Background: The role of phosphatidylinositol-3-kinase member PI3K-C2α in actions of S1P is unknown, although it is implicated in membrane trafficking.
Results: PI3K-C2α is essential for S1P-induced S1P1 internalization, endosomal Rac1 activation, and cell migration in endothelial cells.
Conclusion: Internalized S1P1 generates Rac signal and participates in cell migration.
Significance: PI3K-C2α controls receptor-mediated cell signaling and migration by regulating vesicular trafficking.
Keywords: Cell Migration, Endosomes, Phosphatidylinositol 3-Kinase, Rac, Sphingosine 1-Phosphate, S1P1 Receptor
Abstract
The phosphatidylinositol (PtdIns) 3-kinase (PI3K) family regulates diverse cellular processes, including cell proliferation, migration, and vesicular trafficking, through catalyzing 3′-phosphorylation of phosphoinositides. In contrast to class I PI3Ks, including p110α and p110β, functional roles of class II PI3Ks, comprising PI3K-C2α, PI3K-C2β, and PI3K-C2γ, are little understood. The lysophospholipid mediator sphingosine 1-phosphate (S1P) plays the important roles in regulating vascular functions, including vascular formation and barrier integrity, via the G-protein-coupled receptors S1P1–3. We studied the roles of PI3K-C2α in S1P-induced endothelial cell (EC) migration and tube formation. S1P stimulated cell migration and activation of Akt, ERK, and Rac1, the latter of which acts as a signaling molecule essential for cell migration and tube formation, via S1P1 in ECs. Knockdown of either PI3K-C2α or class I p110β markedly inhibited S1P-induced migration, lamellipodium formation, and tube formation, whereas that of p110α or Vps34 did not. Only p110β was necessary for S1P-iduced Akt activation, but both PI3K-C2α and p110β were required for Rac1 activation. FRET imaging showed that S1P induced Rac1 activation in both the plasma membrane and PtdIns 3-phosphate (PtdIns(3)P)-enriched endosomes. Knockdown of PI3K-C2α but not p110β markedly reduced PtdIns(3)P-enriched endosomes and suppressed endosomal Rac1 activation. Also, knockdown of PI3K-C2α but not p110β suppressed S1P-induced S1P1 internalization into PtdIns(3)P-enriched endosomes. Finally, pharmacological inhibition of endocytosis suppressed S1P-induced S1P1 internalization, Rac1 activation, migration, and tube formation. These observations indicate that PI3K-C2α plays the crucial role in S1P1 internalization into the intracellular vesicular compartment, Rac1 activation on endosomes, and thereby migration through regulating vesicular trafficking in ECs.
Introduction
Phosphatidylinositol (PtdIns)3 3-kinases (PI3Ks), the family of enzymes responsible for the generation of 3′-phosphorylated phosphoinositides, have been extensively studied, and it is now established that PI3Ks are crucial components of many signaling pathways (1–3). Among the three classes of PI3K members, the majority of these studies have been focused on class I PI3Ks, and their main in vivo product is PtdIns 3,4,5-trisphosphates (PtdIns(3,4,5)P3). Vps34, the sole member of class III, generates PtdIns 3-phosphate (PtdIns(3)P) to regulate vesicular trafficking/autophagy (4). Class II PI3Ks comprise three members, PI3K-C2α (C2α), PI3K-C2β (C2β), and PI3K-C2γ, and mainly produce PtdIns(3)P in vivo (5–7). C2α is distinct from the other members of PI3Ks due to its unique structure of the presence of a clathrin-binding domain in the N terminus (8). C2α is enriched in clathrin-coated endocytic vesicles, endosomes, and the trans-Golgi network and has been implicated in the regulation of intracellular vesicular trafficking (9, 10). C2α is expressed in a restricted pattern (i.e. mainly in the epithelium, vascular endothelium, and smooth muscle) (11, 12).
Recently, we showed that C2α plays a crucial role in developmental and pathological angiogenesis and maintenance of the endothelial barrier function in an EC-autonomous manner through regulating vesicular trafficking (13). Global and endothelial cell (EC)-specific C2α-null mice were embryonic lethal due to defects in angiogenesis. C2α knockdown in ECs reduced PtdIns(3)P-enriched endosomes, impaired endosomal trafficking, and caused defective delivery of VE-cadherin to EC junctions and its assembly. C2α knockdown also impeded cell signaling, including vascular endothelial growth factor receptor internalization and endosomal RhoA activation. These together led to defective EC migration, proliferation, tube formation, and barrier integrity. Endothelial PI3K-C2α deletion suppressed postischemic and tumor angiogenesis and compromised vascular barrier integrity with augmented susceptibility to anaphylaxis and a higher incidence of dissecting aortic aneurysm formation (13).
Sphingosine 1-phosphate (S1P), a bioactive lysophospholipid mediator, induces diverse cellular responses, including cell migration, proliferation, survival, and differentiation, mainly through five S1P-specific G-protein-coupled receptors, S1P1–5 (14–19). S1P1, S1P2, and S1P3 are widely expressed in various tissues and are major receptors in the vasculature (18, 20). In vascular ECs, S1P stimulates cell migration and facilitates intercellular adherens junction formation mainly via S1P1 and, to a lesser extent, S1P3 (21–24). Targeted disruption of the S1P1 gene in mice impairs vascular angiogenesis, maturation, or stabilization (25–27), indicating the significance of S1P-S1P1 signaling axis in vascular formation.
Rho family GTPases have emerged as key regulators of cell migration and cell adhesion. Specifically, Rac activity is increased at the leading edge of migrating cells (28). This activity drives the formation of lamellipodial protrusion and subsequent forward movement of cells (29, 30). Rac activity also directs the formation of focal complexes (30–32). Cell motility critically relies on localized signaling. Recent studies suggested that Rac activation occurs on early endosomes, where a Rac-guanine nucleotide exchange factor (GEF) is also recruited (33–35). Further studies showed that controlled intracellular trafficking of Rac is important for spatially proper activation and actions of Rac (29, 35). Thus, the mechanism of endosomal Rac activation may be required for the spatial resolution of Rac-dependent mitogenic signals. Previous studies suggested that S1P1-mediated activation of Rac plays a crucial role in S1P-directed migration of ECs (20, 22). However, it is unknown how S1P1 regulates Rac activity spatially within cells.
In this study, we investigated the role of C2α for S1P-induced EC migration and activation of cellular signaling. Our data demonstrate for the first time that C2α is pivotal for S1P-induced cell migration, lamellipodium formation, and tube formation in ECs. Importantly, C2α is also essential for the particular cell signaling, Rac1 activation; S1P-induced Rac1 activation occurs on endosomes, in which internalized S1P1 is co-localized, in a C2α-dependent manner. Thus, these observations indicate that, different from the classical role of receptor internalization as a mechanism for receptor desensitization or inactivation, internalized S1P1 serves a role for signaling on endosomes and thereby migration and morphogenesis and that these S1P-induced processes are orchestrated by C2α.
EXPERIMENTAL PROCEDURES
Reagents and Constructs
The following antibodies and reagents were used: anti-C2α antibody (catalog no. 611047, BD Pharmingen); anti-C2β antibody (P91720, BD Transduction); anti-p110α antibody (catalog no. 4249, Cell Signaling Technology (CST)); anti-p110β antibody (catalog no. 3011, CST); anti-Rac1 antibody (catalog no. 05-389, Upstate-Millipore); anti-RhoA antibody (clone 26C4, sc-418, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-active GTP-Rac1 antibody (catalog no. 26903, NewEast Biosciences); anti-phospho-paxillin antibody (Tyr-118) (catalog no. 2541, CST); anti-α-tubulin antibody (MS-581-P0, NeoMarker); anti-Vps34 antibody (catalog no. 3358, CST); anti-phospho-p42/p44 MAPK antibody (ERK1/2, Thr-202/Tyr-204, catalog no. 4370, CST), anti-total p42/p44 MAPK antibody (ERK1/2, catalog no. 9102, CST), anti-phospho-Akt antibody (Ser-473, catalog no. 4060, CST), anti-total Akt antibody (catalog no. 9272, CST), anti-GSK-3β (catalog no. 5676, CST), anti-phospho-GSK-3β (Ser-9) (catalog no. 5558, CST), all AlexaFluor-conjugated secondary antibodies and AlexaFluor594-phalloidin (Molecular Probes, Inc.), 4′,6-diamidino-2-phenyliindole (DAPI) (Invitrogen), dynasore hydrate (Sigma), S1P (catalog no. SL-140, Enzo Life Sciences), W146 (catalog no. 10009109, Cayman), and Akt1/2 inhibitor VIII (catalog no. 124018, Calbiochem). GFP-2xFYVE and mRFP-2XFYVE expression vectors were obtained from Dr. H. Stenmark (University of Oslo) and Dr. Y. Ohsumi (Tokyo Institute of Technology), respectively. The expression vector for Rac1-FRET probe, Raichu-Rac (pRaichu-Rac1), was obtained from Dr. M. Matsuda (Kyoto University). S1P1-mRFP expression vectors were constructed by standard PCR methods.
