Background: RasGRP1 is a protein involved in Ras activation, but its physiological function is largely unknown.
Results: Knockdown of RasGRP1 impairs development of the lymphatic system in zebrafish embryos, shown as defective thoracic duct formation, pericardial and truck edema, and altered sprouts of lymphatic vessels.
Conclusion: RasGRP1 is involved in lymphangiogenesis in zebrafish.
Significance: RasGRP1 has a physiological function in early development.
Keywords: Development, Lymphangiogenesis, Ras, Vascular Biology, Vascular Endothelial Growth Factor (VEGF), Zebrafish, PAQR10, RasGRP1
Abstract
The molecular basis of the lymphatic development remains largely unknown. Using zebrafish as a model, we discovered a novel role for the Ras guanine-releasing protein 1 (RasGRP1), a protein involved in Ras activation in lymphangiogenesis. Secondary lymphatic sprouts from the posterior cardinal vein give rise to thoracic duct which is the first lymphatic vessel in zebrafish. Knockdown of rasgrp1 by injecting morpholino in zebrafish embryos impaired formation of thoracic duct accompanied by pericardial and truck edema, whereas blood vessel development of the embryos was largely unaffected. In rasgrp1-knockdown embryos, the number of sprouts producing the string of parachordal lymphangioblast cells was reduced. Meanwhile the total number of the secondary sprouts was not changed. As a result, the number of venous intersegmental vessels was increased, whereas the number of lymphatic vessel was reduced at a later stage. The lymphatic developmental defects caused by rasgrp1 knockdown could be rescued by ectopic expression of a constitutively active HRas. Further analysis revealed that RasGRP1 knockdown could synergize with flt4/vegfr3 knockdown to induce defects in lymphangiogenesis. Taken together, this finding demonstrates a critical role for RasGRP1 in lymphatic development in zebrafish.
Introduction
The lymphatic vascular system regulates interstitial fluid homeostasis, fat absorption, and immune surveillance (1). Growing evidence has revealed that the lymphatic system is also involved in multiple disease processes such as lymphedema, tumor metastasis, inflammation, obesity, and hypertension (2). However, the molecular basis of lymphatic development remains poorly understood. During the last two decades, a few molecules and signaling pathways have been found to regulate differentiation of lymphatic endothelial cells and lymphatic migration, including the Sox18/Prox1 transcriptional pathway (3, 4), the VEGF-C/Flt4 signaling pathway (5, 6), Δ-like-4/Notch signaling (7), synectin (8), and collagen and calcium-binding EGF domain-1 (ccbe1) (9, 10). However, additional molecules contributing to lymphatic development remain to be discovered.
Ras guanine-releasing protein 1 (RasGRP1) is a member of the guanine nucleotide exchange factors that are involved in the activation of Ras proteins (11, 12). Previous studies on RasGRP1 were mainly focused on its regulation on Ras activation in lymphocytes (13). RasGRP1 is essential for T cell receptor signaling, thymocyte differentiation, and B cell proliferation (14–16). However, RasGRP1 likely possesses other physiological functions in addition to its regulation on lymphocytes. RasGRP1 is expressed in neurons in the brain (17) and in the kidney (18) in addition to its expression in a variety of blood cells, including T cells and B cells (14). However, whether or not RasGRP1 is involved in vascular or lymphatic development remains elusive. Our previous work revealed that PAQR10/11, a member of the progestin and AdipoQ receptor (PAQR)2 family, imparts a spatial regulation on the Ras signaling pathway and that RasGRP1 is implicated in the regulation (19). We next analyzed the potential physiological function using the zebrafish system and discovered that PAQR11 is involved in early heart development in zebrafish embryos (20). In this study, we analyzed the potential function of RasGRP1 during zebrafish development and discovered a novel function of RasGRP1 in early lymphangiogenesis.
EXPERIMENTAL PROCEDURES
Maintenance of Zebrafish
WT AB and Tg (fli1:EGFP)y1 zebrafish (21) (obtained from J. Du, Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China) were maintained under standard conditions. Zebrafish studies were approved by the Institute of Neuroscience, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
Morpholino and Microinjection
Morpholinos (MOs) were purchased from Gene Tools (Philomath, OR). The first rasgrp1 morpholino (RasGRP1-MO) was designed to cover the start codon region and 5′ untranslated region with a sequence of 5′-TGCGATTCATGGTGTCAAATCCCAT-3′. The second rasgrp1 morpholino (RasGRP1-MO2) was targeted at a splice junction between exon 13 and intron 13 of rasgrp1 pre-mRNA with a sequence of 5′-GTTGGTTTAAATACGTACAAATCCT-3′. The flt4 morpholino (Flt4-MO) was used as reported previously with a sequence of 5′-CTCTTCATTTCCAGGTTTCAAGTCC-3′ (8). The control morpholino was a standard control MO with no target in zebrafish embryos, and its sequence was 5′-CCTCTTACCTCAGTTACAATTTATA-3′. The morpholinos were diluted in nuclease-free water (Ambion, Austin, TX), pressure-injected into 1- or 2-cell-stage zebrafish embryos using an mn-151 joystick micromanipulator and an IM-300 microinjector (Narishige, Japan). RasGRP1-MO was injected at a concentration of 6 ng/embryo, and RasGRP1-MO2 was used in 14 ng/embryo after titration with various concentrations. Flt4-MO was injected at a concentration of 1 ng/embryo. A lymphatic dye uptake assay was performed as described previously (22). In brief, embryos at 6 days post-fertilization (dpf) were injected below the skin in the trunk region with a fluorescent dye Alexa-Fluor 568 dextran (Invitrogen) using glass capillary needles and were photographed 3–6 h after injection.
Probe Synthesis and Whole-mount in Situ Hybridization
Probes labeled with digoxigenin (Roche) were synthesized with linearized plasmids by using Sp6 or T7 RNA polymerase (Ambion). Whole mount in situ hybridization (WISH) was carried out as described previously (23). For cross-section analysis, the stained embryos were treated with 75, 80, 95, and 100% ethanol, followed by paraffin treatment before being embedded in paraffin and sectioned (8 μm in thickness) using a Lecia RM 2126 microtome.
Preparation and Injection of Zebrafish rasgrp1 mRNA
To make a morpholino-resistant form of RasGRP1, a full-length zebrafish RasGRP1 expression plasmid was generated to contain five same-sense mutations localized in the targeting region of RasGRP1-MO. The rasgrp1 mRNA was generated with the mMESSAGE mMACHINE kit (Ambion). In the rescue experiments, each 1-cell stage embryo was injected with 1–5 pg of synthesized zebrafish rasgrp1 mRNA.
Microscopy and Imaging
The zebrafish embryos were mounted in 1.0–1.5% low-melt agarose for observation and photographing. The images were acquired by a laser-scanning confocal microscope (Zeiss LSM 510, Zeiss, Jena, Germany) or Olympus DP72 (Tokyo, Japan).
Statistic Analysis
The phenotypic severities of the zebrafish morphants was analyzed by Chi square analysis. The statistic difference between two groups was analyzed by unpaired Student's t test.
