Background: Signaling mechanisms underlying prolactin-induced adult neurogenesis are unknown.
Results: Prolactin activates ERK5 in SVZ; suppression of ERK5 expression in vitro and erk5 deletion in vivo attenuates prolactin-induced adult neurogenesis.
Conclusion: ERK5 is an important mediator in prolactin-stimulated adult neurogenesis.
Significance: Elucidation of signaling pathways underlying prolactin-induced adult neurogenesis is critical for understanding the fundamental role of adult neurogenesis in reproductive functions and behaviors.
Keywords: Cell Signaling, ERK, MAP Kinases (MAPKs), Neurogenesis, Prolactin, Adult Neurogenesis, ERK5 MAPK
Abstract
Prolactin-stimulated adult neurogenesis in the subventricular zone (SVZ) and olfactory bulb (OB) mediates several reproductive behaviors including mating/pregnancy, dominant male pheromone preference in females, and paternal recognition of offspring. However, downstream signaling mechanisms underlying prolactin-induced adult neurogenesis are completely unknown. We report here for the first time that prolactin activates extracellular signal-regulated kinase 5 (ERK5), a MAP kinase that is specifically expressed in the neurogenic regions of the adult mouse brain. Knockdown of ERK5 by retroviral infection of shRNA attenuates prolactin-stimulated neurogenesis in SVZ-derived adult neural stem/progenitor cells (aNPCs). Inducible erk5 deletion in adult neural stem cells of transgenic mice inhibits neurogenesis in the SVZ and OB following prolactin infusion or mating/pregnancy. These results identify ERK5 as a novel and critical signaling mechanism underlying prolactin-induced adult neurogenesis.
Introduction
The forebrain subventricular zone (SVZ)2 is one of the two principal neurogenic areas in the adult mammalian brain. Newly generated neurons in the SVZ migrate to the olfactory bulb (OB), where they may play a role in olfaction- and pheromone-based behaviors (1). SVZ/OB neurogenesis is regulated by physiological processes distinct from those for hippocampal dentate gyrus (DG) neurogenesis. For example, pregnancy or pseudo-pregnancy following mating induces SVZ/OB neurogenesis but does not affect DG neurogenesis (2). Neurogenesis stimulated by pregnancy is mediated by prolactin (PRL) (2), a peptide hormone that can be locally released from neuronal structures in several brain regions or peripherally transported via the choroid plexus through the blood-brain barrier (2, 3).
Prolactin is a critical mediator linking the endocrine and behavioral systems. Plasma levels of prolactin are elevated during early stages of pregnancy in mammals (4, 5) and following orgasm in humans (6, 7). Prolactin, by itself, is sufficient to induce SVZ neurogenesis in both male and female mice (2). It also mediates the induction of adult neurogenesis in the SVZ of female mice that is stimulated by pregnancy or dominant male pheromones (8–11). Paternal recognition of offspring following offspring interactions is also mediated, in part, by prolactin stimulation of adult neurogenesis in the SVZ/OB of males (12). Thus, prolactin-induced adult neurogenesis in the SVZ/OB seems to play an important role in several aspects of reproduction. However, the downstream signaling mechanisms underlying prolactin-induced adult neurogenesis are completely unknown. Because reproductive success and offspring nurturing are essential for the existence and maintenance of animal species, it is important to define mechanisms underlying prolactin-induced adult neurogenesis.
ERK5 is a member of the MAP kinase family including ERK1/2 (13). It is highly expressed in the developing brain where it has been implicated in specifying cortical neural stem/progenitor cells toward a neuronal fate, promoting the survival of newly generated neurons, and regulating OB development and olfactory behavior (14–20). In the adult mouse brain, ERK5 is specifically expressed in the neurogenic regions: SVZ and the subgranular zone (SGZ) of the dentate gyrus (21). Inducible and targeted deletion of erk5 specifically in adult neurogenic regions impairs hippocampal neurogenesis and several challenging forms of hippocampus-dependent memory formation (21). Based on these findings and the fact that several cell-surface receptor systems activate ERK5 (22), we asked whether ERK5 signaling plays a role in prolactin-stimulated adult neurogenesis in the SVZ-OB axis.
Here, we report that prolactin activates ERK5 in SVZ-derived aNPCs but not in SGZ-derived aNPCs. Using retroviral infection of shRNA to suppress ERK5 MAP kinase expression in culture as well as inducible and conditional gene targeting to delete erk5 specifically in neural stem cells of adult transgenic mice, our data demonstrate an essential role for ERK5 in prolactin-induced adult neurogenesis in the SVZ and OB.
EXPERIMENTAL PROCEDURES
Reagents
Mouse recombinant prolactin was from National Hormone & Peptide Program (Torrance, CA). Osmotic pump (Alzet 1007D) was from Durect Corporation (Cupertino, CA). MEK5 inhibitor BIX02188 was kindly provided by Boehringer Ingelheim Pharmaceuticals (Ridgefield, CT) (23). STAT5 inhibitor (N′-((4-oxo-4H-chromen-3-yl)methylene) nicotinohydrazide) (24) was from EMD Millipore (Billerica, MA). The shERK5 and shNS retroviruses were previously described (19). The following primary antibodies and dilutions were used for immunohistochemistry or immunocytochemistry: rat monoclonal anti-BrdU (1:500, AbD Serotec); mouse monoclonal antibodies against PCNA (1:500, Millipore), Sox2 (1:200, R&D Systems), Pax6 (1:200, Developmental Studies Hybridoma Bank), PSA-NCAM (1:200, Developmental Studies Hybridoma Bank), NeuN (1:500, Millipore), GFP (1:5,000, Invitrogen), and anti-β-III tubulin (1:1,000, Promega); goat polyclonal antibody against doublecortin (DCX, 1:200, Santa Cruz Biotechnology); and sheep polyclonal antibody against prolactin receptor (1:200, AbD Serotec). Rabbit polyclonal anti-ERK5 (1:500) was generated previously and affinity purified using recombinant MBP-ERK5 protein (25).