Cell Culture, Short Interfering RNAs (siRNAs), and Transfection
Human umbilical vein endothelial cells (HUVEC) (Lonza) of passages 2–5 were grown in endothelial basal medium supplemented with 2% fetal bovine serum (FBS) and a growth factor supplement mixture (EBM2TM; Lonza catalog no. CC-3162) on type I collagen-coated dishes. The cells were transfected with 20 nm siRNA using Lipofectamine 2000 (Invitrogen) in Opti-MEM (Invitrogen) according to the manufacturer's instructions. After 4 h, the media were replaced with EBM2. The cells were cultured for a further 24–48 h before processing for analysis. The human C2α siRNA target sequence was 5′-AAGGTTGGCACTTACAAGAAT-3′ (the first nucleotide of the target sequence is positioned in the nucleotide 2182 when “A” of the start codon ATG of the mRNA sequence is numbered as 1). The human Vps34 target sequence was 5′-AAACTCAACACTGGCTAATTA-3′ (the first nucleotide is positioned in the nucleotide 1513). The human p110α target sequence was 5′-GGACAACTGTTTCATATAG-3′ (D-003018-7, Dharmacon). The human C2β target sequence was 5′-AAGCCGGAAGCTTCTGGGTTT-3′ (the first nucleotide is positioned in the nucleotide 2889). The human p110β target sequence was 5′-AATCCCTCTAAATATCAGACC-3′ (the first nucleotide is positioned in the nucleotide 1328). As a control, a scrambled duplex oligonucleotide with a C/G content equivalent to the positive oligonucleotide was used. All siRNAs were designed and synthesized using the siRNA construction kit (Ambion) according to the manufacturer's instructions. Efficacy of RNA interference was confirmed by Western blotting using specific antibodies. The cells were transfected with the expression vectors for GFP/mRPF-2xFYVE and mRFP-S1P1 by Amaxa Nucleofector (Lonza) according to the manufacturer's instructions.
Transwell Migration Assay
Transwell cell migration across a type I collagen-coated polycarbonate filter with 8-μm pores (Neuroprobe) was determined using a modified Boyden chamber (a 96-blind well chamber; Neuroprobe) as described previously (36, 37). The indicated concentrations of S1P in M199 medium (Invitrogen) containing 0.25% fatty acid-free bovine serum albumin (BSA) (Sigma-Aldrich) were loaded into the lower wells. The siRNA-transfected HUVEC (1 × 105 cells/well) resuspended in 0.25% BSA-containing M199 medium were placed in the upper wells and allowed to migrate toward the lower chamber at 37 °C in 5% CO2 for 6 h. The cells attached on the filter were fixed with methanol and stained with a Diff-Quick staining kit (Baxter Scientific Products). The upper side of the filter was then scraped free of cells. The number of cells on the lower side of membranes was determined by measuring optical densities at 595 nm using a 96-well microplate reader model 3550 (Bio-Rad).
Pull-down Assay of Small G-protein Activity
The pull-down assays for GTP-bound Rac1 and RhoA were performed as described previously (36, 38–40). In brief, HUVEC were stimulated and lysed with the Rac/Rho extraction buffer (50 mm Tris-HCl (pH7.5), 500 mm NaCl, 10 mm MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 1 mm Na3VO4, 10 mm NaF, 10 mm sodium β-glycerophosphate, and Complete protease inhibitor mixture (Roche Applied Science)). The lysates were sonicated and cleared by centrifugation, and the resultant supernatants were incubated for 60 min at 4 °C with glutathione-Sepharose 4B beads (GE Healthcare) coupled to either the CRIB domain of PAK1 for Rac1 or the Rho binding domain of rhotekin for RhoA. The beads were washed, and bound proteins were solubilized by the addition of 50 μl of 2× Laemmli's SDS-PAGE loading buffer, followed by separation on 15% SDS-polyacrylamide gels and Western blotting using anti-Rac1 or anti-RhoA antibodies. The band densities of Rac1 and RhoA were determined by densitometry. The amounts of GTP-Rac1 and GTP-RhoA were corrected for those of total Rac1 and RhoA, respectively, and the normalized quantified data were expressed as multiples over the values of non-treated control cells.
Tube Formation Assay
siRNA-transfected HUVEC (2.5 × 104 cells) in M199 containing 1% FBS were seeded onto 200 μl of growth factor-reduced Matrigel (BD Biosciences) in a 24-well plate in the absence or presence of S1P and dynasore and incubated for 6–12 h (41). Tube formation was quantified by measuring cumulative tube length in five random microscopic fields/well with ImageJ software (National Institutes of Health).
Immunostaining
HUVEC cultured on cover slides or glass-bottomed dishes were serum-starved in M199 medium including 0.5% BSA overnight or for 6 h and stimulated with S1P (41). The cells were fixed in prewarmed 4% paraformaldehyde in Dulbecco's phosphate-buffered saline (PBS) for 15 min and then permeabilized in 0.2% Triton X-100 in PBS for 15 min when necessary. After blocking, the cells were incubated with primary antibodies overnight and then incubated for 1 h with goat AlexaFlour488- or AlexaFlour594-conjugated secondary antibodies. The cells were counterstained with DAPI (Molecular Probes) for 5 min and mounted on Fluoromount. The stained cells were observed using a confocal laser-scanning microscope (Zeiss Axiovert 200M with LSM5 Pascal) equipped with a ×63/numerical aperture 1.4 PlanApo oil immersion objective. For quantification of the amount of positive pixels, the positive area in stained images in each frame was calculated using ImageJ software. Adobe Photoshop was used to adjust image levels and process image overlays.
Actin Filament (F-actin) Staining
HUVEC were treated with either vehicle or S1P, fixed in 3.7% formaldehyde or 4% paraformaldehyde in PBS, and permeabilized in 0.2% Triton X-100 in PBS, followed by staining with AlexaFluor594-conjugated phalloidin for F-actin, as described previously (37). Total cellular perimeter and lamellipodial perimeter per cell were measured by using ImageJ software, and the lamellipodial index (lamellipodial perimeter length/total cell perimeter × 100) was determined.
FRET Imaging
HUVEC were transfected with the Rac FRET probe expression vector pRaichu-Rac1 (42), using the Amaxa Nucleofector system according to the manufacturer's instructions. The chimeric FRET probe protein consists of N-terminal yellow fluorescent protein (YFP), the CRIB domain of PAK1, Rac1, and C-terminal cyan fluorescent protein (CFP). When Rac1 in the chimeric FRET probe protein is bound to GDP, fluorescence of 475 nm emanates from CFP with excitation at 433 nm. When Rac1 is bound to GTP, intramolecular binding of GTP-loaded Rac to the CRIB domain brings YFP into close proximity to CFP, which causes FRET and fluorescence of 527 nm from YFP. For confocal FRET imaging, cells were serum-starved in phenol red-free M199, including 0.25% BSA, for 6 h. Then, the cells were incubated in a fresh medium with the same compositions and observed/imaged on a heated stage (37 °C) (Thermo Plate, Tokai-Hit) in an atmosphere of 5% CO2 and 95% air (2GF-MIXER, Okolab) on an inverted microscope (model IX70, Olympus)-based high resolution confocal laser microscope system (CSU system, Yokogawa) equipped with a UPLSAPO ×60/numerical aperture 1.35 oil objective, an electron-multiplying charge-coupled device digital camera (iXon, Andor), and a light engine (Lumencor, Inc.) configured with a CFP/YFP filter set (Di01-T442/514/647–13 × 15 × 0.5, Semrock). The acquisition and process were controlled by iQ software (Andor). Images were taken at every 10-s interval, and movies were prepared at a frequency of 5 frames/s. Pseudo-gray scale ratio images were generated from images from CFP and FRET channels using Andor iQ software.
Imaging of GFP- and RFP-tagged Proteins
HUVEC were transfected with the expression vectors for GFP/mRFP-2xFYVE and S1P1-mRFP, using the Amaxa Nucleofector system (Lonza), plated on glass-bottomed dishes (catalog no. P35G-1.5–20-C, MatTek), and allowed to adhere for 16 h before imaging. The cells were serum-starved in M199 medium, including 0.5% BSA, for 6 h and stimulated with S1P. The cells were fixed in 4% paraformaldehyde for 15 min and observed with a high resolution confocal laser microscope system (CSU System, Yokogawa), as described above.
Quantitative Real-time PCR (qPCR)
Total RNAs were extracted using TRIzol reagent (Invitrogen). Total RNA (1 μg) was reverse-transcribed using a QuantiTect Transcription Kit (Qiagen). Absolute qPCR (absolute copy number quantification using the standard curve for each gene) was performed using qPCRMasterMix plus for SYBR assay (Eurogentec, RT-SY2X-03+WOU) in the ABI7300 real-time PCR system (Applied Biosystems), as described previously (13). The primers employed were as follows: for S1P1, S1P1-F (tgcgggaagggagtatgttt) and S1P1-R (cgatggcgaggagactgaac); for S1P2, S1P2-F (gcctctctacgccaagcatta) and S1P2-R (ttgagcggaccacgcagta); for S1P3, S1P3-F (ggtgattgtggtgagcgtgtt) and S1P3-R (aggccacatcaatgaggaaga); for S1P4, S1P4-F (aagaccagccgcgtcta) and S1P4-R (ccaggcagaagaggatgt); for S1P5, S1P5-F (ggaaatgcagccaaagg) and S1P5-R (ccattatttcatcaccgagtt); and for β-actin, β-actin-F (tctacaatgagctgcgtgtg) and β-actin-R (atggctggggtgttgaag). We have validated that these primer pairs are highly specific for each target without cross-amplification. Absolute quantification determined the actual copy numbers of target genes by relating the Ct value to a standard curve. The levels of S1P receptor mRNAs were expressed as multiples of mRNA level of the reference gene, β-actin.