RESULTS
Expression Profile of rasgrp1 during Zebrafish Development
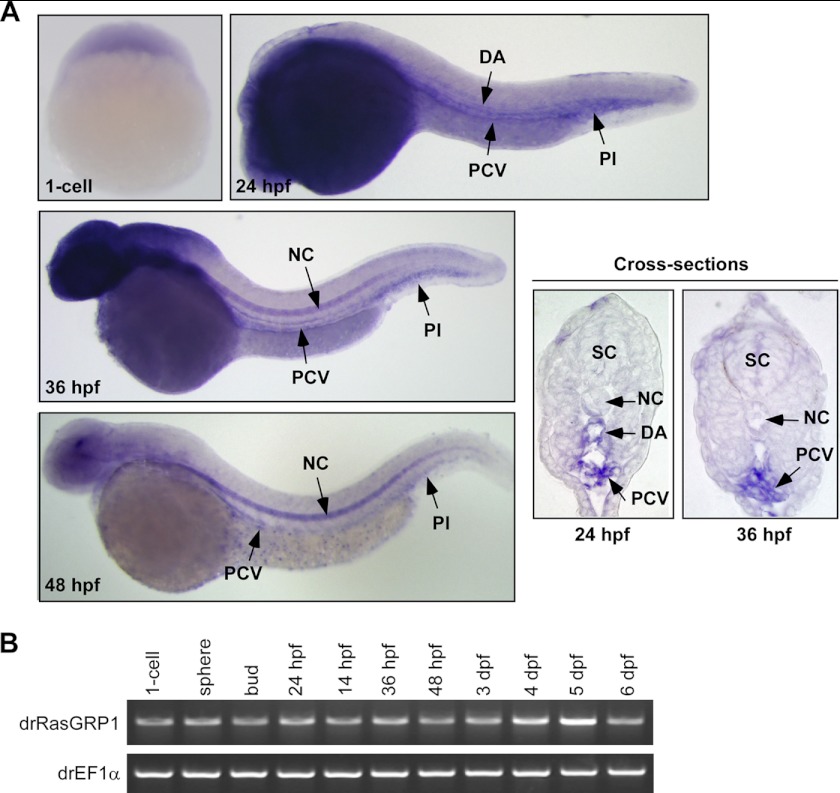
We used WISH and RT-PCR in wild-type zebrafish embryos to investigate the spatial and temporal expression pattern of rasgrp1. Both WISH and RT-PCR results revealed that rasgrp1 was maternally provided, as its mRNAs were detected at the 1-cell stage before onset of zygotic gene expression (Fig. 1). From 24 h post fertilization (hpf) on, restricted expression of rasgrp1 was observed in the dorsal aorta (DA), posterior cardinal vein (PCV), and cardinal vein plexus (Pl) regions. The signal of rasgrp1 expression was mostly lost in DA but still persistent in PCV and Pl regions at 36 hpf (Fig. 1A), at a time when the lymphatic precursors begin to migrate toward the horizontal myoseptum region from the PCV (9). By 48 hpf, rasgrp1 expression in the PCV and Pl regions was decreased markedly (Fig. 1A). In addition, we found apparent staining of rasgrp1 in the notochord from approximately at 36 hpf, as well as in the head region (Fig. 1A). In control experiments, no hybridization signal was seen in the DA, PCV, and Pl regions with rasgrp1 sense probe (supplemental Fig. 1). The tissue-specific expression pattern of RasGRP1 in endothelia cells and the nervous system is consistent with the findings in mammals (17). Besides, we analyzed rasgrp1 mRNA expression in zebrafish embryos at different stages from the 1-cell stage to 6 dpf using RT-PCR (Fig. 1B). It appeared that rasgrp1 was expressed in all stages of early embryos and larvae up to 6 dpf (Fig. 1B). These data, therefore, indicate that RasGRP1 may play a functional role in the development of neural system, notochord, vascular, or lymphatic vessels.
FIGURE 1.
RasGRP1 is expressed in PCV in zebrafish. A, WISH was used to analyze the embryonic expression pattern of RasGRP1. At 24 hpf, RasGRP1 expression was detected in the DA, PCV, and Pl region of the embryos. At 36 and 48 hpf, RasGRP1 expression was restricted to the PCV and Pl region. From 36 hpf on, RasGRP1 was also detected in the notochord (NC) and head region. A cross-section of stained embryos showed DA and PCV expression of RasGRP1 at 24 hpf and PCV expression at 36 hpf. SC, spinal cord. B, the expression of RasGRP1 was analyzed by RT-PCR from the 1-cell stage to 6 dpf. The rasgrp1 mRNA (a 1059-bp PCR product) is shown in the top gel, and the mRNA of EF1α (a 524-bp PCR product) served as an internal control (bottom gel).
Knockdown of rasgrp1 Results in Pericardial and Truck Edema
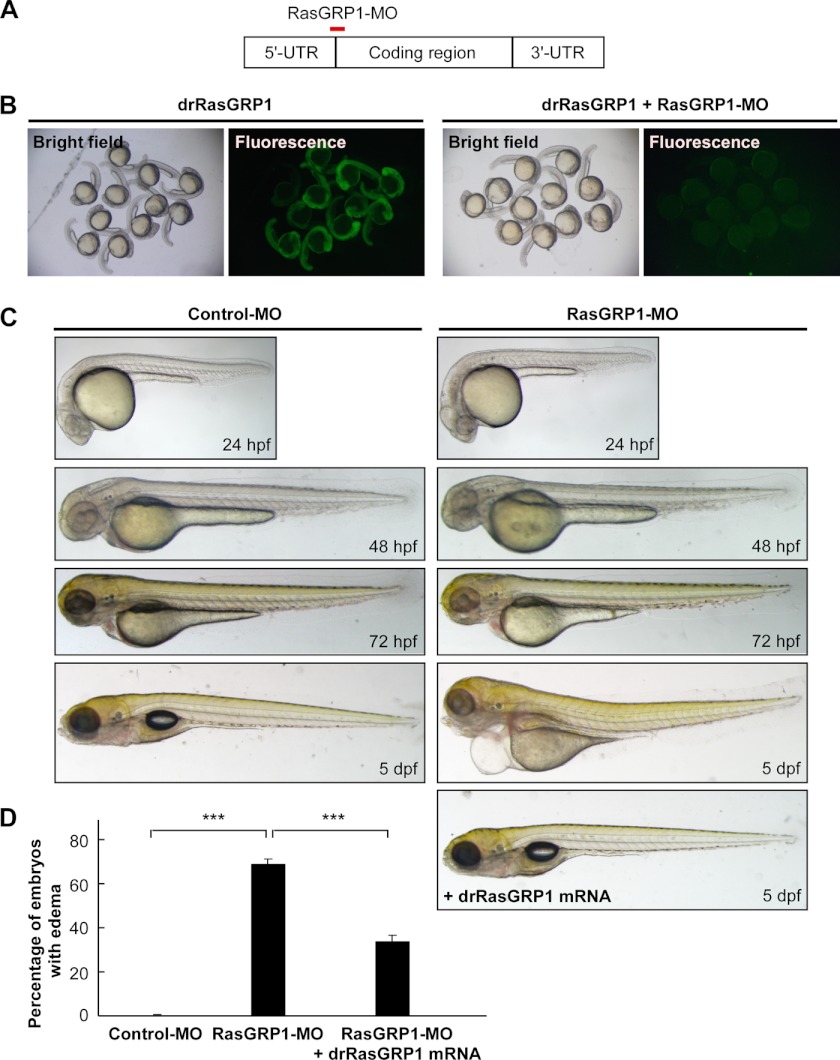
To study the roles of RasGRP1 during early zebrafish embryogenesis, we used an antisense morpholino oligonucleotide (RasGRP1-MO) to block the translation of RasGRP1 (Fig. 2A). To validate the knockdown efficiency, we constructed a fusion gene, drRasGRP1-GFP, with the 5′ untranslated region sequence of rasgrp1 that included the targeting sequence of RasGRP1-MO and a GFP reporter. The expression of the GFP fusion protein was abolished when RasGRP1-MO was injected into the zebrafish embryos, indicating efficient silence of rasgrp1 expression by the morpholino (Fig. 2B). As rasgrp1 was expressed in the vascular/lymphatic system during early zebrafish development (Fig. 1), we investigated the possible function of RasGRP1 in the development of vessel systems. The zebrafish embryos injected with 10 ng or a higher dose of RasGRP1-MO exhibited blood accumulation, curl tails, and blood flow retardation (supplemental Fig. 2). These defects could be partially rescued by rasgrp1 mRNA (supplemental Fig. 2) that was mutated in the region targeted by RasGRP1-MO so that it was resistant to the morpholino. Intriguingly, embryos injected with a lower dose of RasGRP1-MO (at 6 ng/embryo) gradually developed severe edema in the pericardial sac and around gut after 4 dpf (Fig. 2A), resembling the phenotype of thoracic duct (TD) defect, as described previously (9, 22). However, these morphants showed normal morphology during the first 3 days of development (Fig. 2C). In summary, about 34% of RasGRP1 morphants developed edema at 5 dpf (Fig. 2D). In contrast, almost all embryos injected with 6 ng of control morpholino developed normally (Fig. 2D). To assure the specificity of RasGRP1-MO in the induction of edema, we analyzed another morpholino that affected splicing of rasgrp1 mRNA (RasGRP1-MO2, supplemental Fig. 3). Injection of the zebrafish embryos with RasGRP1-MO2 was able to successfully disrupt the splicing of rasgrp1 mRNA (supplemental Fig. 2). As expected, similar edema phenotypes were observed with RasGRP1-MO2 (supplemental Fig. 4). Furthermore, coinjection of rasgrp1 mRNA (at 1 pg/embryo) could significantly rescue the edema phenotype caused by RasGRP1-MO (Fig. 2B). Taken together, these observations indicate that RasGRP1 likely plays an important role in zebrafish lymphatic development.
FIGURE 2.
Knockdown of rasgrp1 results in severe edema. A, RasGRP1-MO was designed to block the translation of RasGRP1 by targeting at the start codon region (+1 to +25). B, embryos injected with drRasGRP1-GFP mRNA (50 pg) had expression of green fluorescent signals. Coinjection of RasGRP1-MO (6 ng) with drRasGRP1-GFP mRNA (50 pg) abolished drRasGRP1-GFP expression. The data were obtained from 28-hpf embryos. C, zebrafish embryos were injected with control MO (6 ng) or RasGRP1-MO (6 ng), and the embryonic development was monitored at 24, 48, 72, and 5 dpf. Note that severe edema occurred in the pericardial sac and trunk region at 5 dpf in the RasGRP1 morphants and that such defects were rescued by coinjection of a morpholino-resistant drRasGRP1 mRNA (1 pg). D, the percentage of embryos with edema was calculated from control MO-injected embryos (n = 241), RasGRP1-MO morphants (n = 288), and the embryos coinjected with RasGRP1-MO and drRasGRP1 mRNA (n = 207). The data are shown as mean ± S.D. ***, p < 0.001 by Student's t test.
Rasgrp1 Knockdown Leads to Defects in Thoracic Duct Formation
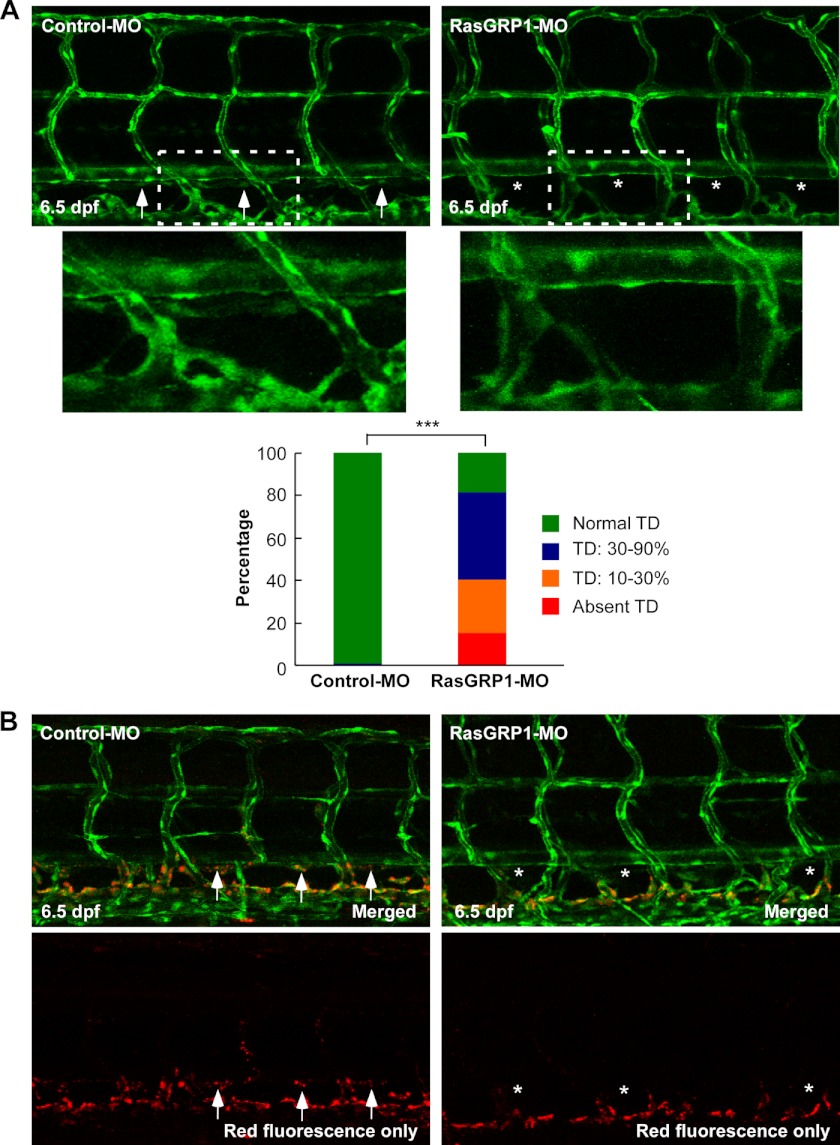
To explore whether RasGRP1 played a functional role in lymphatic development, we silenced RasGRP1 in Fli1:eGFPy1 zebrafish embryos in which enhanced GFP (eGFP) was expressed in both blood and lymphatic vessels (21). We first examined the formation of the TD, which was the first and largest lymphatic vessel formed between the DA and PCV. The TD was analyzed by measuring its length in 10 somites (supplemental Fig. 5) as described previously (7, 8). Upon injection of 6 ng of RasGRP1-MO, 16% of the embryos failed to form any TD by 6.5 dpf, a stage when this vessel is fully formed (22). In 25% of RasGRP1-MO-injected embryos, only 10% to 30% of TD was formed, whereas 40% of the morphants had 30% to 90% TD formation (Fig. 3A). In contrast, TD was completely formed in 93% of control morpholino-injected embryos by 6.5 dpf (Fig. 3A). Similar results were also observed in RasGRP1-MO2-injected embryos (supplemental Fig. 6).
