 3 min after stimulation via the BCR (right panel). B, transfectants described above and dok-3−/− DT40 cells expressing Dok-3-GFP or Dok-3(YF)-GFP were treated as described above and subjected to anti-GFP immunopurification (IP). Western blot analysis was performed using anti-phospho tyrosine (α-pTyr, upper panel) or anti-GFP antibodies (lower panel). C, BCR-induced Ca2+ mobilization of the same cells was analyzed by flow cytometry as described above.
The Dok-3/Grb2 Module Can Affect Multiple Lyn Targets
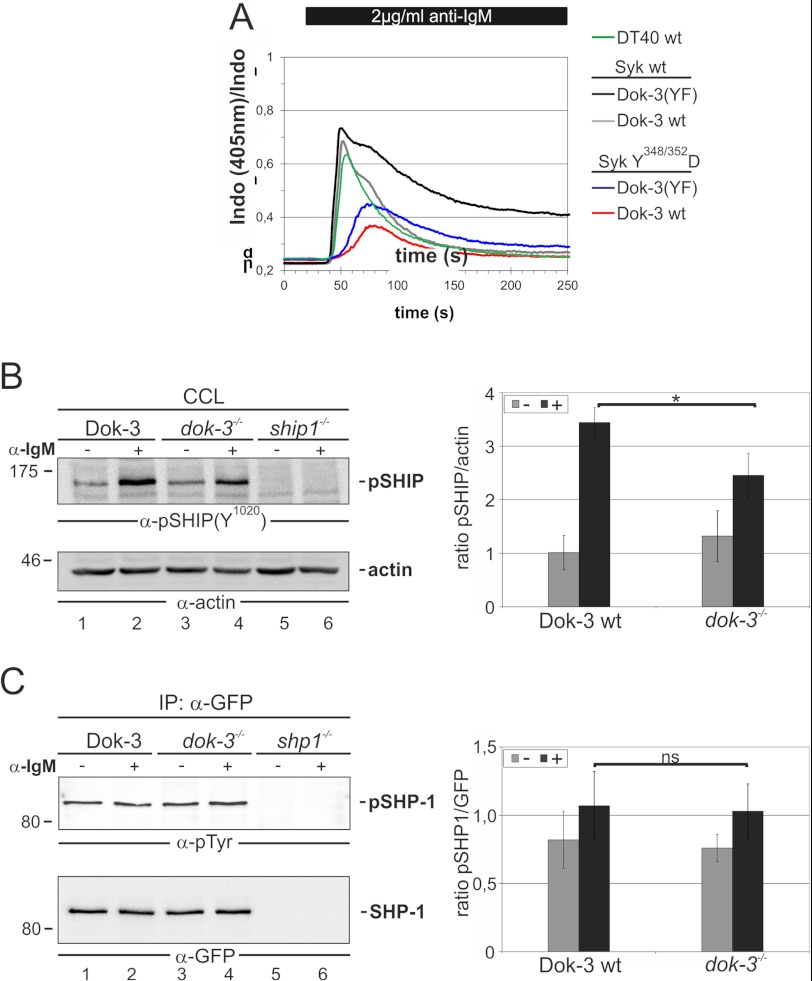
We have elucidated previously the B lymphoid interactome of Grb2, which encompasses negative as well as positive signal effector molecules (36). It was thus likely that besides Syk (see above), the Dok-3/Grb2 module possesses additional targets. Indeed, when we analyzed BCR-induced Ca2+ mobilization in DT40 B cells that expressed either wild-type Syk or a Syk variant in which the activating tyrosine residues 348 and 352 were replaced with aspartate, we still observed robust signal inhibition by a functional Dok-3/Grb2 module even though it cannot act on the Tyr-to-Asp variant because the aspartate mimics the phosphotyrosine, rendering that mutant independent of its upstream activation by Lyn (Fig. 6A). Well known Lyn targets within the Grb2 interactome are the negative regulatory phosphatases SHIP and SHP-1. Using the DT40 B cell reconstitution system, we analyzed the BCR-induced phosphorylation of these two Lyn targets in the presence or absence of a functional Dok-3/Grb2 signaling module (Fig. 6, B and C, respectively). Lack of Dok-3 expression in DT40 B cells was associated with a moderate reduction of SHIP phosphorylation, whereas phosphorylation of SHP-1 appeared to be independent of Dok-3. In summary, our data suggest that Dok-3/Grb2 can control the phosphorylation of multiple Lyn targets, leading to signal inhibition by suppressing positive regulators such as Syk and by facilitating the activation of negative regulators such as the lipid phosphatase SHIP.