Western Blot Analysis
The cells were washed in PBS and lysed in cell lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 1 mm Na3VO4, 10 mm NaF, 10 mm sodium β-glycerophosphate, and Complete protease inhibitor mixture) by scraping, and cell lysates were centrifuged for 10 min at 16,000 × g at 4 °C, the resulting supernatants taken for Western blotting. Cell lysates were subjected to 8 or 15% SDS-PAGE, followed by electrotransfer to polyvinylidene difluoride membranes (Millipore). After transfer, the membranes were blocked in 5% BSA, 0.1% Tween 80 in Tris-buffered saline (10 mm Tris (pH 7.5) and 150 mm NaCl). Target proteins were detected with specific antibodies. After incubation with the appropriate alkaline phosphatase-conjugated secondary antibodies, protein bands were visualized by the color reaction using the nitro blue tetrazolium chloride/5-bromo-4-chloro-3′-indolylphosphatase p-toluidine system (Sigma).
Metabolic Cell Labeling, Lipid Extraction, and Phosphoinositide Measurements
The amounts of cellular phosphoinositides were determined as described (43, 44). In brief, HUVEC in 10-cm dishes were serum-starved in phosphate-free Dulbecco's minimal essential medium (DMEM) for 1 h and labeled by [32P]orthophosphate (1 mCi/dish; PerkinElmer Life Sciences) for 4 h in phosphate-free DMEM. The labeled cells were stimulated as indicated. The cells were washed twice with ice-cold PBS and quickly scraped in 800 μl of 2% HClO4. Resulting extracts were mixed with 3 ml of chloroform/methanol (1:2) and vortexed. The mixture were then incubated at room temperature for 10 min and mixed with each 1 ml of CHCl3 and 2% HClO4 to separate the organic and aqueous phases. After centrifugation, the lower organic phase was recovered and mixed with 1 ml of CHCl3-saturated 0.5% NaCl and 1% HClO4. The mixture was vortexed well and then centrifuged. After centrifugation, the lower organic phase was recovered and dried in a vacuum centrifuge. Dried lipids were deacylated with 1 ml of 40% methylamine/H2O/methanol/n-butanol (24:16:40:10), incubated at 53 °C for 1 h, dried in a vacuum centrifuge, and resuspended in 800 μl of n-butanol/petroleum ether/ethyl formate (20:40:1) and 800 μl of H2O. After centrifugation, the aqueous phase was transferred and dried in a vacuum centrifuge. The resulting samples were resuspended in 130 μl of 10 mm (NH4)2HPO4 and analyzed by high performance liquid chromatography (HPLC) using a Partisphere SAX column (Whatman), as described (43, 44). The radioactivity of each fraction was determined by scintillation counting. The amounts of PtdIns(3)P and PtdIns(3,4,5)P3 were expressed as the ratios of the radioactivities of PtdIns(3)P or PtdIns(3,4,5)P3 over those of PtdIns(4)P plus PtdIns(4,5)P2, as described previously (43, 44).
Statistical Analysis
All the data are presented as the means ± S.E. and were analyzed using Prism 5 software (GraphPad Software Inc., San Diego, CA). All statistical analysis was performed either by one-way or two-way analysis of variance with a subsequent post hoc test. Results with p < 0.05 were considered statistically significant.
RESULTS
S1P1-mediated Cell Migration of HUVEC Is Dependent on Class II PI3K-C2α
The HUVEC expressed S1P1 most abundantly and S1P3 less prominently but did not detectably express S1P2, S1P4, or S1P5, as determined by qPCR analysis (Fig. 1A). As reported previously (22–24), S1P stimulated transwell migration of HUVEC with increased phosphorylation of Akt, a protein kinase activated downstream of PI3K (Fig. 1, B and C). Because S1P1 mediates cell migration through a PI3K-dependent mechanism (36, 45), we examined whether S1P-stimulated migration and Akt phosphorylation in HUVEC were mediated by S1P1 or not. The S1P1-selective antagonist W146 abolished S1P-induced cell migration (Fig. 1B) and Akt phosphorylation (Fig. 1C), indicating that both responses were mediated by S1P1.
FIGURE 1.
S1P stimulates cell migration and Akt activation through S1P1 in HUVEC. A, real-time qPCR analysis of S1P receptor mRNAs in HUVEC. Total RNA was isolated, and qPCR was performed. The mRNA levels of S1P receptors (S1PR) are expressed as S1P receptor/β-actin mRNA ratio. ND, non-detectable. B, S1P-directed migration of HUVEC is inhibited by an S1P1-selective antagonist. Transwell cell migration toward S1P was determined using a modified Boyden chamber. Serum-starved HUVEC were placed in the upper chamber. S1P (0.3 μm) was present only in the lower chamber, whereas the S1P1 antagonist W146 (3 μm) was present in both the upper and lower chambers when indicated. C, S1P-induced Akt phosphorylation is inhibited by an S1P1 antagonist. Serum-starved HUVEC were pretreated or non-pretreated with W146 (3 μm) for 10 min and stimulated with S1P (0.3 μm) or not for 2 min, followed by Western blot analysis using anti-phospho-Akt (p-Akt; Ser-473) and anti-total Akt antibody. D, siRNA-mediated knockdown of PI3K protein expression. HUVEC were transfected with either of C2α-, C2β-, p110α-, p110β-, and Vps34-specific siRNAs or scrambled (sc) siRNA duplex and 48 h later underwent Western blot analysis using anti-PI3K isoform-specific antibodies. α-Tubulin was analyzed as a loading control. E, effects of knockdown of different PI3K isoforms on S1P-induced migration of HUVEC. Various concentrations of S1P were added in the lower chamber. F and G, HPLC analysis of PtdIns(3)P and PtdIns(3,4,5)P3. HUVEC that had been transfected with either C2α-specific (F) or p110β-specific (G) siRNAs or scrambled siRNA duplex were metabolically labeled with [32P]orthophosphate for 4 h, and total cellular lipids were extracted and analyzed for the amounts of PtdIns(3)P and PtdIns(3,4,5)P3. In A, B, C, F, and G, n = 3, and in E, n = 4. In B, C, and E, * and *** denote statistical significance at the levels of p < 0.05 and p < 0.001, respectively, compared with the values in the absence of S1P and W146. In B and C, ### denotes statistical significance between the indicated groups at the level of p < 0.001. Error bars, S.E.
We studied the role of C2α in S1P1-mediated cell migration. The HUVEC were transfected with either of the specific siRNAs targeting class I p110α and p110β, class II C2α and C2β, and class III Vps34 or scrambled siRNA (sc-siRNA) duplex as a control. Each specific siRNA effectively inhibited the expression of respective PI3K proteins (Fig. 1D). Knockdown of p110β or C2α substantially inhibited S1P-directed cell migration, and that of C2β, to a lesser extent, inhibited S1P-directed migration (Fig. 1E). Knockdown of p110α or Vps34 showed no inhibitory effect. We previously showed in HUVEC that C2α knockdown reduced GFP-FYVE-positive vesicles and inhibited their motility (13). In this study, we determined the effects of knockdown of C2α and p110β on the total cellular levels of PtdIns(3)P and PtdIns(3,4,5)P3 in HUVEC by HPLC analysis of cellular lipids. C2α knockdown did not affect the cellular contents of PtdIns(3)P or PtdIns(3,4,5)P3 in non-treated HUVEC (Fig. 1F). S1P simulation did not change the cellular levels of PtdIns(3)P or PtdIns(3,4,5)P3. Knockdown of p110β did not affect the cellular contents of PtdIns(3)P or PtdIns(3,4,5)P3 in S1P-stimulated or non-stimulated HUVEC (Fig. 1G).
S1P-induced Lamellipodium Formation Is Markedly Inhibited in C2α-depleted HUVEC
Because lamellipodium formation in migrating cells is caused by actin filament reorganization, which is the most crucial process for cell migration (46), we studied the effects of knockdown of PI3K isoforms on S1P-induced lamellipodium formation. S1P induced the robust formation of lamellipodia in sc-siRNA-transfected HUVEC (Fig. 2A). Knockdown of either C2α, C2β, or p110β reduced the formation of lamellipodia. The inhibitory effect of C2α knockdown was greater compared with that of C2β knockdown. Migrating cells form focal complexes at lamellipodia, in which phosphorylated paxillin serves as a docking site for clustering of other focal complex proteins (31, 47–49). S1P stimulation increased the number of focal complexes at the lamellipodia of control HUVEC, as determined with anti-phospho-paxillin immunofluorescent staining (Fig. 2B). In C2α-depleted cells, S1P-induced formation of focal complexes was markedly diminished compared with that in control HUVEC.