FIGURE 3.
Knockdown of rasgrp1 leads to defects in thoracic duct formation. A, representative confocal images of 6.5 dpf in Fli1:eGFPy zebrafish embryos injected with control MO or RasGRP1-MO. Normal TD formation in the control embryos is marked by arrows, and the absence of the TD in RasGRP1-MO-injected embryos is marked by asterisks. The insets are magnified pictures of the dotted-line boxes. TD formation was quantitatively analyzed by measuring its presence from segments of somite 5 to somite 15, and the result is shown in the bottom panel (n = 120 for control embryos, and n = 187 for RasGRP1 morphants). ***, p < 0.001 by Chi square analysis. B, lymphatic dye uptake assay in Fli1:eGFPy embryos. Alexa Fluor 568 dextran (red) was injected into zebrafish embryos at 6 dpf, and the uptake of the dye was found in control embryos (arrows) but not in RasGRP1-MO-injected embryos (asterisks).
To confirm the lack of TD formation in RasGRP1-MO-injected embryos, we performed a subcutaneous dye uptake experiment by injecting zebrafish embryos at 6.5 dpf blow the skin in the trunk region with a fluorescent dye Alexa Fluor 568 dextran. The Alexa Fluor 568 dextran was absorbed by the TD in control embryos but not in the RasGRP1-MO-injected embryos (Fig. 3B). These results, therefore, provide further evidence that TD formation is disrupted by rasgrp1 knockdown in the zebrafish embryos.
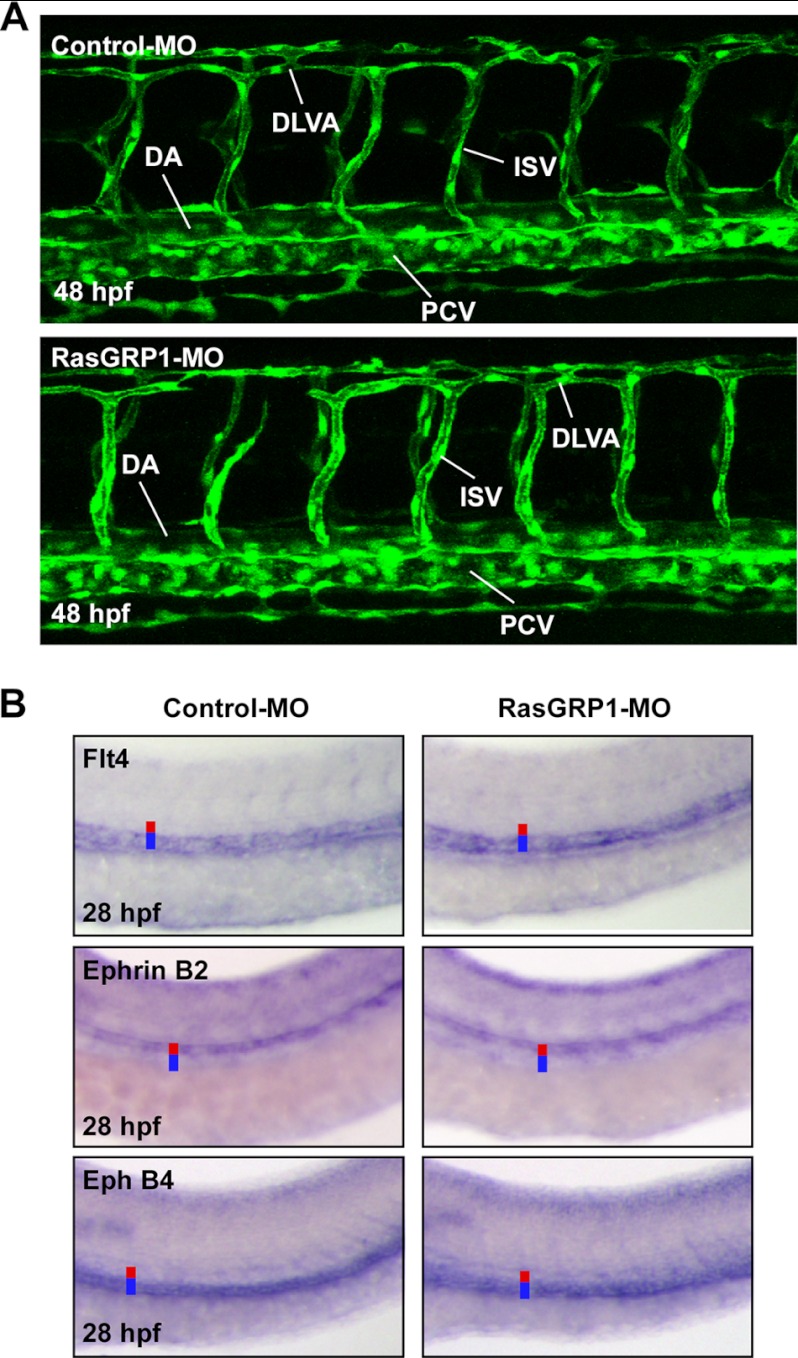
Knockdown of rasgrp1 in Zebrafish Does Not Affect Angiogenesis
To verify that the observed lymphatic defects caused by RasGRP1 knockdown was not because of a secondary effect of angiogenic defects, we investigated whether knockdown of RasGRP1 could impair early vascular development. We did not observe any apparent cardiovascular defects upon injection of 6 ng of RasGRP1-MO (Fig. 4A). Furthermore, arteriovenous differentiation of the large axial vessels was apparently not altered in the RasGRP1-MO-injected embryos, as evidenced by normal expression patterns of arterial and venous markers including Flt4, Ephrin B2, and Eph B4 at 28 hpf (Fig. 4B). Similarly, no vascular defects were observed with RasGRP1-MO2-injected embryos (supplemental Fig. 7). Collectively, these data indicate that the lymphatic defects upon rasgrp1 knockdown are not due to a secondary effect of disrupted angiogenesis by the morpholino.
FIGURE 4.