Animals and Tamoxifen Administration
The generation of Nestin-CreERTM/ERK5loxP/loxP mice has been described (21). Male Nestin-CreERTM/ERK5loxP/loxP and female ERK5loxP/loxP animals were used for experimental breeding. Nestin-CreERTM/ERK5loxP/loxP and ERK5loxP/loxP littermates (8–10-week-old females) were identically treated with tamoxifen for 7 days and handled throughout the study. Tamoxifen (Sigma) was made fresh daily and dissolved in 2% glacial acetic acid in corn oil solution (Sigma). Pre-warmed tamoxifen solution was administered orally (200 mg/kg) once a day for 7 days. Mice were allowed to recover for 14 days after last dosing before further experimentation.
Animals were housed under standard conditions (12 h light/dark cycle) with food and water provided ad libitum. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee. 10–12-week-old wild type C57Bl/6 mice were used for studies in Figs. 6 and 7 to characterize ERK5 co-expression with various cellular markers.
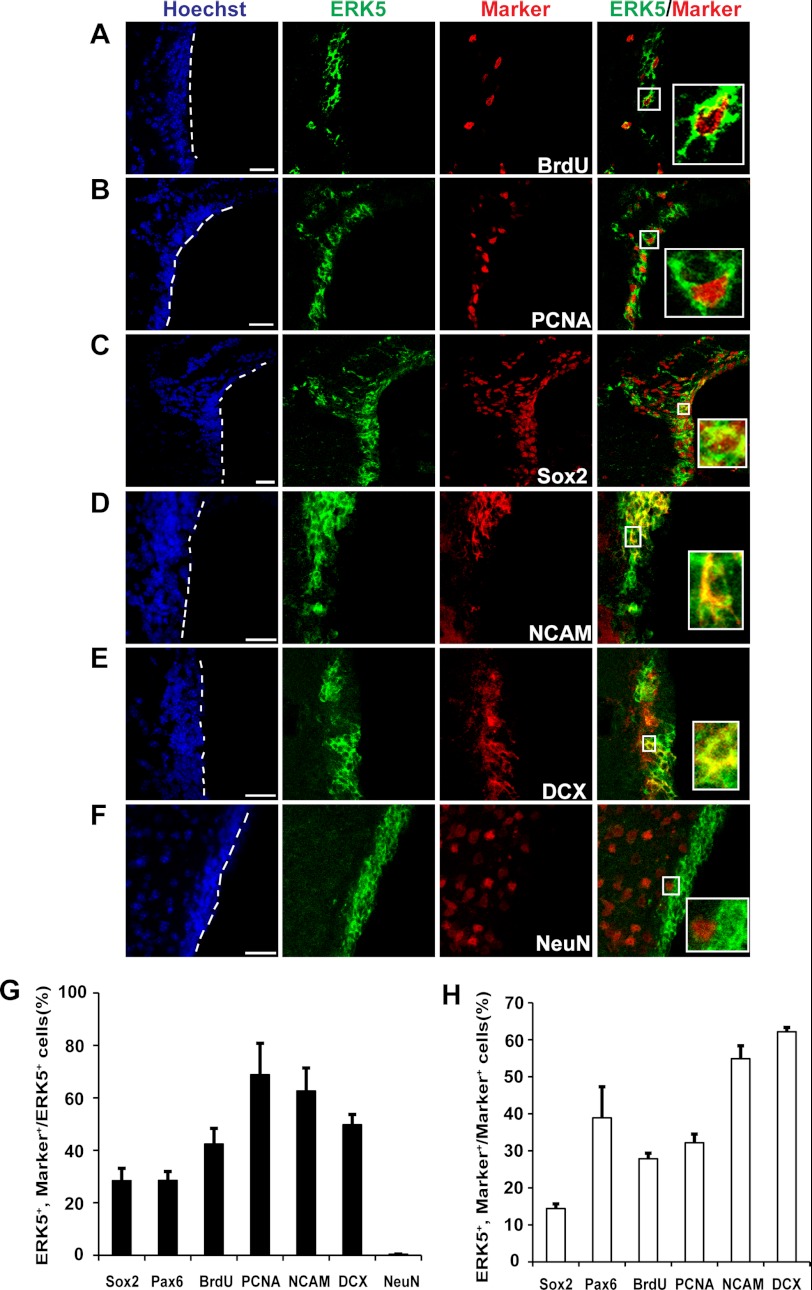
FIGURE 6.
Characterization of ERK5 expression in the SVZ of the adult mouse brain. A&–F, representative confocal images of brain sections co-stained for ERK5 and BrdU (A), PCNA (B), Sox2 (C), PSA-NCAM (D), DCX (E), and NeuN (F). Hoechst staining (blue) was used to identify all cell nuclei. Scale bar: 25 μm. G, quantification of marker-positive cells in total ERK5+ cell population along the SVZ. H, quantification of ERK5+ cells in total marker-positive cell population along the SVZ. Data represent mean percentage of double-labeled cells.
FIGURE 7.
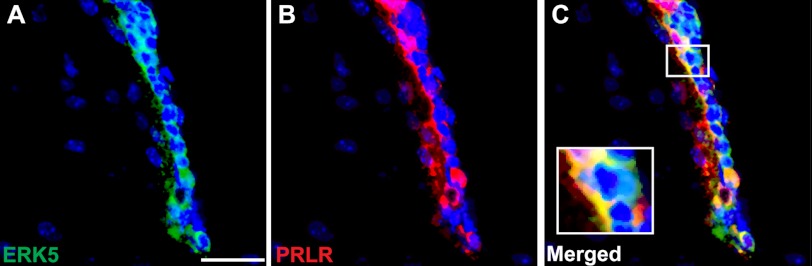
Co-localization of ERK5 and prolactin receptor in the SVZ of the adult mouse brain. Shown are representative confocal images of brain sections with Hoechst staining (blue) to identify all cell nuclei, or co-stained for ERK5 (green) (A), for prolactin receptor (PRLR, red) (B), and merged images (C). Scale bar: 25 μm.