FIGURE 2.
PI3K-C2α knockdown inhibits the formation of lamellipodia and focal complexes. A, confocal fluorescent microscopic imaging of F-actin (red) in HUVEC. Cells were transfected with either C2α-, C2β-, or p110β-specific siRNAs or sc-siRNA. Twenty-four h later, the cells were serum-starved overnight and then stimulated with S1P (0.3 μm) or not for 10 min, followed by AlexaFluor594-conjugated phalloidin staining. Nuclei were stained by DAPI. The yellow arrows indicate lamellipodia. Lamellipodial formation was quantified as described under “Experimental Procedures” (right). n = 20–24 cells. Scale bar, 20 μm. B, immunofluorescent staining of phosphopaxillin (p-paxillin (Tyr-118)) (green) and F-actin (red) in HUVEC. Serum-starved cells were transfected and stimulated with S1P or not, as in A. Scale bar, 20 μm. Higher magnification views of the boxed areas are shown in the right panels. Scale bar, 10 μm. In A and B, *, **, and *** denote statistical significance at the levels of p < 0.05, p < 0.01, and p < 0.001, respectively, compared with respective non-stimulated control. #, ##, and ### denote statistical significance between the indicated groups at the levels of p < 0.05, p < 0.01, and p < 0.001, respectively. NS, not significant. Error bars, S.E.
Rac1 Activity Is Inhibited in C2α-depleted HUVEC
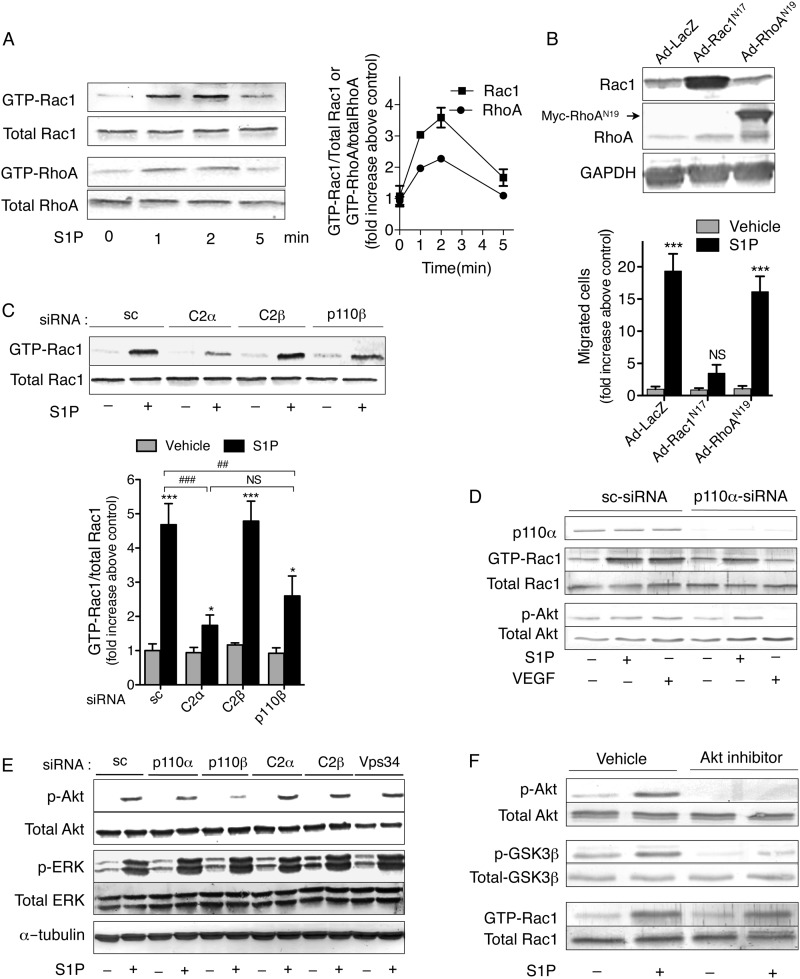
The Rho family GTPases function as molecular switches for cytoskeletal reorganization, focal adhesion/complex assembly, and motility (50). S1P induced time-dependent increases in the amounts of GTP-bound, active forms of Rac1 and RhoA in control HUVEC with 2–3.5-fold peak rises over the basal levels (Fig. 3A). The expression of dominant negative Rac1 mutant (Rac1N17) but not dominant negative RhoA mutant (RhoAN19) inhibited S1P-directed cell migration under our experimental conditions, indicating the essential role of Rac1 in S1P-directed cell migration (Fig. 3B). Because S1P-induced formation of lamellipodia and focal complexes were impaired in C2α-depleted HUVEC, we studied the effects of knockdown of C2α and other PI3K isoforms on the activity of Rac1. C2α depletion markedly suppressed S1P-induced Rac1 activation, and p110β depletion inhibited Rac1 activation less prominently, whereas knockdown of either C2β or p110α failed to inhibit it (Fig. 3, C and D). In contrast, C2α knockdown did not inhibit S1P-induced Akt phosphorylation, whereas p110β knockdown strongly suppressed S1P-induced Akt phosphorylation (Fig. 3E). Knockdown of p110α, C2β, or Vps34 was without any inhibitory effect on S1P-induced Akt activation. In addition, knockdown of either of these PI3Ks did not alter S1P-induced ERK activation. Because previous studies showed the involvement of Akt in Rac activation (51, 52), we studied the involvement of Akt in S1P-induced Rac activation. The Akt inhibitor strongly inhibited S1P-induced phosphorylation of its downstream target GSK3β but had no inhibitory effect on Rac1 activation (Fig. 3F). These observations suggest that C2α and p110β but not C2β are involved in regulating Rac1 activity and thereby cell migration.
FIGURE 3.
PI3K-C2α knockdown inhibits S1P-induced Rac1 activation. A, time-dependent activation of Rac1 and RhoA by S1P in HUVEC. Serum-starved cells were stimulated with S1P (0.3 μm) for the indicated time periods. The cells were subjected to a pull-down assay for GTP-RhoA and GTP-Rac1. B, effects of expression of dominant negative RhoGTPase mutants Rac1N17 and RhoAN19 on S1P-directed migration of HUVEC. Cells were infected with adenoviruses carrying the cDNAs of LacZ (Ad-LacZ), Rac1N17 (Ad-Rac1N17), and Myc-tagged RhoAN19 (Ad-RhoAN19); 24 h later serum-starved overnight; and underwent a transwell migration assay. S1P (0.3 μm) was added to the lower chamber. The protein expression of Rac1N17, Myc-tagged RhoAN19, and GAPDH as endogenous loading control are shown at the top. C–E, effects of knockdown of PI3K isoforms on S1P-induced Rac1 activation and Akt phosphorylation. HUVEC were transfected with either C2α-, C2β-, p110α-, or p110β-specific siRNAs or sc-siRNA. Forty-eight h later, the cells were serum-starved for 6 h and then stimulated with S1P (0.3 μm) for 2 min, stimulated with VEGF (20 ng/ml) for 10 min, or non-treated, followed by the pull-down assay for GTP-Rac1 (C), the pull-down assay for GTP-Rac1, Western blot analysis of phosphorylation of Akt (p-Akt) (D), and Western blot analysis of phosphorylation of Akt and ERK (p-ERK) (E). F, serum-starved HUVEC were pretreated or not with Akt inhibitor VIII (3 μm) for 15 min and stimulated with S1P (0.3 μm) for 2 min, followed by the pull-down assay for GTP-Rac1 and Western blot analysis of phosphorylation of Akt and GSK3β. In A and B, n = 3, and in C, n = 4. D–F represent three independent experiments. In B and C, * and *** denote statistical significance at the levels of p < 0.05 and p < 0.001, respectively, compared with respective non-stimulated control. In C, ## and ### denote statistical significance between the indicated groups at the levels of p < 0.01 and p < 0.001, respectively. Error bars, S.E.
C2α Is Required for Rac1 Activation on Endosomes
We investigated the intracellular sites in which active Rac1 is located or in which Rac1 is activated by S1P within HUVEC by using two different techniques. We first performed immunofluorescent staining using anti-active Rac1 (GTP-Rac1) antibody. We tested the specificity of the anti-active Rac1 antibody. The non-transfected HUVEC and the Cell Tracker Green-labeled HUVEC transfected with Rac1N17, which remains bound to a guanine nucleotide exchange factor and thereby prevents GTP loading of endogenous Rac1, were mixed and cultured. The mixed cell culture was stimulated with S1P and underwent anti-active Rac1 immunofluorescent staining. The non-transfected cells were intensely stained with anti-active Rac1, whereas Rac1N17-transfected cells were nearly negative for anti-active Rac1 staining (Fig. 4A), indicating that the antibody specifically detected active Rac1. The non-stimulated, sc-siRNA-transfected HUVEC showed anti-active Rac1 staining in the plasma membrane, particularly lamellipodia, and in the intracellular compartment, the latter being stained in a granular or fine speckled pattern (Fig. 4B, top). S1P-stimulated cells displayed much more intense staining in both the lamellipodial area and the intracellular compartment compared with non-stimulated cells. Knockdown of C2α markedly attenuated anti-active Rac1 staining in both the lamellipodia and the intracellular compartment of non-stimulated and S1P-stimulated cells.