Knockdown of rasgrp1 does not affect angiogenesis. A, representative confocal images of 48 hpf Fli1:eGFPy zebrafish embryos. DLAV, dorsal longitudinal anastomosing vessel. B, WISH was performed with control MO- and RasGRP1-MO-injected embryos 28 hpf to analyze expression of the arterial marker (Ephrin B2) and venous markers (Flt4, Eph B4). Red lines denote the DA, and blue lines denote the PCV.
Knockdown of rasgrp1 Impairs Early Lymphatic Development
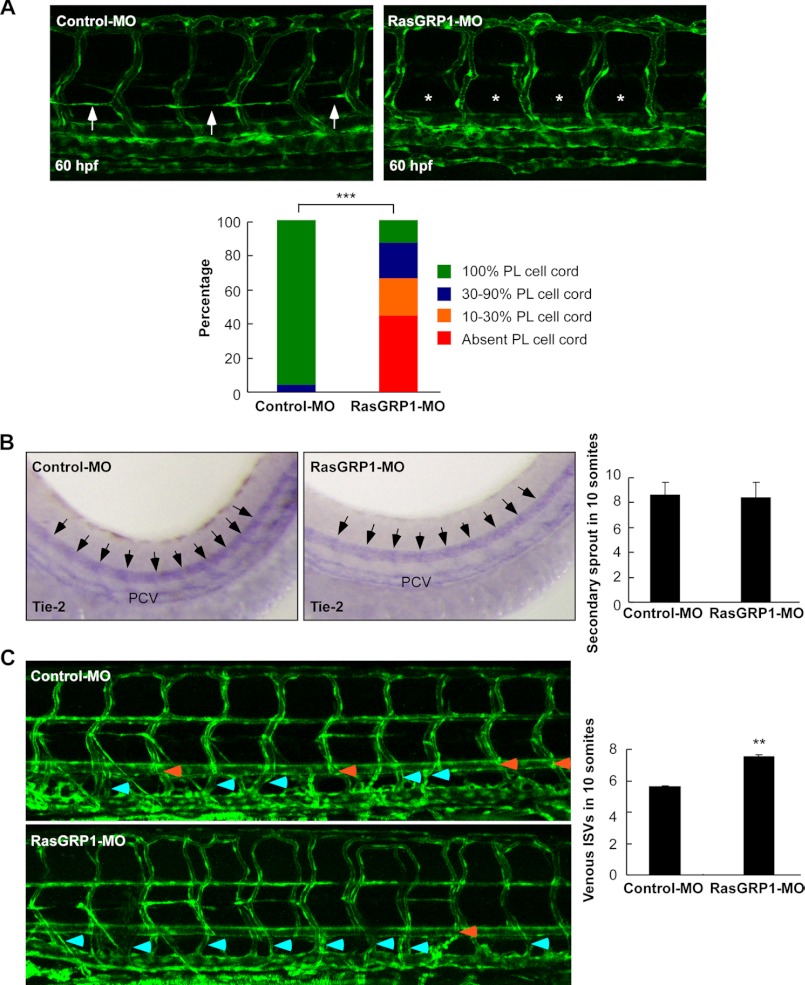
We next analyzed whether knockdown of RasGRP1 could impair development of the parachordal lymphangioblast (PL) cells, which contribute to TD formation as they are precursors for lymphatic vessels (9). Similar to TD analysis, PL string formation was analyzed by measuring its length in 10 somites. At 60 hpf in control embryos, PL cells were detected in nearly every somite segment (Fig. 5A). In contrast, PL cells were absent in 44% of RasGRP1-MO-injected embryos, whereas 22% of embryos had 10–30% of the normal length of PL cells, and 21% embryos had 30–90% of the normal length of PL cells (Fig. 5A). Similarly, the embryos injected with RasGRP1-MO2 were also defective in PL cell development (supplemental Fig. 8). These data, therefore, provide additional evidence that RasGRP1 is involved in early lymphatic development in zebrafish embryos.
FIGURE 5.
Knockdown of rasgrp1 impairs early lymphatic development in zebrafish. A, representative confocal images of Fli1:eGFPy zebrafish embryos at 60 hpf upon injection of control MO or RasGRP1-MO. The presence of a PL string in control embryos is marked by arrows, and its absence in RasGRP1 morphants is marked by asterisks. The percentage of PL cell formation was quantitatively analyzed by measuring its presence in 10 somites, and the results are shown in the lower panel (n = 121 for control and n = 245 for RasGRP1 morphants). ***, p < 0.001 by Chi square analysis. B, WISH of control embryos and RasGRP1 morphants at 48 hpf for Tie-2 expression. The secondary sprouts are marked by arrows. The number of secondary sprouts in 10 somites at 48 hpf was quantified (n = 10 for control embryos and n = 10 for RasGRP1-MO-injected embryos). The data are shown as mean ± S.D. There is no significant difference between the two groups. C, quantification of unilateral venous ISVs that connect to PCV in Fli1:eGFPy zebrafish embryos at 6 dpf. The number of venous ISVs was measured from the 10-somite region of control embryos (n = 71) and RasGRP1-MO-injected embryos (n = 107). The data are shown as mean ± S.D. **, p < 0.01 by Student's t test. Representative confocal images of Fli1:eGFPy zebrafish embryos at 6 dpf are shown in the left panels. Light blue arrows indicate venous ISVs, and orange arrows indicate arterial ISVs.
To determine whether the absence of PL cells was caused by lymphangiogenic sprouts defects, we analyzed the expression of Tie2, which marks both angiogenic and lymphangiogenic secondary sprouts at 48 hpf. The WISH results revealed that RasGRP1-MO-injected embryos had normal the total number of secondary sprouts compared with control embryos (Fig. 5B). We also counted the numbers of intersegmental vessels (ISVs) that connected to the PCV in Fli1:eGFPy1 embryos at 6 dpf. It appeared that the proportion of venous intersomitic vessels (vISVs) in rasgrp1 morphants was increased significantly (Fig. 5B). In control embryos, about 50% of the ISVs were vISVs, whereas about 75% of ISVs were vISVs in rasgrp1 morphant embryos (Fig. 5C). Because vISVs are formed through connection of secondary angiogenic sprouts, the rest of the sprouts should be the secondary lymphangiogenic sprouts contributing to TD formation. An increase in the number of vISVs would indicate a decrease in secondary lymphangiogenic sprouts forming the TD, in agreement with the finding that TD formation was disrupted by rasgrp1 knockdown (Fig. 3). Taken together, these results demonstrate that RasGRP1 is required for early lymphangiogenesis in zebrafish.