Pump Implantation and Hormone Infusion
This was performed as described (2) with minor modifications. Briefly, mouse recombinant prolactin was dissolved in 1 mg/ml mouse serum albumin (MSA)/0.9% saline at 0.67 mg/ml and filled with ∼90 μl/osmotic pump (Alzet 1007D). The minipumps were implanted subcutaneously. Each animal was infused for 7 consecutive days with either prolactin (8 μg/day) or MSA as vehicle control.
Bromodeoxyuridine (BrdU) Labeling
BrdU was administered to mice the day after the completion of prolactin infusion or at gestation day 7 (i.e. 6.5 days after vaginal plug). BrdU (Sigma) was dissolved in 0.007% NaOH in sterile saline solution and administered at 100 mg/kg by intraperitoneal injection 5 times (every 2 h for 10 h) in 1 day (2). Mice were sacrificed 0.5 h or 4 weeks after the last injection to identify proliferating or BrdU-retaining, adult-born cells, respectively.
Immunohistochemistry
Mice were sacrificed and perfused transcardially with 4% paraformaldehyde (PFA) in phosphate buffer saline (PBS), pH 7.4. Brains were post-fixed in 4% PFA overnight, followed by 30% (w/v) sucrose/PBS solution at 4 °C. IHC was performed on 30 μm-thick coronal subventricular sections using a free-floating antibody staining method or on 20 μm-thick coronal OB sections using a mounted staining method. Briefly, after washing with PBS and PBST (PBS + 0.25% Triton X-100), brain slices were incubated in blocking buffer (PBST + 0.1% bovine serum album (BSA) + 10% normal serum) for 2 h at room temperature. For BrdU staining, tissue sections were first subjected to HCl treatment by sequential incubation in H2O for 5 min, ice-cold 1 N HCl for 10 min, and 2 N HCl for 30 min at 37 °C. Slices were then neutralized by rinsing 2 × 15 min in 0.5 m borate buffer. This was followed by PBST washes and blocking as described above. Sections were incubated with primary antibodies for 48–60 h at 4 °C, washed in PBST 4 × 10 min after primary antibody incubation and incubated with secondary antibodies conjugated with Alexa-Fluor-dyes (1:500 dilution, Invitrogen) in blocking buffer 2 h at room temperature. Sections were then washed 4 × 10 min in PBST and incubated with 2.5 μg/ml Hoechst 33342 for 30 min and washed 3 × 5 min with PBST. Free-floating brain sections were then mounted on gelatin-coated Superfrost plus slides (VWR) and both free-floating SVZ-containing sections and mounted OB sections were coverslipped with anti-fade Aqua Poly/Mount (Polysciences).
Immunocytochemistry
Cells were fixed in PBS containing 4% PFA/30% sucrose at room temperature for 30 min. Fixed cells were washed with PBS for 3 × 5 min, permeabilized with 1% SDS for 5 min, and washed again with PBS for 3 × 5 min. Cells were then blocked in 5% BSA/PBST (PBS with 0.1% Triton X-100) for 2 h, followed by incubation with primary antibodies overnight at 4 °C. Cells were then washed with PBST for 3 × 10 min, followed by incubation with secondary antibodies at 1:5,000 dilution (Alexa-Fluor-488) or 1:2,000 dilution (Alexa-Fluor-594) for 2 h in blocking buffer. Cells were then washed with PBST for 3 × 10 min followed by a 30 min of incubation in Hoechst 33342 for nuclei visualization and a final wash of 10 min in PBST prior to mounting onto slides using anti-fade Aqua Poly/Mount solution.
Retrovirus Construction, Preparation, and Infection
The construction, preparation, and infection procedures of the ERK5 shRNA (shERK5) and control nonspecific shRNA (shNS) retroviruses have been previously described (19, 26).
Adult Neural Progenitor cell Culture and in Vitro Analysis
Primary cell cultures were prepared from 8-week-old C57Bl/6 mice as described (27). Briefly, tissue samples containing the subventricular zone or the dentate gyrus were micro-dissected and enzymatically digested with 0.125% trypsin-EDTA for 7 min at 37 °C followed by incubation with equal volume of 0.014% trypsin inhibitor (Sigma). Tissue samples were then spun down and resuspended in serum-free culture media consisting of DMEM/F12 (Invitrogen), 1 × N2 supplement (Invitrogen), 1 × B27 supplement without retinoic acid (Invitrogen), 100 units/ml penicillin/streptomycin (Invitrogen), 2 mm l-glutamine (Invitrogen), 2 μg/ml heparin (Sigma), 20 ng/ml EGF (EMD Chemicals), and 10 ng/ml bFGF (Millipore). Tissue was mechanically triturated and filtered through a 40 μm cell sieve and plated in non-coated Petri dishes and cultured for 7–14 days until primary neurospheres formed. EGF and bFGF were replenished every 3 days during this period. Spheres collected from secondary passage were dissociated and plated as a monolayer culture on fibronectin and poly-l-ornithine (BD Biosciences)-coated culture plates or Aclar coverslips (Electron Microscopy Sciences) for experiments.
For in vitro knockdown of ERK5 expression, cells were infected with shNS or shERK5 retroviruses as previously described (19). Cells were then subjected to proliferation or differentiation assays. For cell proliferation, cells were plated at a density of 50,000 cells per well in coated coverslips in 48-well plates. Cells were then switched into EGF- and bFGF-free culture medium, and treated with vehicle or prolactin (1 μg/ml) for 18 h, followed by BrdU (10 μm) incorporation for 3 h. Cells were then fixed with 4% PFA/sucrose for 30 min at room temperature, followed by immunocytochemical analysis. For differentiation assay, cells were changed into medium free of EGF and bFGF with or without prolactin treatment for 5 days, followed by fixation and immunocytochemical analysis.