FIGURE 4.
PI3K-C2α knockdown inhibits S1P-induced Rac1 activation in the intracellular vesicular compartment in HUVEC. A, effects of Rac1N17 expression on anti-active Rac1 immunofluorescent staining in HUVEC. Cells were infected with Ad-Rac1N17, 24 h later labeled with Cell Tracker Green, and mixed with Ad-LacZ-infected control HUVEC at a 1:1 ratio. The mixed cells were plated and cultured overnight. Then the cells were serum-starved for 6 h and stimulated by S1P (0.3 μm) for 2 min, followed by immunofluorescent staining for GTP-Rac1 using anti-active Rac1-specific antibody. Scale bar, 20 μm. B, immunofluorescent staining of active Rac1 in control and C2α-depleted HUVEC. Cells transfected with either C2α-siRNA or sc-siRNA were serum-starved overnight and stimulated with S1P (0.3 μm) or not for 2 min, followed by immunofluorescent staining for GTP-Rac1 as in A. Scale bar, 20 μm. C, effects of C2α-knockdown on anti-active Rac1 immunostaining and GFP-2xFYVE distribution in HUVEC. Cells were transfected with GFP-2xFYVE expression vector, cultured overnight, and then serum-starved for 6 h. The cells were stimulated with S1P (0.3 μm) or not for 2 min and underwent anti-active Rac1 immunofluorescent staining. Scale bar, 20 μm. Higher magnification of the boxed area in the merged view is also shown. Scale bar, 5 μm.
The intracellular granular staining of active Rac1 suggested that Rac1 activation might occur in endosomes, which are relatively rich in PtdIns(3)P (2, 4). Therefore, we studied whether active Rac1 and PtdIns(3)P co-localized or not by determining the localization of active Rac1 and fluorescence of the PtdIns(3)P-specific probe GFP-tagged 2xFYVE domain. HUVEC were transfected with the expression vector for GFP-tagged 2xFYVE domain and subjected to anti-active Rac1 immunofluorescent staining. In S1P-stimulated sc-siRNA-transfected HUVEC, a significant portion of the anti-active Rac1 signal overlapped with the GFP-2xFYVE signal, indicating that at least a part of S1P-activated Rac1 exists in PtdIns(3)P-enriched endosomes (Fig. 4C). S1P stimulation of sc-siRNA-transfected HUVEC did not alter the number, distribution, or motility of GFP-2xFYVE+ vesicles. C2α knockdown substantially reduced the number of GFP-2xFYVE+ endosomes and anti-active Rac1 signal, resulting in a marked decrease of active Rac1 in GFP-2xFYVE+ endosomes.
We determined cellular sites of Rac1 activation in HUVEC by adopting a FRET imaging technique. Rac1 was activated in both the intracellular vesicular compartment and the plasma membrane, particularly membrane ruffles, in S1P-stimulated sc-siRNA-treated HUVEC (Fig. 5A and supplemental Movie 1). There was the tendency that Rac1 activation was first observed in the membrane ruffles and then in the intracellular vesicles. C2α depletion markedly suppressed Rac1-FRET signals in both the plasma membrane and the intracellular compartment in non-stimulated and S1P-stimulated HUVEC (Fig. 5A and supplemental Movie 2). In S1P-stimulated sc-siRNA-treated HUVEC that were co-transfected with the expression vectors for mRFP-tagged 2xFYVE domain and the Rac1-FRET probe, a substantial portion of the intracellular Rac1-FRET signals were co-localized with RFP-2xFYVE signals (Fig. 5, B (top left and bottom (white and yellow arrowheads, respectively)) and C). The Rac1-FRET signal was also intense at cell-cell contacts (Fig. 5B (bottom left, red arrowheads)). However, Rac1-FRET signal+ cell-cell contacts were negative for RFP-2xFYVE. C2α depletion greatly attenuated Rac1-FRET signals in the RFP-2xFYVE+ endosomes (Fig. 5, B (top middle and bottom) and C), indicating that C2α is essential for Rac1 activation in the PtdIns(3)P-enriched endosomes and the plasma membrane. In contrast, knockdown of p110β did not reduce RFP-2xFYVE+ vesicles or intracellular vesicular Rac1-FRET signals but attenuated Rac1-FRET signal in the plasma membrane (Fig. 5, B (top right and bottom) and C).
FIGURE 5.
FRET imaging of inhibition by PI3K-C2α knockdown of S1P-induced Rac1 activation in HUVEC. A, FRET imaging of Rac1 activation in control and C2α-depleted HUVEC. Cells that were co-transfected with pRaichu-Rac1 and either C2α-siRNA or sc-siRNA were cultured overnight and then serum-starved for 6 h, followed by FRET imaging. The cells were stimulated with S1P (0.3 μm). B, co-localization of the Rac1-FRET signal and mRFP-2xFYVE signal. Cells co-transfected with pRaichu-Rac1 and the mRFP-2xFYVE expression vector were cultured overnight and then serum-starved for 6 h. The cells were stimulated with S1P (0.3 μm) for 2 min with fluorescent microscopic observations. The white arrowheads in the 2xFYVE images and the yellow arrowheads in the FRET images are overlapped. The red arrowheads indicate Rac1 activation at cell-cell contacts. C, quantification of mRFP-2xFYVE+ and Rac1-FRET signal+ vesicles. n = 12–20 cells. Scale bar, 20 μm. In C, *** denotes statistical significance at p < 0.001 compared with non-stimulated control, and ### denotes statistical significance between the indicated groups at a level of p < 0.001. NS, not significant. Error bars, S.E.
S1P-induced Internalization of S1P1 Is Dependent on C2α and Required for Rac1 Activation and Migration in HUVEC
We studied the role of C2α in S1P1 internalization upon S1P stimulation in HUVEC co-transfected with the expression vectors for mRFP-S1P1 and GFP-2xFYVE. S1P stimulation induced robust internalization of S1P1 with substantial co-localization of the internalized S1P1 and the intracellular GFP-2xFYVE signals in sc-siRNA-transfected control HUVEC (Fig. 6A and supplemental Movie 3). In C2α-depleted cells, S1P-induced S1P1 internalization was much attenuated (Fig. 6A and supplemental Movie 4), indicating that C2α is required for S1P1 internalization. In contrast to C2α-depleted cells, S1P induced the internalization of S1P1 in p110β-depleted cells as well as in control HUVEC, indicating that p110β is not required for S1P-induced S1P1 internalization. In HUVEC co-transfected with sc-siRNA and the expression vectors for mRFP-S1P1 and the Rac1-FRET probe, S1P induced time-dependent increases in Rac1-FRET signals in the endosomes and the plasma membrane with S1P1 internalization (Fig. 6B and supplemental Movie 1). At least a portion of S1P-induced FRET signals co-localized with mRFP-S1P1 in the endosomes as well as in the plasma membrane. C2α depletion markedly suppressed S1P1 internalization as well as Rac1-FRET signal (Fig. 6B and supplemental Movie 2).
FIGURE 6.
S1P induces S1P1 internalization into GFP-2xFYVE+ vesicles in a PI3K-C2α-dependent manner and activates Rac1 in S1P1+ vesicles in HUVEC. A, effects of knockdown of C2α and p110β on S1P-induced S1P1 internalization. Cells were co-transfected with the expression vectors for mRFP-S1P1, GFP-2xFYVE, and either C2α-siRNA, p110β-siRNA, or sc-siRNA. Twenty-four h later, the cells were serum-starved overnight and stimulated with S1P (0.3 μm) for 30 min. The cells were observed with a confocal fluorescent microscope as described under “Experimental Procedures.” B, co-localization of mRFP-S1P1 and the Rac1-FRET signal (white (bottom) and yellow (top) arrowheads, respectively). Cells co-transfected with mRFP-S1P1 expression vector and pRaichu-Rac1 were cultured overnight and then serum-starved for 6 h. The cells were stimulated with S1P (0.3 μm) for 30 min with fluorescent microscopic observations. Scale bar, 20 μm.
We further investigated the role of S1P1 internalization in S1P1-mediated cell signaling and migration by studying the effects of dynasore, an inhibitor of dynamin, which is a molecule essential for vesicle formation in endocytosis (53). Treatment of HUVEC with dynasore strongly inhibited S1P-induced internalization of S1P1 and reduced GFP-2xFYVE+ vesicles (compare Fig. 7A with Fig. 6A). Cell migration is also inhibited by dynasore treatment (Fig. 7B). Similar to the effects of C2α knockdown, dynasore inhibited S1P-induced activation of Rac1 but not of Akt or ERK (Fig. 7, C and D). These observations lend further support for the notion that the internalized S1P1 activates Rac1 on endosomes and thereby cell migration.
FIGURE 7.