FIGURE 6.
The Lyn-dependent phosphorylation of SHIP is augmented by Dok-3/Grb2. A, Syk/Dok-3-double deficient DT40 cells were transfected with plasmids encoding citrine-tagged Syk (black and gray lines) or Syk Y348/352D (blue and red lines). BCR-induced Ca2+ mobilization of these cells, additionally expressing either Dok-3 (gray and red line) or Dok-3(YF) as a control (black and blue lines) and DT40 wild-type cells (green line) was analyzed as described above. B and C, to examine the impact of Dok-3/Grb2 on the phosphorylation of Lyn targets Dok-3-deficient DT40 cells were used that express either HA-Dok-3 or empty vector as control. These cells were left untreated (−) or stimulated via their BCR for 3 min (+). DT40 cells lacking expression of respective proteins served as negative controls. The phosphorylation efficiency was examined by Western blot analysis of either cleared cellular lysates (CCL) using a site-specific anti-phosphotyrosine antibody against Y1020 of SHIP (B) or immunopurification (IP) of heterologously expressed SHP-1-GFP (C) using anti-phosphotyrosine antibodies. As loading control membranes were reprobed with anti-actin (B) or anti-GFP (C). The Western blot data were quantified, and the mean values ± S.D. of normalized phosphotyrosine signal intensities are plotted in the right panels of B and C. * p < 0.05; ns, not significant.
DISCUSSION
Here we elucidated molecular details of how Dok-3/Grb2 complexes attenuate BCR signals and provide evidence that Dok-3/Grb2 translocates to microsignalosomes of activated B cells. Dok-3/Grb2 presence in these areas is associated with a shift in Lyn-dependent signaling processes, leading to decreased activation of Syk and increased activation of the negative regulator SHIP.
We provide several lines of evidence that Dok-3/Grb2 attenuates the activity of Syk, which is a pivotal effector for the initiation of the BCR signaling cascade. First, we found Dok-3/Grb2 to reduce Syk tyrosine phosphorylation in DT40 and DG75 B cells, which results, second, in attenuation of Syk-kinase activity and third, in decreased phosphorylation of the Syk substrate SLP-65. These data are consistent with our previous finding that Dok-3/Grb2 regulates the BTK-dependent phosphorylation of PLC-γ2 (21), because in the BCR signaling cascade this process is downstream of Syk and SLP-65. The Dok-3/Grb2-dependent regulation of Syk is on the basis of reduced phosphorylation of a single tyrosine residue at position 352, as revealed by our mass spectrometry-based phospho-analysis as well as Western blot analysis and flow cytometry. This residue and Tyr-348 are located within the interdomain B and have been shown to be the most dynamic phospho-acceptor sites in BCR-activated cells (31). These sites are conserved in the Syk/ZAP70 family of protein tyrosine kinases and important for allosteric regulation of Syk and ZAP70 (5, 37). AlthoughTyr-352 has been reported to be autophosphorylated (5), our analysis revealed that Dok-3/Grb2 still reduces Syk phosphorylation in the presence of the Syk inhibitor Bay 61-3606 but not in the absence of Lyn. These data strongly imply that Lyn is important for the phosphorylation of Syk Tyr-352, which is consistent with reports showing that Lyn augments Syk activation (38). Moreover, these results show that Dok-3/Grb2 regulates the Lyn-dependent Syk phosphorylation rather than the autophosphorylation process, although the Lyn kinase activity remains unaltered. Hence, we propose that the access of Lyn to the Syk tyrosine residue Tyr-352 is perturbed when Dok-3/Grb2 complexes are present in BCR microsignalosomes. It has been shown that the corresponding site in the Syk paralog ZAP-70 is a component of an autoinhibitory hydrophobic pocket, which is disrupted upon tyrosine phosphorylation (39). Dok-3/Grb2 might stabilize this hydrophobic structure and reduce the efficiency of Lyn-dependent phosphorylation of Tyr-352. Recently, Dok-3 was described as a suppressor of lung tumors (40). Given the fact that Syk was reported to act as a tumor suppressor in breast and lung cancers (41, 42) and that a Syk variant lacking a linker insert that precedes Tyr-352 is predominantly expressed in breast cancer cells (43), it is tempting to speculate that the tumor suppressor function of Dok-3 is related to its Syk-regulating properties.