Western Analysis
Adult neural progenitor cells were plated at a density of 1 × 106 cells per well in fibronectin and poly-l-ornithine-coated 12-well plates. At 48 h after plating, cells were changed into EGF- and bFGF-free medium overnight to reduce background, followed by treatment with vehicle or prolactin at indicated times and concentrations. For mechanistic studies using pharmacological inhibitors, cells were pretreated with STAT5 inhibitor or MEK5 inhibitor for 1 h prior to prolactin stimulation. Cells were then washed with cold PBS and lysed with Triton-X lysis buffer. Cell lysates were clarified by centrifugation and protein concentration determined using BCA protein assay (Pierce). Samples containing 10 μg protein were separated on 10% SDS-PAGE gels and transferred to PVDF membrane (Millipore), followed by immunoblotting and ECL Plus (GE Healthcare) detection. The following antibodies were used as primary antibodies for Western analysis: rabbit anti-ERK5 (25), rabbit anti-phospho-ERK5 (Thr218/Tyr220, Cell Signaling), rabbit anti-Stat5 (Cell Signaling), rabbit anti-phospho-Stat5 (Tyr694, Cell Signaling), and anti-β-actin (Sigma).
Confocal Imaging and Analysis
All images were captured with an Olympus Fluoview-1000 laser scanning confocal microscope with numerical aperture (NA) 0.75, 20× lens or NA 1.3, 40× oil immersion lens. Optical Z-sections (1 μm) were collected and processed using ImageJ software (NIH). Images were uniformly adjusted for color, brightness, and contrast with Adobe Photoshop CS4 (Adobe Systems Inc).
Quantification of Immunostained Cells
Immunopositive cells were counted using an inverted fluorescence microscope (Leitz DMIRB, Leica) with a 40× objective (Leica) for cultured cells after immunocytochemistry. For immunohistochemistry of the SVZ, one in every 8 serial coronal sections (30 μm) through the entire rostral-caudal extent of the lateral ventricle was immunostained and counted. For OB, one in every 5 serial sections (20 μm) was counted from rostral to caudal using Optical Fractionator Probe of Stereology Investigator Software (MBF Bioscience). For each section, the region of interest was outlined under 5× objective of Zeiss upright fluorescence microscope, and cell counting was performed under 40X objectives with counting frame set as 50×50 μm2. For cellular characterization in Fig. 6, at least 800 ERK5-immunopositive cells were randomly selected and analyzed for each cellular marker using confocal microscopy. Similarly, greater than 800 marker-immunopositive cells were randomly selected and analyzed for ERK5 co-expression.
Statistical Analysis
All data were expressed as means ± S.E. Pair-wise comparison of the means was analyzed by Student's t test, two-tailed analysis. n.s. not significant; *, p < 0.05; **, p < 0.01.
RESULTS
ERK5 Is Activated by Prolactin in aNPCs Derived from SVZ but Not Those from SGZ
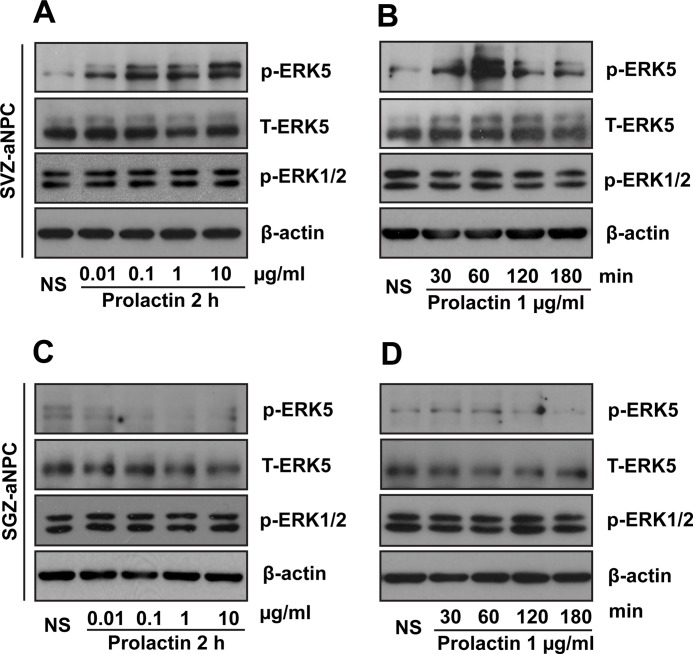
We first determined if prolactin activates ERK5 in SVZ- or SGZ-derived adult neural stem/progenitor cells (aNPCs), prepared from the subventricular zone and the dentate gyrus, respectively, of 8-week-old adult C57Bl/6 mice. ERK5 activation was monitored by Western analysis using an antibody that recognizes phosphorylated (p-) and activated ERK5. Treatment of SVZ-derived aNPCs with recombinant mouse prolactin induced ERK5 phosphorylation, indicative of ERK5 activation, in a dose- and time-dependent manner (Fig. 1, A and B). In contrast, ERK5 was not activated by prolactin in SGZ-derived aNPCs under the same conditions (Fig. 1, C and D), consistent with the observation that prolactin stimulates adult neurogenesis in the SVZ but not SGZ (2, 3).
FIGURE 1.
Prolactin activates ERK5 in aNPCs derived from the SVZ but not those from the SGZ. Cells were treated with prolactin as indicated, and cell lysates were analyzed by Western blotting for phosphorylated and active ERK5 (p-ERK5), total ERK5 (T-ERK5), and phospho-ERK1/2. β-Actin was used as a loading control. A, dose-dependent activation of ERK5 by prolactin in SVZ-aNPCs. B, time course of prolactin-induced ERK5 activation in SVZ-aNPCs. C, prolactin does not activate ERK5 in SGZ-aNPCs at 0.01–10 μg/ml. D, prolactin does not activate ERK5 in SGZ-aNPCs at indicated times. NS, no stimulation.