Activated HRas Rescues Lymphatic Defects Caused by RasGRP1 Knockdown
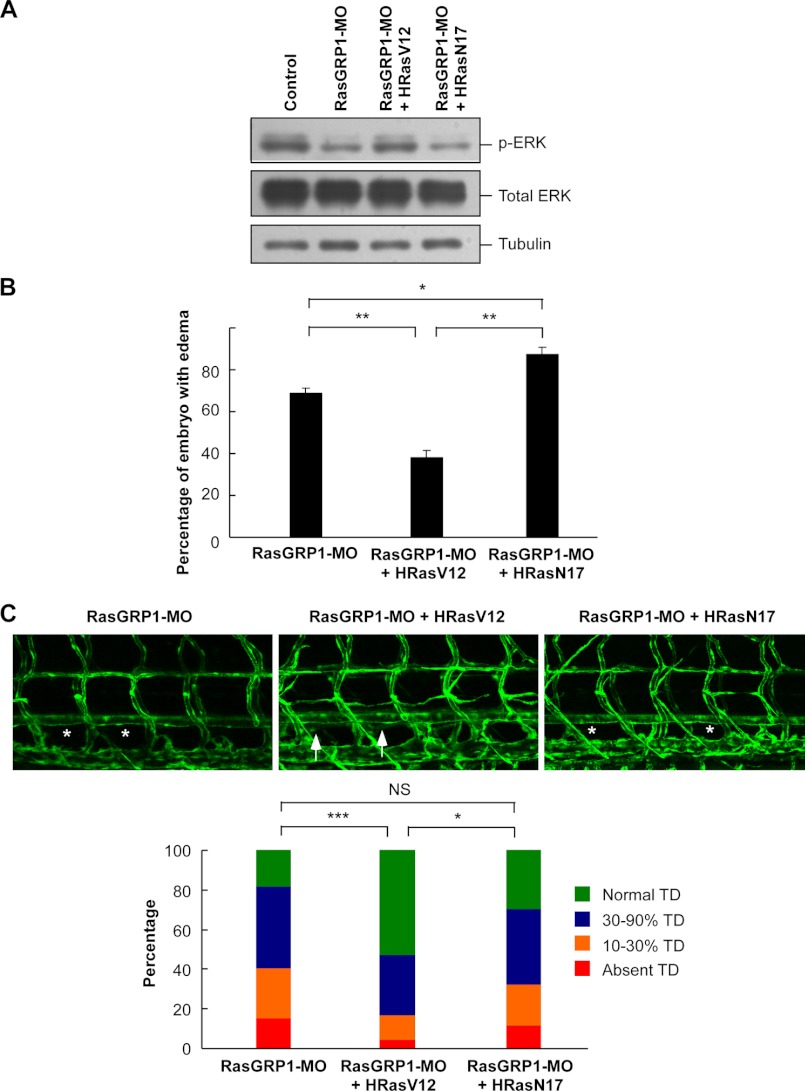
Next we tried to explore the molecular basis underlying RasGRP1 knockdown-mediated lymphatic vessel defects during early development in zebrafish. As RasGRP1 is an activator of Ras proteins, we thus analyzed whether RasGRP1 affects lymphangiogenesis through modulation of Ras/ERK signaling. As expected, ERK phosphorylation was reduced by RasGRP1-MO in zebrafish embryos at 36 hpf (Fig. 6A). We constructed a constitutively active HRas (HRasV12) and a dominant negative HRas (HRasN17) (24). When these two Ras constructs were expressed in zebrafish embryos, they could alter ERK phosphorylation, consistent with the activity of the Ras proteins (Fig. 6A and supplemental Fig. 9). In agreement with these observations, we also found that ERK phosphorylation in the PCV region was reduced by RasGRP1-MO (supplemental Fig. 10). As rasgrp1 knockdown is associated with a decrease in Ras/ERK signaling, we hypothesized that the lymphatic developmental defects caused by rasgrp1 down-regulation would be rescued by the constitutively active Ras. As expected, the percentage of edema caused by RasGRP1-MO was reduced significantly in the embryos coinjected with HRasV12 mRNA, whereas the percentage of edema was increased in embryos coinjected with HRasN17 mRNA (Fig. 6B). We also analyzed the effect of coexpression of Ras proteins on TD formation in Fli1:eGFPy1 zebrafish at 6.5 dpf. Consistently, HRasV12 could partially rescue the TD defects induced by RasGRP1-MO, whereas HRasN17 could not rescue the defects (Fig. 6C). The percentage of embryos without any TD formation upon rasgrp1 knockdown was reduced from 15.9% to 4.5% by HRasV12 overexpression (Fig. 6C). Collectively, these data revealed that an alteration of Ras/ERK signaling pathway is, at least partially, implicated in the observed lymphatic developmental defects caused by rasgrp1 knockdown.
FIGURE 6.
The lymphatic defects upon rasgrp1 knockdown is rescued by activated Hras. A, Western blot analysis for ERK phosphorylation. Zebrafish embryos were injected with the mRNAs as indicated, and the whole embryo protein extract was used in Western blotting to detect the levels of phosphorylated ERK1/2 and total ERK1/2. The doses of injection were as follows: 6 ng/embryo for control-MO and RasGRP1-MO, 1 pg/embryo for HRasV12 mRNA, and 2.5 pg/embryo for HRasN17 mRNA. B, the percentage of edema at 5 dpf. The data are shown as mean ± S.D. **, indicates p < 0.01 by Student's t test. C, representative confocal images of 6.5 dpf Fli1:eGFPy zebrafish embryos. The properly formed TD is marked by arrows, and the absence of TD is marked by asterisks. D, quantification of TD formation at 6.5 dpf embryos (n = 187 for RasGRP1-MO, n = 165 for RasGRP1-MO plus HRasV12, and n = 195 for RasGRP1-MO plus HRasN17). *, p < 0.05; ***, p < 0.001; NS, not significant; Chi square test.
Synergistic Effect of Silencing Both Flt4/VEGFR3 and RasGRP1 on Lymphatic Development
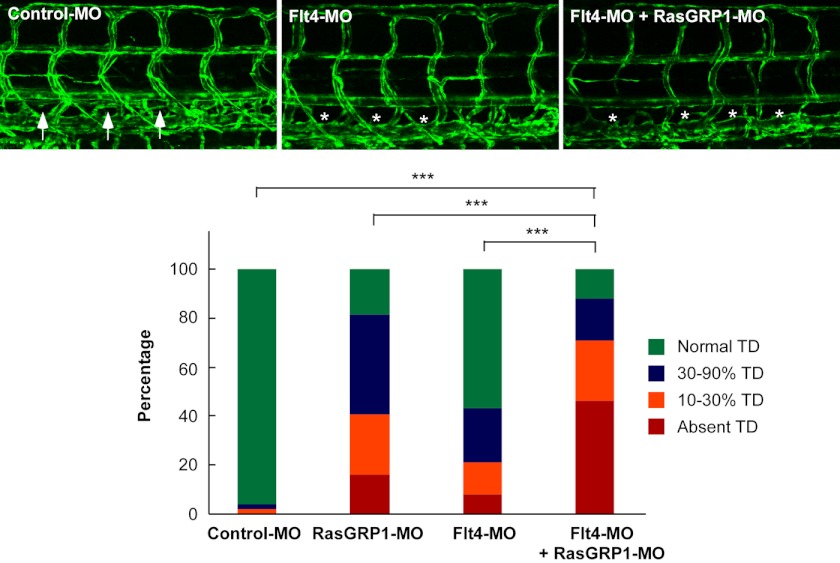
VEGFC and Flt4 (VEGFR3) are known to be essential for the formation of TD in zebrafish (25). Recently, it has been demonstrated that Ras proteins regulate lymphatic vessel growth by modulating Flt4 signaling in mice (26). As RasGRP1 is an activator of Ras proteins, we then explored whether RasGRP1 genetically interacts with Flt4. We used a morpholino oligonucleotide to block the translation of flt4, as characterized previously (8). We found that a low dose of Flt4-MO induced a minimal defect in TD formation, consistent with previous findings (8). Notably, knockdown of both flt4 and rasgrp1 caused much more severe defects in lymphatic development than knockdown of either gene alone (Fig. 7), shown as absence of TDs in 46% of embryos with double knockdown (Fig. 7). Thus, our findings provide a preliminary model that RasGRP1 may have a genetic interaction with Flt4 during lymphatic development in zebrafish.