BCR-proximal signaling processes have been shown to be confined to areas of BCR microclusters, which are spontaneously formed after binding of antigen (4, 44, 45). Hence, a Lyn-proximal localization of Dok-3/Grb2 within these BCR microsignalosomes is required to attenuate the Lyn-dependent Syk activation. We show that the correct localization of Dok-3/Grb2 is an interdependent process. AlthoughDok-3 recruits Grb2 to the plasma membrane, Grb2 controls the translocation of Dok-3/Grb2 complexes into BCR microsignalosomes. This conclusion rests on the findings that first, in contrast to wild-type Dok-3, the non-Grb2-binding variant Dok-3(YF) is hardly abundant at sites of BCR microclusters, and second, the same domains that are essential for Dok-3/Grb2 function confer the correct localization within the plasma membrane. The latter was shown by a chimeric Dok-3 protein encompassing the PH and phosphotyrosine-binding (PTB) domains of Dok-3 and the cSH3 domain of Grb2, which, in marked contrast to the variant with a dysfunctional cSH3 domain, inhibits BCR signals and colocalizes with BCR microclusters. Our data are consistent with recent findings that Dok-3 does not colocalize with BCR microclusters in Grb2-deficient cells (46) and with the fact that known cSH3 binding partners like Vav-3 and SLP-65 reside at sites of BCR microclusters (4, 36). Within BCR microsignalosomes, the Lyn-proximal localization of Dok-3·Grb2 complexes allows for efficient phosphorylation of Dok-3. Because we have shown earlier that phosphorylation of the tyrosine residue C-terminal of the Dok-3 PH domain (Tyr-140) enables the formation of Dok-3 oligomers by virtue of phosphotyrosine/PTB domain interactions (21), augmented phosphorylation further increases the abundance of Dok-3·Grb2 complexes in microsignalosomes. Despite our previous findings showing that PTB domain function and Tyr-140 are dispensable for BCR inhibition, oligomerization might support Dok-3/Grb2 function in conditions with low antigen concentration. This interpretation is supported by the fact that the Dok-3 cSH3 chimeric protein with a Tyr-140-to-Phe exchange inhibits BCR signals less efficiently than wild-type Dok-3 cSH3 (data not shown).