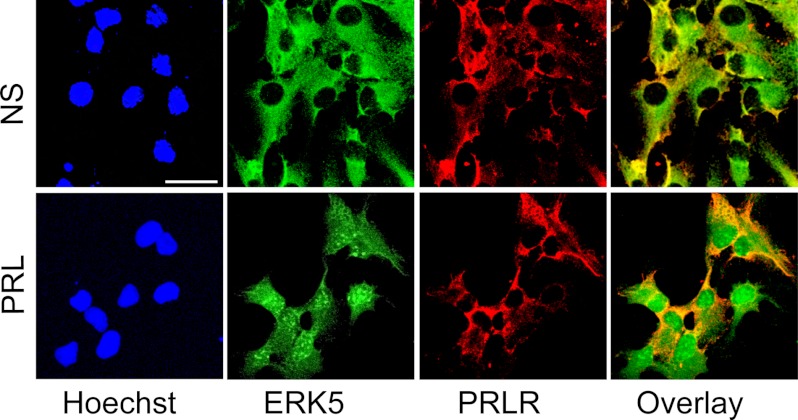
Immunocytochemical staining confirmed that SVZ-aNPCs express prolactin receptor (Fig. 2). Furthermore, prolactin receptor-positive cells also co-expressed ERK5 that translocated to the nucleus after prolactin treatment (Fig. 2). ERK5 nuclear translocation is another indicator of ERK5 activation (28).
FIGURE 2.
ERK5 translocates to the nucleus upon prolactin stimulation. Confocal images showed that SVZ-aNPCs express both ERK5 (green) and prolactin receptor (PRLR) (red). ERK5 translocates to the nucleus after prolactin (PRL) stimulation. Hoechst (blue) staining was used to identify all nuclei. NS, no stimulation. Scale bar: 25 μm.
Significantly, ERK1/2, MAP kinases closely related to ERK5, were not activated by prolactin in aNPCs derived from either SVZ or SGZ. However, ERK1/2 phosphorylation was readily detectable after treatment with neurotrophins including BDNF in both SVZ- and SGZ-aNPCs (data not shown). Thus the lack of phospho-ERK1/2 signal increase after prolactin treatment is not due to limitations of detection. Taken together, these data suggest that prolactin specifically activates ERK5 in SVZ-derived aNPCs, and that ERK5 may mediate prolactin-induced SVZ neurogenesis.
Prolactin-induced ERK5 Activation Is Independent of STAT5 Signaling
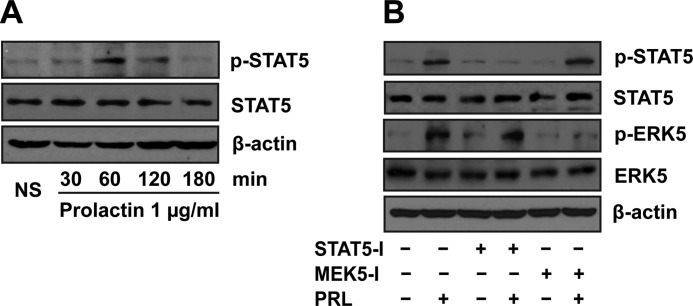
Binding of prolactin to its receptors usually leads to the activation of the canonical STAT5 pathway (29). To gain insights into the mechanisms by which prolactin activates ERK5, we examined if there is crosstalk between prolactin activation of the STAT5 and ERK5 signaling pathways. Prolactin induced STAT5 phosphorylation at Tyr-694, and presumably activation in SVZ-aNPCs (Fig. 3A). Similar to ERK5 activation, STAT5 phosphorylation peaked at 60 min. However, unlike ERK5, phosphorylation of STAT5 was no longer elevated at 3 h. Thus, prolactin activation of ERK5 is more persistent than its activation of the canonical STAT5 pathway.
FIGURE 3.
Prolactin-induced ERK5 activation is independent of STAT5. A, time course of prolactin-induced STAT5 phosphorylation and activation in SVZ-aNPCs. B, no crosstalk between ERK5 and STAT5 signaling pathways. SVZ-aNPCs were pretreated for 1 h with or without a STAT5 inhibitor (STAT5-I) or MEK5 inhibitor (MEK5-I, BIX02188) prior to prolactin stimulation. β-Actin was used as a loading control. NS, no stimulation.
SVZ-aNPCs were pretreated for 1 h before prolactin stimulation with a MEK5 inhibitor (BIX02188, 10 μm) (23) or a STAT5 inhibitor (100 μm) that directly inhibits STAT5 phosphorylation and activation by targeting its SH2 domain (24). MEK5 is an upstream kinase that phosphorylates and activates ERK5 (30, 31), and BIX02188 is a selective MEK5/ERK5 inhibitor (22, 23). Prolactin-induced phosphorylation of STAT5 was inhibited by this STAT5 inhibitor but not by the MEK5 inhibitor (Fig. 3B). Conversely, the MEK5 inhibitor blocked ERK5 phosphorylation without affecting STAT5 phosphorylation. These results suggest that prolactin-induced ERK5 activation is independent of the canonical STAT5 pathway.
Prolactin Stimulation of Neurogenesis in SVZ-aNPCs Requires ERK5
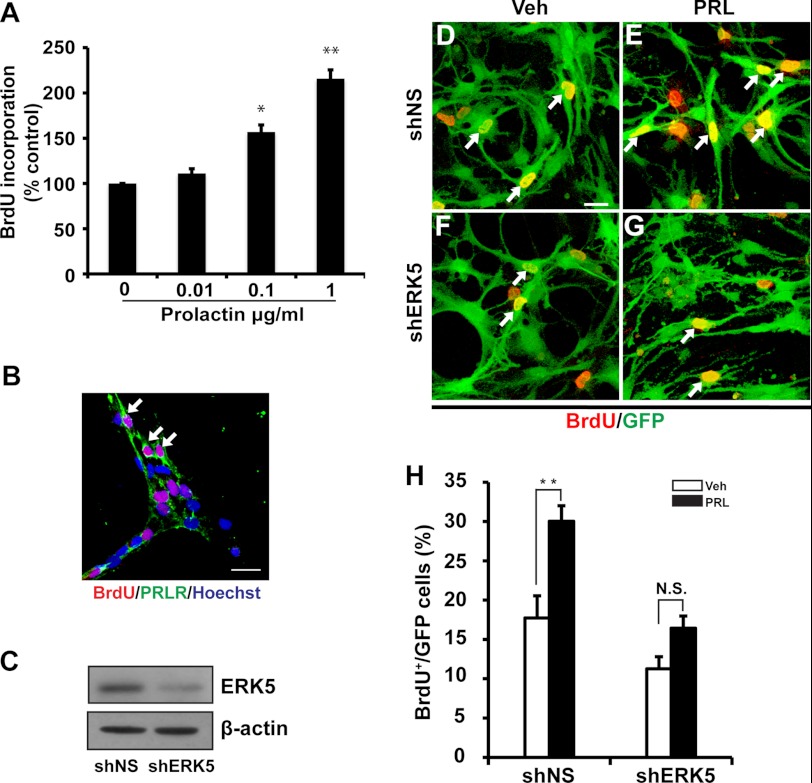
Prolactin promotes proliferation and neuronal differentiation of SVZ-derived aNPCs in vitro as well as stem/progenitor cells in vivo in the SVZ/OB (2, 8, 9). Using BrdU incorporation to measure DNA synthesis in the S-phase and cell proliferation, we confirmed that prolactin treatment induces SVZ-aNPC proliferation in a dose-dependent manner (0.01 μg/ml to 1 μg/ml) (Fig. 4A). Furthermore, almost all BrdU+ cells in culture expressed prolactin receptor (Fig. 4B). To investigate the role of ERK5 in prolactin-induced adult neurogenesis, we utilized a retrovirus expressing shRNA against ERK5 (shERK5) to suppress the expression of endogenous ERK5 in SVZ-derived aNPCs (Fig. 4C). This shERK5 is specific to ERK5 and does not affect the expression of the closely related ERK1/2 (19). The retroviral infection rate was ∼70%. Prolactin caused a statistically significant increase in BrdU incorporation in SVZ-aNPCs infected with the control virus (shNS) but not with the shERK5 virus (Fig. 4, D--H).