FIGURE 7.
RasGRP1 genetically interacts with Flt4. Representative confocal images of 6.5 dpf Fli1:eGFPy zebrafish embryos injected with control MO, Flt4-MO (1 ng), and RasGRP1-MO (6 ng) as indicated. The normally formed TD is marked by arrows, and the absence of TD is marked by asterisks. TD formation was quantitatively analyzed (n = 52 for control embryos, n = 187 for RasGRP1-MO, n = 121 for Flt4-MO, and n = 138 for RasGRP1-MO plus Flt4-MO). ***, p < 0.001 by Chi square analysis.
DISCUSSION
So far there are four RasGRP genes identified in mammals, whereas zebrafish have three RasGRPs, including RasGRP1, RasGRP2, and RasGRP3 (27). The physiological functions of the RasGRP family are largely unknown. RasGRP1 has been found to be required for T cell receptor signaling, thymocyte differentiation, and B cell proliferation (14–16). RasGRP2 is required for proper platelet aggregation in mice (28), whereas RasGRP3 was reported to regulate angiogenesis in response to VEGF stimulation (29). However, mice depleted of rasgrp1 were phenotypically normal except for defects in T cell development (15, 16). This study reveals for the first time a new physiological function of RasGRP1 in lymphatic development in zebrafish embryos. Knockdown of rasgrp1 impaired the formation of lymphangiogenic sprouts, development of PL cells, and formation of TD. It is worth noting that at a low dose of RasGRP1-MO, the development of blood vessels was not affected (Fig. 4), and the development of notochord, somite, and heart are also not affected (supplemental Fig. 11). Meanwhile, injection of a high dose of rasgrp1 mRNA appeared to have no effect to promote lymphangiogenesis in zebrafish embryos (supplemental Fig. 12). Interestingly, silencing of both rasgrp1 and flt4/vegfr3 in zebrafish embryos aggravated the lymphatic defects. Collectively, these observations have pinpointed a critical role of RasGRP1 in lymphatic development in zebrafish.
Zebrafish is a well established model to study lymphatic development. According to previous reports (8, 9, 22, 30), TD development is initiated at ∼36 hpf when the secondary sprouts branch off from the PCV. About half of these sprouts are angiogenic, and they further develop into venous ISVs, whereas the other half of the sprouts are lymphangiogenic and further develop into the string of PC cells at the horizontal myoseptum region. Beyond 60 hpf, PL cells migrate along the arterial intersegmental vessels dorsally to contribute to the formation of the dorsal longitudinal lymphatic vessel or ventrally to contribute to the formation of the TD between the DA and PCV. On the basis of this study, lymphatic formation defects upon rasgrp1 knockdown were unlikely due to a secondary effect of alterations in blood vascular development. In RasGRP1-MO-injected embryos, the differentiation of PCV and DA occurred normally, as revealed by properly formed venous-arterial-specific markers (Fig. 4). In addition, the formation of the PCV, DA, and primary ISVs were all unaltered by RasGRP1 knockdown (Fig. 4). Our observation is consistent with previous studies demonstrating that disruption of arterial development in zebrafish cannot impair lymphangiogenic sprouting nor cause TD defects (9, 31).
Although we found that Ras/ERK signaling is implicated in RasGRP1-mediated lymphangiogenesis (Fig. 6), the molecular details underlying the regulation of lymphatic development by RasGRP1 remains to be determined in the future. As RasGRP1 expression was detected in the PCV from 24–40 hpf, at a time when the secondary sprouts occurs, we speculate that RasGRP1-mediated Ras/ERK signaling may directly induce venous endothelial cells to form lymphangiogenic sprouts. As we found that knockdown both flt4 and rasgrp1 has a synergistic effect on the defects of lymphatic development (Fig. 7), we propose that RasGRP1 may function downstream of Flt4 to activate Ras proteins, thereby regulating lymphangiogenic sprout formation in zebrafish. It is also possible that Ras activation by RasGRP1 is implicated in the expression of Flt4, which in turn modulates lymphangiogenesis, as supported by a recent report showing that Ras proteins are involved in Flt4 expression and lymphatic development in mice (26). Another possibility is that the lymphangiogenic sprout formation is due to the inhibition of venous angiogenic sprouts by RasGRP1-mediated Ras/ERK signaling, similar to a model proposed to explain the segregation and generation of the DA and PCV in early vascular development in zebrafish embryos (32). It is also noteworthy that Ras/ERK signaling is required for both angiogenesis and lymphangiogenesis. Intriguingly, injection of RasGRP1-MO at a low dose only affects development of lymphatic system but not angiogenesis (Fig. 4). We speculate that the subtle change of the Ras/ERK signaling pathway mediated by RasGRP1 may dictate whether angiogenesis or lymphangiogenesis is affected. Nevertheless, the finding that RasGRP1 is implicated in lymphangiogenesis not only reveals a new physiological function for RasGRP1 but also paves the way for future studies to unravel the unique role of RasGRP1-mediated Ras/ERK signaling in lymphatic development.
This work was supported by Ministry of Science and Technology of China Research Grant 2012CB524900 (to Y. C.) and 2010CB529506 (to Y. P. and Z. W.); by National Natural Science Foundation of China Grants 30830037, 81021002, and 81130077 (to Y. C.) and 30971660 (to Y. P.); and by Chinese Academy of Sciences Grant KSCX2-EW-R-08 (to Y. C.).

This article contains supplemental Figs. 1–12.
- PAQR
- progestin and AdipoQ receptor
- MO
- morpholino
- dpf
- days post-fertilization
- hpf
- hours post-fertilization
- DA
- dorsal aorta
- PCV
- posterior cardinal vein
- Pl
- cardinal vein plexus
- TD
- thoracic duct
- eGFP
- enhanced GFP
- PL
- parachordal lymphangioblast
- ISV
- intersegmental vessel
- vISV
- venous intersomitic vessel.