Beyond providing correct Dok-3 localization within the plasma membrane, the Grb2 cSH3 domain appears to contribute to the inhibitory function of Dok3-/Grb2. This conclusion is supported by our experiments using tSH2-Dok-3 chimeric proteins. These chimeras are correctly translocated to BCR microsignalosomes by virtue of the Syk SH2 domains, which leads to an efficient and Grb2-independent phosphorylation of the Dok-3 part. Nevertheless, the Grb2-binding chimera inhibits BCR signals more efficiently than the Tyr-to-Phe variant. These data imply that Grb2 contributes to signal inhibition in BCR microsignalosomes accommodating important negative regulators of BCR signaling like SHP-1, SHP-2, Cbl (Casitas B lineage lymphoma), and SHIP, all of which are known to bind the Grb2 cSH3 domain (36). As for SHP-1 we did not find a significant impact of Dok-3/Grb2 on SHP-2 and Cbl (data not shown). However, we observed an augmented SHIP phosphorylation in the presence of Dok-3/Grb2, which is in line with previous findings that the BCR-induced SHIP phosphorylation is attenuated in B cells from dok-3−/− mice (22). SHIP is known to bind the Dok-3 PTB domain in addition to the cSH3 domain of Grb2 (36, 47, 48), which could facilitate an increased recruitment of SHIP into BCR microsignalosomes. Similar effects are implicated after concomitant engagement of the BCR and the inhibitory coreceptor fragment crystalline γ receptor IIb, where a stronger SHIP phosphorylation correlates with increased complex formation between Dok-3/Grb2 and SHIP (49). Hence, SHIP-mediated disruption of phosphatidyl-inositol-3,4,5-trisphosphate destabilizes the Ca2+ initiation complex which, in addition to the attenuated Syk activity, appears to complement the inhibitory function of Dok-3/Grb2. This conclusion is further supported by the fact that we still observed an impact of Dok-3/Grb2 on BCR signals of cells that express the Syk T348,352D variant, which should be unsusceptible for Dok-3/Grb2.
Interestingly, SHIP is also a target of Lyn, implying that Dok-3/Grb2 abundance in BCR microsignalosomes shifts the balance between activatory and inhibitory Lyn functions toward signal inhibition by attenuating Lyn-dependent Syk activation on one hand and augmenting Lyn-dependent SHIP activation on the other hand. This conclusion is supported by the fact that Dok-3-cSH3 chimeras have no impact on BCR-induced Ca2+ mobilization in the absence of Lyn. Hence, the complex diversity of Lyn effector functions may be controlled by the composition of BCR microsignalosomes, in particular by the number of Dok-3·Grb2 complexes that are recruited to these sites.
DT40 B cells represent an immature B cell stage where Grb2 recruitment is strictly Dok-3-dependent (21). In B cells of other developmental stages, Grb2 can be recruited to the plasma membrane by various other proteins, e.g. LAT2 in mature B cells or the BCR immunoreceptor tail tyrosine motifs in memory B cells (23, 36, 50). Once Grb2 is sequestered from Dok-3 by those proteins, providing alternative docking sites for the Grb2 SH2 domain, Dok-3·Grb2 complex formation is perturbed, and Dok-3 is not efficiently integrated into the BCR signaling cascade. This explains why LAT2 expression augments BCR signals in mature B cells (16, 23) and why different modes of Grb2 translocation might be the basis for differential BCR signaling.
Collectively, we deciphered the sequence of events leading to BCR signal attenuation by Dok-3/Grb2 and show that Dok-3/Grb2 abundance in BCR microsignalosomes not only requires Lyn but also modulates Lyn function in a feedback manner. This example shows how adapter proteins can modulate the complex processes occurring in BCR signaling by changing the composition of microsignalosomes and/or the accessibility of effector proteins for Lyn and adjust the BCR-induced cellular response to a given developmental B cell stage.
Acknowledgments
We thank Ines Heine, Johanna Lehne, Uwe Plessmann, Tobias Schmidt, Kai Dittmann, and Johannes Lutz for excellent technical assistance and critical reading of the manuscript, respectively.
This work was supported by the Deutsche Forschungsgemeinschaft research group FOR 521; by the State of Lower Saxony; and by the Volkswagen Foundation, Hannover, Germany.
- BCR
- B cell antigen receptor
- Syk
- spleen tyrosine kinase
- Lyn
- protein tyrosine kinase
- PH
- pleckstrin homology
- SHIP
- SH2 domain-containing inositol 5′ phosphatase.