FIGURE 4.
Retroviral shRNA knockdown of ERK5 attenuates prolactin-induced proliferation in SVZ-aNPCs. A, prolactin treatment (18 h) induced SVZ-aNPC proliferation in a dose-dependent manner, measured by BrdU incorporation. B, immunocytochemistry showed that prolactin receptor (PRLR, green) is expressed in cells incorporating BrdU (red). C, Western analysis demonstrating that retrovirus expressing shRNA against ERK5 (shERK5) suppresses the expression of endogenous ERK5 in SVZ-derived aNPCs. shNS: retroviruses encoding nonspecific shRNA control. β-Actin was used as a loading control. D–G, representative fluorescence photomicrographs. Cells were infected with shNS (D, E) or shERK5 (F, G). Three days after retroviral infection, cells were treated with vehicle (Veh, D, F) or prolactin (PRL, 1 μg/ml, E, G) for 18 h followed by a 3 h BrdU incorporation (red). Virus-infected cells were identified by GFP immunostaining (green). Scale bar: 25 μm. H, quantification of data from panels D–G, as the percentage of BrdU+ cells in total GFP+ population. *, p < 0.05; **, p < 0.01; n.s. not statistically significant.
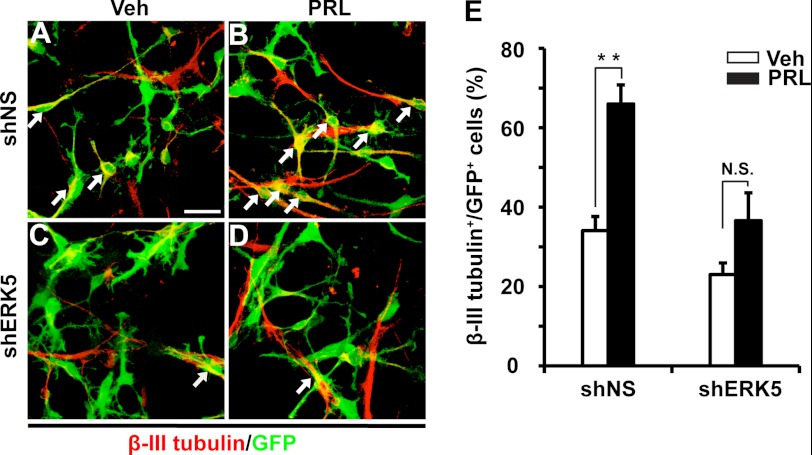
We further investigated the role of ERK5 in prolactin-induced SVZ-aNPC neuronal differentiation. After retroviral infection with shERK5 or shNS, SVZ-aNPCs were placed in EGF- and bFGF-free medium with or without prolactin treatment for 5 days. Withdrawal of the mitogenic EGF and bFGF alone induced spontaneous neuronal differentiation in both virus infected and non-infected cells (Fig. 5A). Basal levels of cells expressing β-III tubulin, a marker for newborn immature neuron, were 34 and 30% in shNS-virus infected cells (Fig. 5E) and the overall population (data not shown) respectively. These numbers are quite comparable, suggesting that retroviral infection per se does not affect neuronal differentiation. Addition of prolactin to the mitogen-free medium significantly enhanced neuronal differentiation (Fig. 5, B and E). Knockdown of ERK5 expression by retroviral infection of shERK5 significantly attenuated prolactin-stimulated neuronal differentiation (Fig. 5, C–E). These data suggest that ERK5 is required for prolactin-induced neurogenesis in vitro.
FIGURE 5.
shRNA knockdown of ERK5 decreases prolactin-induced neuronal differentiation in cultured SVZ-aNPC cells. Cells were infected with shNS (A, B) or shERK5 (C, D). Three days later, cells were treated with vehicle (Veh, A, C) or prolactin (PRL, 1 μg/ml, B, D) for 5 days. Neuronal differentiation was identified by cells expressing β-III tubulin. A–D, representative fluorescence photomicrographs of cells expressing β-III tubulin (red) and GFP (green). Arrows identify co-labeled cells. E, quantification of data from panels A–D, as the percentage of β-III tubulin+ cells in total GFP+ population. **, p < 0.01; n.s. not statistically significant. Scale bar: 25 μm.
ERK5 Expression in Different Cell Types in the SVZ of Adult Mouse Brain
ERK5 protein expression is high during early embryonic development but declines as the brain matures (14). Interestingly, we recently found that ERK5 protein is specifically expressed in the two neurogenic areas in adult brain, the SVZ and SGZ, suggesting a fundamentally important role of ERK5 in regulating adult neurogenesis (21).