REFERENCES
- 1. Tammela T., Alitalo K. (2010) Lymphangiogenesis. Molecular mechanisms and future promise. Cell 140, 460–476 [DOI] [PubMed] [Google Scholar]
- 2. Cueni L. N., Detmar M. (2008) The lymphatic system in health and disease. Lymphat. Res. Biol. 6, 109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wigle J. T., Oliver G. (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 [DOI] [PubMed] [Google Scholar]
- 4. François M., Caprini A., Hosking B., Orsenigo F., Wilhelm D., Browne C., Paavonen K., Karnezis T., Shayan R., Downes M., Davidson T., Tutt D., Cheah K. S., Stacker S. A., Muscat G. E., Achen M. G., Dejana E., Koopman P. (2008) Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 [DOI] [PubMed] [Google Scholar]
- 5. Karkkainen M. J., Ferrell R. E., Lawrence E. C., Kimak M. A., Levinson K. L., McTigue M. A., Alitalo K., Finegold D. N. (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 [DOI] [PubMed] [Google Scholar]
- 6. Karkkainen M. J., Haiko P., Sainio K., Partanen J., Taipale J., Petrova T. V., Jeltsch M., Jackson D. G., Talikka M., Rauvala H., Betsholtz C., Alitalo K. (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 [DOI] [PubMed] [Google Scholar]
- 7. Geudens I., Herpers R., Hermans K., Segura I., Ruiz de Almodovar C., Bussmann J., De Smet F., Vandevelde W., Hogan B. M., Siekmann A., Claes F., Moore J. C., Pistocchi A. S., Loges S., Mazzone M., Mariggi G., Bruyère F., Cotelli F., Kerjaschki D., Noël A., Foidart J. M., Gerhardt H., Ny A., Langenberg T., Lawson N. D., Duckers H. J., Schulte-Merker S., Carmeliet P., Dewerchin M. (2010) Role of -like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscl. Thromb. Vasc. Biol. 30, 1695–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hermans K., Claes F., Vandevelde W., Zheng W., Geudens I., Orsenigo F., De Smet F., Gjini E., Anthonis K., Ren B., Kerjaschki D., Autiero M., Ny A., Simons M., Dewerchin M., Schulte-Merker S., Dejana E., Alitalo K., Carmeliet P. (2010) Role of synectin in lymphatic development in zebrafish and frogs. Blood 116, 3356–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogan B. M., Bos F. L., Bussmann J., Witte M., Chi N. C., Duckers H. J., Schulte-Merker S. (2009) Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 [DOI] [PubMed] [Google Scholar]
- 10. Bos F. L., Caunt M., Peterson-Maduro J., Planas-Paz L., Kowalski J., Karpanen T., van Impel A., Tong R., Ernst J. A., Korving J., van Es J. H., Lammert E., Duckers H. J., Schulte-Merker S. (2011) CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ. Res. 109, 486–491 [DOI] [PubMed] [Google Scholar]
- 11. Ebinu J. O., Bottorff D. A., Chan E. Y., Stang S. L., Dunn R. J., Stone J. C. (1998) RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 [DOI] [PubMed] [Google Scholar]
- 12. Ehrhardt A., David M. D., Ehrhardt G. R., Schrader J. W. (2004) Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol. Cell. Biol. 24, 6311–6323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genot E., Cantrell D. A. (2000) Ras regulation and function in lymphocytes. Curr. Opin. Immunol. 12, 289–294 [DOI] [PubMed] [Google Scholar]
- 14. Ebinu J. O., Stang S. L., Teixeira C., Bottorff D. A., Hooton J., Blumberg P. M., Barry M., Bleakley R. C., Ostergaard H. L., Stone J. C. (2000) RasGRP links T-cell receptor signaling to Ras. Blood 95, 3199–3203 [PubMed] [Google Scholar]
- 15. Dower N. A., Stang S. L., Bottorff D. A., Ebinu J. O., Dickie P., Ostergaard H. L., Stone J. C. (2000) RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1, 317–321 [DOI] [PubMed] [Google Scholar]
- 16. Coughlin J. J., Stang S. L., Dower N. A., Stone J. C. (2005) RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J. Immunol. 175, 7179–7184 [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki H., Springett G. M., Toki S., Canales J. J., Harlan P., Blumenstiel J. P., Chen E. J., Bany I. A., Mochizuki N., Ashbacher A., Matsuda M., Housman D. E., Graybiel A. M. (1998) A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc. Natl. Acad. Sci. U.S.A. 95, 13278–13283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamashita S., Mochizuki N., Ohba Y., Tobiume M., Okada Y., Sawa H., Nagashima K., Matsuda M. (2000) CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J. Biol. Chem. 275, 25488–25493 [DOI] [PubMed] [Google Scholar]
- 19. Jin T., Ding Q., Huang H., Xu D., Jiang Y., Zhou B., Li Z., Jiang X., He J., Liu W., Zhang Y., Pan Y., Wang Z., Thomas W. G., Chen Y. (2012) PAQR10 and PAQR11 mediate Ras signaling in the Golgi apparatus. Cell Res. 22, 661–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang H., Jin T., He J., Ding Q., Xu D., Wang L., Zhang Y., Pan Y., Wang Z., Chen Y. (2012) Progesterone and adipoQ receptor 11 links ras signaling to cardiac development in zebrafish. Arterioscl. Thromb. Vasc. Biol. 32, 2158–2170 [DOI] [PubMed] [Google Scholar]
- 21. Lawson N. D., Weinstein B. M. (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- 22. Yaniv K., Isogai S., Castranova D., Dye L., Hitomi J., Weinstein B. M. (2006) Live imaging of lymphatic development in the zebrafish. Nat. Med. 12, 711–716 [DOI] [PubMed] [Google Scholar]
- 23. Westerfield M. (1995) The Zebrafish Book, 3rd Ed, p. 144, University of Oregon, Eugene, OR [Google Scholar]
- 24. Shields J. M., Pruitt K., McFall A., Shaub A., Der C. J. (2000) Understanding Ras. 'It ain't over 'til it's over'. Trends Cell Biol. 10, 147–154 [DOI] [PubMed] [Google Scholar]
- 25. Küchler A. M., Gjini E., Peterson-Maduro J., Cancilla B., Wolburg H., Schulte-Merker S. (2006) Development of the zebrafish lymphatic system requires VEGFC signaling. Curr. Biol. 16, 1244–1248 [DOI] [PubMed] [Google Scholar]
- 26. Ichise T., Yoshida N., Ichise H. (2010) H-, N- and Kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice. Development 137, 1003–1013 [DOI] [PubMed] [Google Scholar]
- 27. Gomez G. A., Veldman M. B., Zhao Y., Burgess S., Lin S. (2009) Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS ONE 4, e4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crittenden J. R., Bergmeier W., Zhang Y., Piffath C. L., Liang Y., Wagner D. D., Housman D. E., Graybiel A. M. (2004) CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 10, 982–986 [DOI] [PubMed] [Google Scholar]
- 29. Roberts D. M., Anderson A. L., Hidaka M., Swetenburg R. L., Patterson C., Stanford W. L., Bautch V. L. (2004) A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol. Cell. Biol. 24, 10515–10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bussmann J., Bos F. L., Urasaki A., Kawakami K., Duckers H. J., Schulte-Merker S. (2010) Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653–2657 [DOI] [PubMed] [Google Scholar]
- 31. Lawson N. D., Mugford J. W., Diamond B. A., Weinstein B. M. (2003) phospholipase C γ-1 is required downstream of vascular endothelial growth factor during arterial development. Gene Dev. 17, 1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hong C. C., Peterson Q. P., Hong J. Y., Peterson R. T. (2006) Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr. Biol. 16, 1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]