An inhibitor of endocytosis blocks S1P-induced S1P1 internalization, cell migration, and Rac1 activation in HUVEC. A, effects of dynasore on S1P-induced S1P1 internalization. Cells were co-transfected with the expression vectors for mRFP-S1P1 and GFP-2xFYVE, serum-starved overnight, and stimulated with S1P (0.3 μm) or not for 30 min with or without dynasore (40 μm) pretreatment for 1 h. The cells were observed with a confocal fluorescent microscope as in Fig. 6A. Scale bar, 20 μm. B, effects of dynasore on S1P-directed migration of HUVEC. Serum-starved cells were pretreated with dynasore (40 μm) or not for 1 h and then underwent a transwell migration assay. S1P (0.3 μm) was added in the lower chamber. C, effects of dynasore on S1P-induced Rac1 activation and phosphorylation of Akt (p-Akt) and ERK (p-ERK). Cells were treated as in B and stimulated with S1P (0.3 μm) for 2 min. D, quantified data of phospho-Akt (p-Akt), phospho-ERK (p-ERK), and GTP-Rac1. n = 3. In B and D, ** and *** denote statistical significance at the levels of p < 0.01 and p < 0.001, respectively, compared with respective non-treated control. # and ## denote statistical significance between the indicated groups at the levels of p < 0.05 and p < 0.01, respectively. NS, not significant. Error bars, S.E.
C2α Is Essential for Tube Formation of HUVEC
We studied the role of C2α in S1P-induced tube formation of HUVEC on Matrigel. Depletion of C2α but not C2β in HUVEC inhibited S1P-induced tube formation (Fig. 8A). Knockdown of p110β partially inhibited S1P-induced tube formation. S1P-induced tube formation was dependent on Rac1, as demonstrated by abolition of tube formation by adenovirus-mediated expression of Rac1N17 (Fig. 8B). Finally, dynasore abolished S1P-induced tube formation (Fig. 8C), indicating that S1P-induced tube formation was dependent on an endocytic process.
FIGURE 8.
PI3K-C2α knockdown and dynasore inhibit S1P-induced tube formation in HUVEC. A, effects of knockdown of PI3K isoforms on tube formation. Cells were co-transfected with either C2α-siRNA, C2β-siRNA, p110β-siRNA, or sc-siRNA and 24 h later were serum-starved overnight. The cells were seeded on growth factor-reduced Matrigel in a 24-well plate containing 0.3 μm S1P and allowed to form tube-like structures for 6 h. Right, quantified data of tube length. n = 4. Scale bar, 200 μm. B, effects of the expression of a dominant negative Rac1 mutant on S1P-induced tube formation. Cells were infected with either Ad-LacZ or Ad-Rac1N17 as in Fig. 3B and 24 h later were serum-starved overnight, followed by tube formation assay as in A. C, effects of dynasore on S1P-induced tube formation. Cells were pretreated with dynasore or not for 1 h and underwent tube formation assay as in A. Scale bar, 200 μm. Right, quantified data of tube length. n = 3. In A and C, *, **, and *** denote statistical significance at the levels of p < 0.05, p < 0.01, and p < 0.001, respectively, compared with respective non-stimulated control. ### denotes statistical significance between the indicated groups at a levels of p < 0.001. NS, not significant. Error bars, S.E.
DISCUSSION
The present study demonstrates that class II PI3K member C2α is essential for S1P1-mediated EC migration, capillary-like tube formation, and particular cell signaling (i.e. Rac1 activation, which is a signal crucial for cell motility and endothelial morphogenesis) in a manner different from class I PI3K. We show for the first time that S1P1-mediated Rac1 activation occurs not only in the plasma membrane but also on endosomes and requires C2α. The endosomes where Rac1 activation occurs in response to S1P stimulation are enriched in PtdIns(3)P, a major product of C2α, and bear the internalized S1P1. S1P-induced S1P1 internalization requires C2α. Pharmacological blockade of S1P1 internalization markedly inhibits activation of Rac1 but not Akt or ERK and thereby suppresses cell migration and tube formation. Thus, C2α plays a thus far unrecognized, distinct role in S1P1-mediated endothelial migration and morphogenesis by engaging in S1P1 internalization and subsequent signaling on endosomes.
C2α; another class II member, C2β; and also class III Vps34 all generate PtdIns(3)P, which is mainly accumulated in the intracellular membranes, including early endosomes, multivesicular bodies/late endosomes, phagosomes, and the Golgi apparatus (1, 4, 13). The localized enrichment of PtdIns(3)P provides the platform for recruiting effector proteins containing FYVE, PX, and PH domains, thus regulating membrane trafficking and controlling signal transduction, cytoskeletal reorganization, phagocytosis, and autophagy (54). Our data indicate that C2α is involved in S1P1-mediated, selected signaling through controlling vesicular trafficking-dependent processes; under the condition in which S1P-induced internalization of S1P1 is blocked by either C2α depletion or dynasore (dynamin inhibitor) treatment, S1P-induced activation of Rac1 but not ERK or Akt is greatly suppressed (Figs. 3C and 7, C and D). Furthermore, Rac1 activation in the intracellular compartment is localized to S1P1+ and FYVE+ vesicles (Figs. 5B and 6B). Thus, very likely, internalized S1P1 on PtdIns(3)P-enriched endosomes is engaged in Rac1 activation. Because S1P-nonstimulated, C2α-depleted cells display a marked decrease in the number of FYVE+ vesicles and inhibition of their motility (13) and S1P stimulation does not alter the abundance of FYVE+ vesicles (Figs. 4C and 6A), it is suggested that the basal level of vesicular PtdIns(3)P provided by a constitutive activity of C2α plays a crucial role in S1P-induced endosomal signaling in HUVEC. We found that knockdown of C2α but not C2β or Vps34 reduces the number of FYVE+ vesicles in HUVEC (13).4 In the present study, we did not observe a decrease in the total cellular level of PtdIns(3)P in C2α-depleted HUVEC (Fig. 1F). This finding suggests that a majority of total cellular PtdIns(3)P in HUVEC is synthesized by other PI3Ks, such as Vps34 and C2β, and that C2α may produce PtdIns(3)P in the particular compartments, including early endosomes and the trans-Golgi network. We did not observe an S1P-induced increase in PtdIns(3,4,5)P3 level (Fig. 1, F and G). This could be because S1P might increase the level of PtdIns(3,4,5)P3 only in a subdomain of the plasma membrane, and our method for determining PtdIns(3,4,5)P3 in HUVEC might not have enough sensitivity to detect a localized increase in this lipid in the plasma membrane because of poor metabolic labeling of cellular lipids in this type of cells.
Endocytosis of cell surface receptors has been viewed as a mechanism of signal termination by internalizing and degrading receptors (55, 56). However, recent studies demonstrate that endosomes serve as platforms to assemble membrane receptors and their downstream signaling molecules to generate spatially localized signals (56). For example, Rac activation by certain receptor tyrosine kinase ligands is shown to occur on endosomes, which requires the recruitment of the adaptor protein APPL, the Rac-GEF Tiam-1, and plasma membrane-anchored GDP-bound Rac1 (34, 35). In many cases of G protein-coupled receptor signaling, Rac activation by chemotactic ligands is mediated largely by Gi and through the action of guanine nucleotide exchange factors, such as P-Rex1 (57, 58). The chemotactic receptor S1P1 is exclusively coupled to Gi, and S1P1-mediated Rac1 activation is indeed Gi-dependent (36, 59). It remains to be defined how adaptors and a Rac-GEF assemble in the PtdIns(3)P-enriched S1P1+ endosomes to activate the Rac pathway. A previous study showed that the S1P1 agonist FTY720-phosphate produced persistent S1P1 endocytosis and a prolonged reduction in cellular cyclic AMP level. This study suggests that, besides the Rac1 pathway, endosomal S1P1 also signals to inhibit adenylate cyclase (60).