Here, we further characterized ERK5 expression in the SVZ. We found that ERK5 is expressed in SVZ cells co-labeled with markers for proliferation, BrdU and PCNA (Fig. 6, A, B, G). ERK5 was also co-expressed with Sox2 and Pax6 (Fig. 6, C, G), markers for stem/progenitor cells, and with PSA-NCAM and DCX (Fig. 6, D, E, G), markers for transiently amplifying progenitors that have committed to a neuronal fate and/or for newborn neurons (32, 33). Interestingly, very few (less than 1%) ERK5+ cells co-expressed with the marker for mature neurons, NeuN (Fig. 6, F, G). Furthermore, of those few co-labeled cells, the NeuN staining intensity was much weaker than that in NeuN+/ERK5− cells, suggesting that these co-labeled cells are likely still in the early stage of terminal differentiation, therefore just beginning to express NeuN. Quantification of the data suggests that more than 50% of ERK5+ cells are transiently amplifying progenitors and/or newborn neurons expressing PSA-NCAM and DCX (Fig. 6G). Furthermore, the majority of PSA-NCAM- or DCX-positive cells also express ERK5 (Fig. 6H). This expression pattern suggests an important function for ERK5 in adult neurogenesis in the SVZ/OB axis, particularly in regulating the cell fate of the transiently amplifying progenitors and/or neuronal differentiation of newborn neurons.
It has been reported that prolactin receptor co-labels with DCX but not Sox2 or GFAP in the SVZ of the adult mouse brain (12). Our immunohistochemistry data confirmed that prolactin receptor is expressed in cells along the SVZ of the mouse brain. Importantly, prolactin receptor co-localized in ERK5-expressing cells (Fig. 7). This suggests that prolactin could signal through its receptor and activate ERK5 cell-autonomously in vivo.
Conditional and Targeted Deletion of ERK5 Abolishes Prolactin-induced Neurogenesis in the SVZ and OB
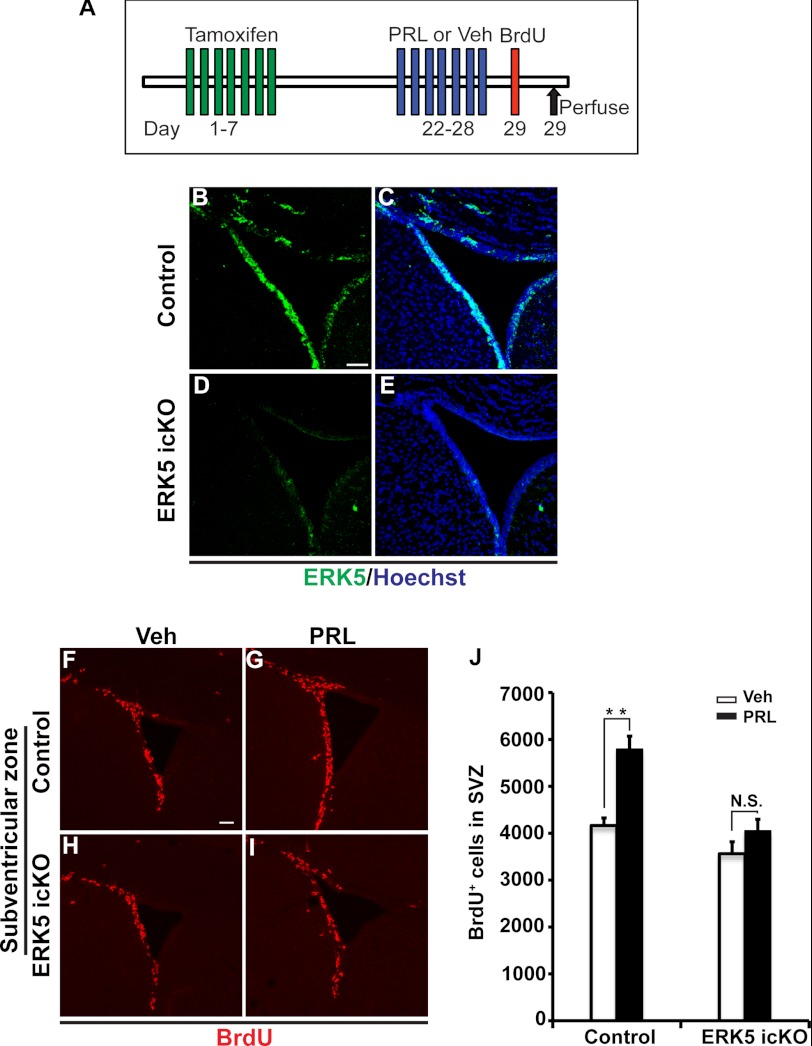
To address the role of ERK5 in SVZ/OB neurogenesis in vivo, we utilized a line of transgenic mouse (Nestin-CreERTM/ERK5loxP/loxP) that we recently developed in which erk5 is conditionally deleted specifically in Nestin-expressing neural stem cells of the adult brain after tamoxifen treatment (21). The use of this inducible and conditional ERK5 knock-out (ERK5 icKO) mouse strain avoids potential deleterious effects on other regions of the brain during development.
8–10-week old female Nestin-CreERTM/ERK5loxP/loxP mice and littermate control ERK5loxP/loxP mice were dosed with tamoxifen by oral gavage for 7 consecutive days to induce Cre-mediated deletion of erk5 in Nestin-expressing neural stem cells (Fig. 8A). Indeed, a 7-day tamoxifen treatment effectively knocked down ERK5 expression in the SVZ of ERK5 icKO mice (Fig. 8, B–E). Two weeks after tamoxifen administration, animals were infused with mouse recombinant prolactin or vehicle (mouse serum albumin) via osmotic pumps for 7 days (8 μg/day, s.c.), followed by BrdU injection to label actively dividing cells. Prolactin infusion caused a statistically significant increase in the number of BrdU+ cells in the SVZ of control mice (+39%, n = 4–5 mice/treatment, p < 0.01, Fig. 8, F, G, J), but not of ERK5 icKO mice (+13%, n = 4–5 mice/treatment, p > 0.05, Fig. 8, H–J).