REFERENCES
- 1. Reth M., Wienands J. (1997) Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15, 453–479 [DOI] [PubMed] [Google Scholar]
- 2. Schamel W. W., Reth M. (2000) Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13, 5–14 [DOI] [PubMed] [Google Scholar]
- 3. Depoil D., Fleire S., Treanor B. L., Weber M., Harwood N. E., Marchbank K. L., Tybulewicz V. L., Batista F. D. (2008) CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol. 9, 63–72 [DOI] [PubMed] [Google Scholar]
- 4. Weber M., Treanor B., Depoil D., Shinohara H., Harwood N. E., Hikida M., Kurosaki T., Batista F. D. (2008) Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J. Exp. Med. 205, 853–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulathu Y., Grothe G., Reth M. (2009) Autoinhibition and adapter function of Syk. Immunol. Rev. 232, 286–299 [DOI] [PubMed] [Google Scholar]
- 6. Kurosaki T., Hikida M. (2009) Tyrosine kinases and their substrates in B lymphocytes. Immunol. Rev. 228, 132–148 [DOI] [PubMed] [Google Scholar]
- 7. Wienands J., Schweikert J., Wollscheid B., Jumaa H., Nielsen P. J., Reth M. (1998) SLP-65. A new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 188, 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu C., Turck C. W., Kurosaki T., Chan A. C. (1998) BLNK. A central linker protein in B cell activation. Immunity 9, 93–103 [DOI] [PubMed] [Google Scholar]
- 9. Goitsuka R., Fujimura Y., Mamada H., Umeda A., Morimura T., Uetsuka K., Doi K., Tsuji S., Kitamura D. (1998) BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J. Immunol. 161, 5804–5808 [PubMed] [Google Scholar]
- 10. Oellerich T., Bremes V., Neumann K., Bohnenberger H., Dittmann K., Hsiao H. H., Engelke M., Schnyder T., Batista F. D., Urlaub H., Wienands J. (2011) The B-cell antigen receptor signals through a preformed transducer module of SLP65 and CIN85. EMBO J. 30, 3620–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashimoto S., Iwamatsu A., Ishiai M., Okawa K., Yamadori T., Matsushita M., Baba Y., Kishimoto T., Kurosaki T., Tsukada S. (1999) Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK. Functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood 94, 2357–2364 [PubMed] [Google Scholar]
- 12. Su Y. W., Zhang Y., Schweikert J., Koretzky G. A., Reth M., Wienands J. (1999) Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 29, 3702–3711 [DOI] [PubMed] [Google Scholar]
- 13. Chiu C. W., Dalton M., Ishiai M., Kurosaki T., Chan A. C. (2002) BLNK. Molecular scaffolding through 'cis'-mediated organization of signaling proteins. EMBO J. 21, 6461–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurosaki T., Tsukada S. (2000) BLNK. Connecting Syk and Btk to calcium signals. Immunity 12, 1–5 [DOI] [PubMed] [Google Scholar]
- 15. Engels N., Engelke M., Wienands J. (2008) Conformational plasticity and navigation of signaling proteins in antigen-activated B lymphocytes. Adv. Immunol. 97, 251–281 [DOI] [PubMed] [Google Scholar]
- 16. Engelke M., Engels N., Dittmann K., Stork B., Wienands J. (2007) Ca(2+) signaling in antigen receptor-activated B lymphocytes. Immunol. Rev. 218, 235–246 [DOI] [PubMed] [Google Scholar]
- 17. Takata M., Sabe H., Hata A., Inazu T., Homma Y., Nukada T., Yamamura H., Kurosaki T. (1994) Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13, 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishizumi H., Taniuchi I., Yamanashi Y., Kitamura D., Ilic D., Mori S., Watanabe T., Yamamoto T. (1995) Impaired proliferation of peripheral B cells and indication of autoimmune disease in Lyn-deficient mice. Immunity 3, 549–560 [DOI] [PubMed] [Google Scholar]