Previous studies showed that C2β is involved in migration of non-endothelial cells (61–63). The results in those studies differ from our observations in EC migration in several aspects (i.e. in the regulation of the PI3K activities, the distribution of the PI3Ks, and the distribution of their product PtdIns(3)P). Migration of ovarian and uterine cervical tumor cells stimulated by the lipid mediator lysophosphatidic acid in a wound healing assay was dependent on C2β but not C2α with increased production of PtdIns(3)P; lysophosphatidic acid stimulation induced translocation of C2β and GFP-2xFYVE probe from the intracellular compartment to the plasma membrane, the latter of which was abolished by knockdown of C2β but not C2α (61). In epidermoid carcinoma cells, C2β was found to associate with epidermal growth factor (EGF) receptor by forming a complex with adaptor proteins and the Rac/Ras-GEF SOS, and EGF stimulated C2β activity as well as Rac1 activity. Overexpression of either wild-type C2β or a dominant negative C2β mutant enhanced or suppressed cell migration, respectively. Moreover, C2β overexpression enhanced EGF-induced Rac1 activation, whereas that of the dominant negative C2β mutant inhibited Rac1 activation (63). In addition, C2β overexpression in human kidney cells stimulated cell migration with activation of Cdc42 but not Rac1, and endogenous C2β was localized in both lamellipodia and intracellular vesicles (62). In our study, although transwell migration of HUVEC toward S1P was inhibited by knockdown of either C2α or C2β, the C2β dependence of cell migration was much less in magnitude compared with that of C2α (Fig. 1E). Consistently, C2β knockdown failed to inhibit S1P-induced Rac1 activation in HUVEC, unlike C2α knockdown (Fig. 3C). In addition, Vps34, which exclusively produces PtdIns(3)P, did not at all inhibit S1P-directed migration (Fig. 1E). Our recent study (13) also showed that C2α but not C2β or Vps34 decreased GFP-2xFYVE+ endosomes in non-stimulated HUVEC and prevented VEGF-induced tube formation. Differently from the case of the tumor cells in which C2β is responsible for stimulus-induced increase in PtdIns(3)P in the plasma membrane, C2α rather contributes to PtdIns(3)P level in the intracellular vesicular compartment under non-stimulated basal conditions in HUVEC (Figs. 4C and 6A). Consistent with this notion, S1P did not induce translocation to the plasma membrane of C2α in HUVEC.4 These observations together suggest that C2α is probably located in a different endosomal compartment from C2β and Vps34, undergoes distinct regulation, and thereby serves specialized functions in HUVEC. Of note is the structural difference between C2α and C2β; only C2α possesses a clathrin-binding site (8). However, the possibility is not excluded that the functions of C2α and C2β are partially redundant in HUVEC. It is possible that the apparent discrepancies regarding the roles of class II PI3K in cell migration between the previous studies and our study could be explained by cell type-specific difference in relative expression levels of these two class II PI3K, cell type- and PI3K isoform-specific differences in the regulatory mechanisms for the activities of the PI3K and a cell type-specific difference in the intracellular localization of the PI3K. Further study is required for defining whether and to what extent the functions of these PI3Ks are overlapping or distinct in ECs and other cells.
S1P1-mediated cell migration is dependent on not only C2α but also p110β (Fig. 1E). S1P-induced Rac1 activation is also dependent on p110β (Fig. 3C). This is consistent with the recent observations that G protein-coupled receptors are coupled to p110β rather than p110α (1, 45, 64). p110β produces mainly PtdIns(3,4,5)P3, unlike class II and III PI3Ks (1). PtdIns(3,4,5)P3 serves to recruit a Rac-GEF to the membrane mainly through interacting with a PH domain of a Rac-GEF and to activate it probably together with Gβγ (57). Impressively, p110β knockdown substantially attenuates Rac1 activation in the plasma membrane but has little effect on endosomal Rac1 activation (Fig. 5B (bottom)), suggesting that an increase in PtdIns(3,4,5)P3 level may not be necessary for the recruitment and assembly of a Rac-GEF on signaling endosomes. In contrast, C2α knockdown inhibits S1P-induced Rac1 activation in both the plasma membrane and endosomes (Fig. 5B, middle). As evaluated with GFP-2xFYVE labeling, PtdIns(3)P is accumulated mainly in the endosomes and other vesicular structures but not the plasma membrane (Figs. 4C and 6A). Moreover, this distribution pattern of PtdIns(3)P is not changed by S1P stimulation. Hence, the dependence of plasmalemmal Rac1 activation on C2α may indicate that one or more processes of Rac1 activation in the plasma membrane (e.g. delivery and assembly of adaptors and a Rac-GEF to the plasma membrane and also recycling of S1P1) require C2α.
C2α could be involved in cell migration through not only facilitating the assembly of Rac1-activating machinery on endosomes but through other mechanisms involving endosomal trafficking; C2α may be engaged in the transport of Rac1 activated on endosomes to the front of migrating cells to ensure the formation of actin-based protrusions (34, 35, 64). Integrins, lipid raft domains, and other small GTPases, such as Arf6, may also traffic to the leading edge, promoting the formation of the focal complex/adhesion and actin polymerization. Also, at the rear of the cells, focal adhesion disassembly and cell contraction may involve endosomal platforms through the assembly of Rho and Rho kinase.
The present study demonstrates for the first time that C2α is involved in selective cell signaling on endosomes. Our data show that C2α is functionally essential for S1P-induced EC migration and morphogenesis, suggesting that the crucial role of C2α in angiogenesis (13) may be in part explained by the C2α requirement in the S1P actions on ECs. We recently showed that C2α also plays an indispensable role in maintaining endothelial barrier function (13). It is known that S1P exerts a barrier-protective effect through regulating Rac1 (21, 55). Therefore, C2α-dependent endosomal Rac1 activation may also serve as a signal to enhance vascular barrier protection. Because angiogenesis and barrier integrity are important vascular functions in both physiology and pathophysiology, our study opens a new avenue for establishing a therapeutic strategy to regulate physiological and pathological vascular processes by controlling C2α.
Acknowledgment
We thank C. Hirose for secretarial assistance.
This work was supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; the Japan Society for the Promotion of Science; the Honjin Foundation; the Mitsubishi Pharma Research Foundation; and the SENSIN Medical Research Foundation.

This article contains supplemental Movies 1–4.
K. Biswas, unpublished observation.
- PtdIns
- phosphatidylinositol
- S1P
- sphingosine 1-phosphate
- C2α
- PI3K-C2α
- C2β
- PI3K-C2β
- EC
- endothelial cell
- HUVEC
- human umbilical vein endothelial cell(s)
- qPCR
- quantitative real-time PCR
- GEF
- guanine nucleotide exchange factor
- CFP
- cyan fluorescent protein
- sc-siRNA
- scrambled siRNA
- PH
- pleckstrin homology.
REFERENCES
- 1. Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 [DOI] [PubMed] [Google Scholar]
- 2. Sasaki T., Takasuga S., Sasaki J., Kofuji S., Eguchi S., Yamazaki M., Suzuki A. (2009) Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res. 48, 307–343 [DOI] [PubMed] [Google Scholar]
- 3. Vanhaesebroeck B., Stephens L., Hawkins P. (2012) PI3K signalling. The path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 13, 195–203 [DOI] [PubMed] [Google Scholar]
- 4. Lindmo K., Stenmark H. (2006) Regulation of membrane traffic by phosphoinositide 3-kinases. J. Cell Sci. 119, 605–614 [DOI] [PubMed] [Google Scholar]
- 5. Mazza S., Maffucci T. (2011) Class II phosphoinositide 3-kinase C2α. What we learned so far. Int. J. Biochem. Mol. Biol. 2, 168–182 [PMC free article] [PubMed] [Google Scholar]
- 6. Falasca M., Hughes W. E., Dominguez V., Sala G., Fostira F., Fang M. Q., Cazzolli R., Shepherd P. R., James D. E., Maffucci T. (2007) The role of phosphoinositide 3-kinase C2α in insulin signaling, J. Biol. Chem. 282, 28226–28236 [DOI] [PubMed] [Google Scholar]
- 7. Falasca M., Maffucci T. (2012) Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem. J. 443, 587–601 [DOI] [PubMed] [Google Scholar]
- 8. Gaidarov I., Zhao Y., Keen J. H. (2005) Individual phosphoinositide 3-kinase C2α domain activities independently regulate clathrin function. J. Biol. Chem. 280, 40766–40772 [DOI] [PubMed] [Google Scholar]
- 9. Gaidarov I., Smith M. E., Domin J., Keen J. H. (2001) The class II phosphoinositide 3-kinase C2α is activated by clathrin and regulates clathrin-mediated membrane trafficking, Mol. Cell 7, 443–449 [DOI] [PubMed] [Google Scholar]
- 10. Domin J., Gaidarov I., Smith M. E., Keen J. H., Waterfield M. D. (2000) The class II phosphoinositide 3-kinase PI3K-C2α is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J. Biol. Chem. 275, 11943–11950 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y., Yoshioka K., Azam M. A., Takuwa N., Sakurada S., Kayaba Y., Sugimoto N., Inoki I., Kimura T., Kuwaki T., Takuwa Y. (2006) Class II phosphoinositide 3-kinase α-isoform regulates Rho, myosin phosphatase, and contraction in vascular smooth muscle. Biochem. J. 394, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Sheikh S. S., Domin J., Tomtitchong P., Abel P., Stamp G., Lalani E.-N. (2003) Topological expression of class IA and class II phosphoinositide 3-kinase enzymes in normal human tissue is consistent with a role in differentiation. BMC Clin. Pathol. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshioka K., Yoshida K., Cui H., Wakayama T., Takuwa N., Okamoto Y., Du W., Qi X., Asanuma K., Sugihara K., Aki S., Miyazawa H., Biswas K., Nagakura C., Ueno M., Iseki S., Schwartz R. J., Okamoto H., Sasaki T., Matsui O., Asano M., Adams R. H., Takakura N., Takuwa Y. (2012) Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular function. Nat. Med. 18, 1560–1569 [DOI] [PubMed] [Google Scholar]
- 14. Spiegel S., Milstien S. (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blaho V. A., Hla T. (2011) Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 111, 6299–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii I., Fukushima N., Ye X., Chun J. (2004) Lysophospholipid receptors. Signaling and Biology. Annu. Rev. Biochem. 73, 321–354 [DOI] [PubMed] [Google Scholar]
- 17. Takuwa Y., Okamoto Y., Yoshioka K., Takuwa N. (2012) Sphingosine-1-phosphate signaling in physiology and diseases. BioFactors 38, 329–337 [DOI] [PubMed] [Google Scholar]
- 18. Means C. K., Brown J. H. (2009) Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fyrst H., Saba J. D. (2010) An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 6, 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryu Y., Takuwa N., Sugimoto N., Sakurada S., Usui S., Okamoto H., Matsui O., Takuwa Y. (2002) Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ. Res. 90, 325–332 [DOI] [PubMed] [Google Scholar]
- 21. Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee M.-J., Thangada S., Claffey K. P., Ancellin N., Liu C. H., Kluk M., Volpi M., Sha'afi R. I., Hla T. (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 [DOI] [PubMed] [Google Scholar]
- 23. Lee J.-F., Gordon S., Estrada R., Wang L., Siow D. L., Wattenberg B. W., Lominadze D., Lee M.-J. (2009) Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am. J. Physiol. Heart Circ. Physiol. 296, H33–H42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang F., Van Brocklyn J. R., Hobson J. P., Movafagh S., Zukowska-Grojec Z., Milstien S., Spiegel S. (1999) Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor. J. Biol. Chem. 274, 35343–35350 [DOI] [PubMed] [Google Scholar]
- 25. Liu U., Wada R., Yamashita T., Mi Y., Deng C.-X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S.-S., Lee M.-J., Liu C. H., Hla T., Spiegel S., Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung B., Obinata H., Galvani S., Mendelson K., Ding B.-S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., Hla T. (2012) Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaengel K., Niaudet C., Hagikura K., Lavina B., Muhl L., Hofmann J. J., Ebarasi L., Nyström S., Rymo S., Chen L. L., Pang M., Jin Y., Raschperger E., Roswall P., Schulte D., Benedito R., Larsson J., Hellström M., Fuxe J., Uhlén P., Adams R., Jakobsson L., Majumdar A., Vestweber D., Uv A., Betsholtz C. (2012) The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-Cadherin and VEGFR2, Dev. Cell 23, 587–599 [DOI] [PubMed] [Google Scholar]
- 28. Ridley A. J. (2011) Life at the leading edge. Cell 145, 1012–1022 [DOI] [PubMed] [Google Scholar]
- 29. Yamada S., Nelson W. J. (2007) Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 178, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heasman S. J., Ridley A. J. (2008) Mammalian Rho GTPases. New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 31. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase. In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 32. Beningo K. A., Hamao K., Dembo M., Wang Y.-L., Hosoya H. (2006) Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch. Biochem. Biophys. 456, 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dobrowolski R., De Robertis E. M. (2012) Endocytic control of growth factor signalling. Multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 13, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palamidessi A., Frittoli E., Garré M., Faretta M., Mione M., Testa I., Diaspro A., Lanzetti L., Scita G., Di Fiore P. P. (2008) Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134, 135–147 [DOI] [PubMed] [Google Scholar]
- 35. Zoncu R., Perera R. M., Balkin D. M., Pirruccello M., Toomre D., De Camilli P. (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136, 1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okamoto H., Takuwa N., Yokomizo T., Sugimoto N., Sakurada S., Shigematsu H., Takuwa Y. (2000) Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol. Cell Biol. 20, 9247–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugimoto N., Takuwa N., Okamoto H., Sakurada S., Takuwa Y. (2003) Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol. Cell Biol. 23, 1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakurada S., Okamoto H., Takuwa N., Sugimoto N., Takuwa Y. (2001) Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am. J. Physiol. Cell Physiol. 281, C571–C578 [DOI] [PubMed] [Google Scholar]
- 39. Eccles S. A., Court W., Patterson L., Sanderson S. (2009) In vitro assays for endothelial cell functions related to angiogenesis. Proliferation, motility, tubular differentiation, and proteolysis, Methods Mol. Biol. 467, 159–181 [DOI] [PubMed] [Google Scholar]
- 40. Usui S., Sugimoto N., Takuwa N., Sakagami S., Takata S., Kaneko S., Takuwa Y. (2004) Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Krüppel-like factor 5. J. Biol. Chem. 279, 12300–12311 [DOI] [PubMed] [Google Scholar]
- 41. Du W., Takuwa N., Yoshioka K., Okamoto Y., Gonda K., Sugihara K., Fukamizu A., Asano M., Takuwa Y. (2010) S1P2, the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 70, 772–781 [DOI] [PubMed] [Google Scholar]
- 42. Itoh R. E., Kurokawa K., Ohba Y., Yoshizaki H., Mochizuki N., Matsuda M. (2002) Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell Biol. 22, 6582–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sasaki T., Irie-Sasaki J., Jones R. G., Oliveira-dos-Santos A. J., Stanford W. L., Bolon B., Wakeham A., Itie A., Bouchard D., Kozieradzki I., Joza N., Mak T. W., Ohashi P. S., Suzuki A., Penninger J. M. (2000) Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1040–1046 [DOI] [PubMed] [Google Scholar]
- 44. Sasaki J., Sasaki T., Yamazaki M., Matsuoka K., Taya C., Shitara H., Takasuga S., Nishio M., Mizuno K., Wada T., Miyazaki H., Watanabe H., Iizuka R., Kubo S., Murata S., Chiba T., Maehama T., Hamada K., Kishimoto H., Frohman M. A., Tanaka K., Penninger J. M., Yonekawa H., Suzuki A., Kanaho Y. (2005) Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type Iα. J. Exp. Med. 201, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Igarashi J., Michel T. (2001) Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase β. J. Biol. Chem. 276, 36281–36288 [DOI] [PubMed] [Google Scholar]
- 46. Cain R. J., Ridley A. J. (2009) Phosphoinositide 3-kinases in cell migration. Biol. Cell 101, 13–29 [DOI] [PubMed] [Google Scholar]
- 47. Xu K., Sacharidou A., Fu S., Chong D. C., Skaug B., Chen Z. J., Davis G. E., Cleaver O. (2011) Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell 20, 526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Broussard J. A., Lin W. H., Majumdar D., Anderson B., Eason B., Brown C. M., Webb D. J. (2012) The endosomal adaptor protein APPL1 impairs the turnover of leading edge adhesions to regulate cell migration. Mol. Biol. Cell 23, 1486–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zaidel-Bar R., Milo R., Kam Z., Geiger B. (2007) A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 120, 137–148 [DOI] [PubMed] [Google Scholar]
- 50. Hall A., Nobes C. D. (2000) Rho GTPases. Molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Higuchi M., Masuyama N., Fukui Y., Suzuki A., Gotoh Y. (2001) Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr. Biol. 11, 1958–1962 [DOI] [PubMed] [Google Scholar]
- 52. Lee M. J., Thangada S., Paik J. H., Sapkota G. P., Ancellin N., Chae S. S., Wu M., Morales-Ruiz M., Sessa W. C., Alessi D. R., Hla T. (2001) Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8, 693–704 [DOI] [PubMed] [Google Scholar]
- 53. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 54. Kutateladze T. G. (2010) Translation of the phosphoinositide code by PI effectors. Nat. Chem. Biol. 6, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oo M. L., Chang S.-H., Thangada S., Wu M.-T., Rezaul K., Blaho V., Hwang S.-I., Han D. K., Hla T. (2011) Engagement of S1P1-degradative mechanisms leads to vascular leak in mice, The Journal of Clinical Investigation 121, 2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sorkin A., von Zastrow M. (2009) Endocytosis and signalling. Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Welch H. C., Coadwell W. J., Ellson C. D., Ferguson G. J., Andrews S. R., Erdjument-Bromage H., Tempst P., Hawkins P. T., Stephens L. R. (2002) P-Rex1, a PtdIns(3,4,5)P3- and G13-regulated guanine-nucleotide exchange factor for Rac. Cell 108, 809–821 [DOI] [PubMed] [Google Scholar]
- 58. Wertheimer E., Gutierrez-Uzquiza A., Rosemblit C., Lopez-Haber C., Sosa M. S., Kazanietz M. G. (2012) Rac signaling in breast cancer. A tale of GEFs and GAPs. Cell Signal. 24, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Windh R. T., Lee M.-J., Hla T., An S., Barr A. J., Manning D. R. (1999) Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J. Biol. Chem. 274, 27351–27358 [DOI] [PubMed] [Google Scholar]
- 60. Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 [DOI] [PubMed] [Google Scholar]
- 61. Maffucci T., Cooke F. T., Foster F. M., Traer C. J., Fry M. J., Falasca M. (2005) Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell Biol. 169, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Domin J., Harper L., Aubyn D., Wheeler M., Florey O., Haskard D., Yuan M., Zicha D. (2005) The class II phosphoinositide 3-kinase PI3K-C2β regulates cell migration by a PtdIns3P-dependent mechanism. J. Cell Physiol. 205, 452–462 [DOI] [PubMed] [Google Scholar]
- 63. Katso R. M., Pardo O. E., Palamidessi A., Franz C. M., Marinov M., De Laurentiis A., Downward J., Scita G., Ridley A. J., Waterfield M. D., Arcaro A. (2006) Phosphoinositide 3-kinase C2β regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms. Mol. Biol. Cell 17, 3729–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gould G. W., Lippincott-Schwartz J. (2009) New roles for endosomes. From vesicular carriers to multi-purpose platforms. Nat. Rev. Mol. Cell Biol. 10, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]