- 19. Hibbs M. L., Tarlinton D. M., Armes J., Grail D., Hodgson G., Maglitto R., Stacker S. A., Dunn A. R. (1995) Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83, 301–311 [DOI] [PubMed] [Google Scholar]
- 20. Chan V. W., Meng F., Soriano P., DeFranco A. L., Lowell C. A. (1997) Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity 7, 69–81 [DOI] [PubMed] [Google Scholar]
- 21. Stork B., Neumann K., Goldbeck I., Alers S., Kähne T., Naumann M., Engelke M., Wienands J. (2007) Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J. 26, 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng C. H., Xu S., Lam K. P. (2007) Dok-3 plays a nonredundant role in negative regulation of B-cell activation. Blood 110, 259–266 [DOI] [PubMed] [Google Scholar]
- 23. Stork B., Engelke M., Frey J., Horejsí V., Hamm-Baarke A., Schraven B., Kurosaki T., Wienands J. (2004) Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity 21, 681–691 [DOI] [PubMed] [Google Scholar]
- 24. Ackermann J. A., Radtke D., Maurberger A., Winkler T. H., Nitschke L. (2011) Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 30, 1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jang I. K., Cronshaw D. G., Xie L. K., Fang G., Zhang J., Oh H., Fu Y. X., Gu H., Zou Y. (2011) Growth-factor receptor-bound protein-2 (Grb2) signaling in B cells controls lymphoid follicle organization and germinal center reaction. Proc. Natl. Acad. Sci. U.S.A. 108, 7926–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niiro H., Maeda A., Kurosaki T., Clark E. A. (2002) The B lymphocyte adaptor molecule of 32 kD (Bam32) regulates B cell antigen receptor signaling and cell survival. J. Exp. Med. 195, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engels N., Wollscheid B., Wienands J. (2001) Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-α. Eur. J. Immunol. 31, 2126–2134 [DOI] [PubMed] [Google Scholar]
- 28. Grabbe A., Wienands J. (2006) Human SLP-65 isoforms contribute differently to activation and apoptosis of B lymphocytes. Blood 108, 3761–3768 [DOI] [PubMed] [Google Scholar]
- 29. Kurosaki T., Takata M., Yamanashi Y., Inazu T., Taniguchi T., Yamamoto T., Yamamura H. (1994) Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J. Exp. Med. 179, 1725–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manders E. M. M., Verbeek F. J., Aten J. A. (1993) Measurement of co-localization of objects in dual-color confocal images. J. Microsc. 169, 375–382 [DOI] [PubMed] [Google Scholar]
- 31. Bohnenberger H., Oellerich T., Engelke M., Hsiao H. H., Urlaub H., Wienands J. (2011) Complex phosphorylation dynamics control the composition of the Syk interactome in B cells. Eur. J. Immunol. 41, 1550–1562 [DOI] [PubMed] [Google Scholar]
- 32. Oellerich T., Grønborg M., Neumann K., Hsiao H. H., Urlaub H., Wienands J. (2009) SLP-65 phosphorylation dynamics reveals a functional basis for signal integration by receptor-proximal adaptor proteins. Mol. Cell. Proteomics 8, 1738–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong S. E., Blagoev B., Kratchmarova I., Kristensen D. B., Steen H., Pandey A., Mann M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto N., Takeshita K., Shichijo M., Kokubo T., Sato M., Nakashima K., Ishimori M., Nagai H., Li Y. F., Yura T., Bacon K. B. (2003) The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61–3606) blocks antigen-induced airway inflammation in rodents. J. Pharmacol. Exp. Ther. 306, 1174–1181 [DOI] [PubMed] [Google Scholar]
- 35. Fleire S. J., Goldman J. P., Carrasco Y. R., Weber M., Bray D., Batista F. D. (2006) B cell ligand discrimination through a spreading and contraction response. Science 312, 738–741 [DOI] [PubMed] [Google Scholar]
- 36. Neumann K., Oellerich T., Urlaub H., Wienands J. (2009) The B-lymphoid Grb2 interaction code. Immunol. Rev. 232, 135–149 [DOI] [PubMed] [Google Scholar]
- 37. Brdicka T., Kadlecek T. A., Roose J. P., Pastuszak A. W., Weiss A. (2005) Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol. Cell Biol. 25, 4924–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsang E., Giannetti A. M., Shaw D., Dinh M., Tse J. K., Gandhi S., Ho H., Wang S., Papp E., Bradshaw J. M. (2008) Molecular mechanism of the Syk activation switch. J. Biol. Chem. 283, 32650–32659 [DOI] [PubMed] [Google Scholar]
- 39. Deindl S., Kadlecek T. A., Brdicka T., Cao X., Weiss A., Kuriyan J. (2007) Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 129, 735–746 [DOI] [PubMed] [Google Scholar]
- 40. Berger A. H., Niki M., Morotti A., Taylor B. S., Socci N. D., Viale A., Brennan C., Szoke J., Motoi N., Rothman P. B., Teruya-Feldstein J., Gerald W. L., Ladanyi M., Pandolfi P. P. (2010) Identification of DOK genes as lung tumor suppressors. Nat. Genet. 42, 216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong S. W., Ma L., Xu N., Yan H. Q., Liu H. Y., Li Y. W., Zhang P. (2011) Research on the reactivation of Syk expression caused by the inhibition of DNA promoter methylation in the lung cancer. Neoplasma 58, 89–95 [DOI] [PubMed] [Google Scholar]
- 42. Coopman P. J., Do M. T., Barth M., Bowden E. T., Hayes A. J., Basyuk E., Blancato J. K., Vezza P. R., McLeskey S. W., Mangeat P. H., Mueller S. C. (2000) The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 406, 742–747 [DOI] [PubMed] [Google Scholar]
- 43. Wang L., Duke L., Zhang P. S., Arlinghaus R. B., Symmans W. F., Sahin A., Mendez R., Dai J. L. (2003) Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 63, 4724–4730 [PubMed] [Google Scholar]
- 44. Depoil D., Weber M., Treanor B., Fleire S. J., Carrasco Y. R., Harwood N. E., Batista F. D. (2009) Early events of B cell activation by antigen. Sci. Signal 2, pt1. [DOI] [PubMed] [Google Scholar]
- 45. Harwood N. E., Batista F. D. (2008) New insights into the early molecular events underlying B cell activation. Immunity 28, 609–619 [DOI] [PubMed] [Google Scholar]
- 46. Schnyder T., Castello A., Feest C., Harwood N. E., Oellerich T., Urlaub H., Engelke M., Wienands J., Bruckbauer A., Batista F. D. (2011) B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity 34, 905–918 [DOI] [PubMed] [Google Scholar]
- 47. Lemay S., Davidson D., Latour S., Veillette A. (2000) Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell Biol. 20, 2743–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robson J. D., Davidson D., Veillette A. (2004) Inhibition of the Jun N-terminal protein kinase pathway by SHIP-1, a lipid phosphatase that interacts with the adaptor molecule Dok-3. Mol. Cell Biol. 24, 2332–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neumann K., Oellerich T., Heine I., Urlaub H., Engelke M. (2011) Fc g receptor IIb modulates the molecular Grb2 interaction network in activated B cells. Cell. Signal. 23, 893–900 [DOI] [PubMed] [Google Scholar]
- 50. Engels N., König L. M., Heemann C., Lutz J., Tsubata T., Griep S., Schrader V., Wienands J. (2009) Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat. Immunol. 10, 1018–1025 [DOI] [PubMed] [Google Scholar]