FIGURE 8.
ERK5 is required in prolactin-induced SVZ neurogenesis in vivo. A, schematic illustration of experimental design. Tamoxifen was orally administered to female control (ERK5loxP/loxP) or ERK5 inducible and conditional knock-out (icKO) mice (Nestin-CreERTM/ERK5loxP/loxP) for 7 consecutive days. Two weeks after the last dose of tamoxifen treatment, mice were infused with either vehicle or prolactin for 7 days followed by BrdU injection to label proliferating cells. Animals were perfused 0.5 h after the last dose of BrdU for immunostaining. B–E, representative immunostaining of adult mouse brain sections showing that tamoxifen treatment effectively knocks down ERK5 expression (green) in the SVZ of ERK5 icKO mice. Scale bar: 50 μm. F–I, confocal images of BrdU staining (red) in the SVZ of control mice (F, G) and ERK5 icKO (H, I) infused with vehicle (F, H) or prolactin (G, I). Scale bar: 50 μm. J, quantification of the total number of BrdU+ cells in the SVZ of control and ERK5 icKO mice. n = 4–5 mice/genotype/treatment. **, p < 0.01; n.s. not statistically significant.
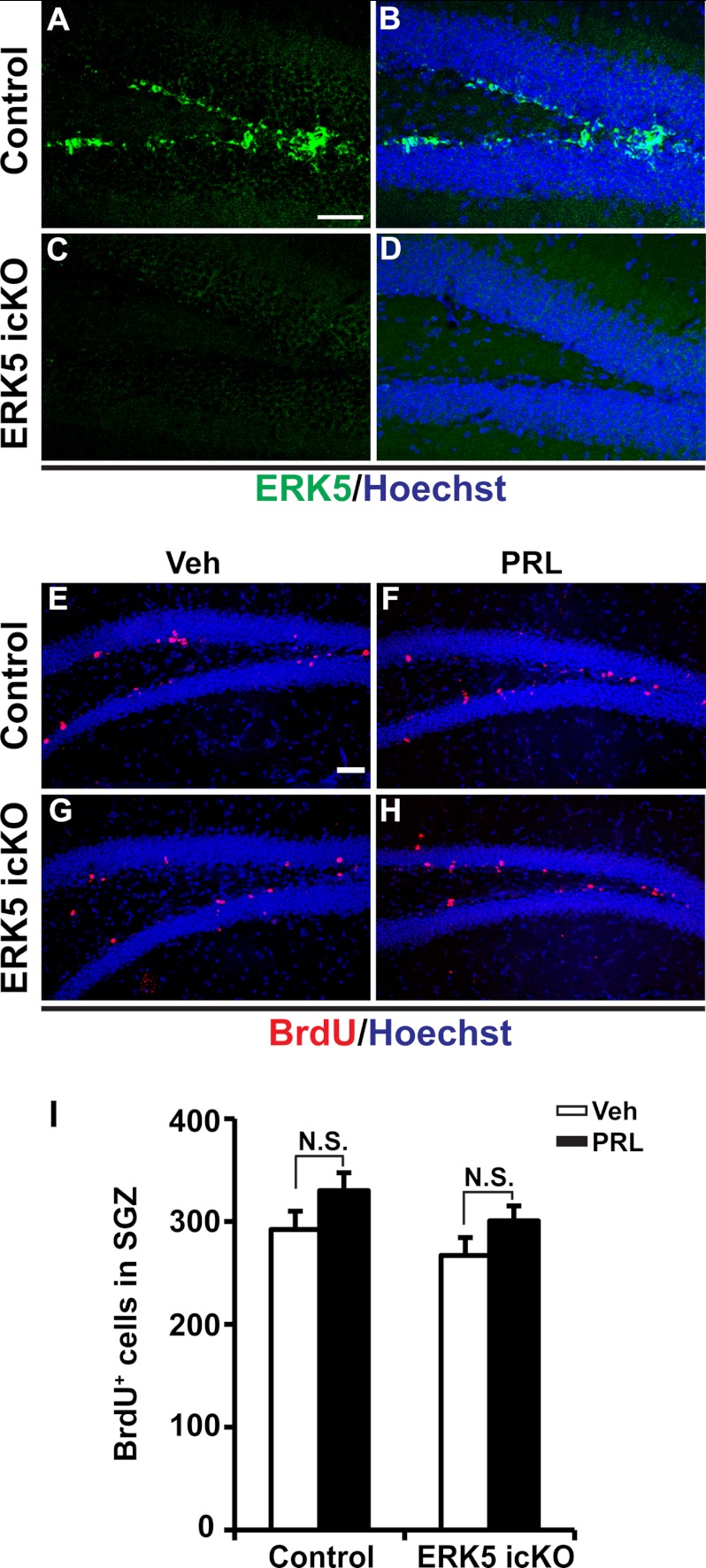
We also investigated the effect of prolactin on cell proliferation in the SGZ. We confirmed that a 7-day tamoxifen treatment effectively knocked down ERK5 expression in the SGZ (Fig. 9, A–D). However, unlike in the SVZ, prolactin infusion did not significantly increase the number of BrdU+ cells in the DG in either control or ERK5 icKO mice (Fig. 9, E–I). These data are consistent with earlier reports that the effect of prolactin on cell proliferation is specific to the SVZ and absent in the SGZ (2, 8).
FIGURE 9.
Prolactin does not induce SGZ neurogenesis in either control or ERK5 icKO mice. A–D, representative immunostaining of adult mouse brain sections showing that tamoxifen treatment effectively knocks down ERK5 expression (green) in the SGZ of ERK5 icKO mice. Scale bar: 50 μm. E–H, confocal images of BrdU staining (red) in the SGZ of control mice (E, F) and ERK5 icKO (G, H) infused with vehicle (E, G) or prolactin (F, H). Scale bar: 50 μm. i, prolactin infusion does not significantly change the number of BrdU+ cells in the SGZ in either control or ERK5 icKO mice. n = 4–5 mice/genotype/treatment; n.s., not statistically significant.
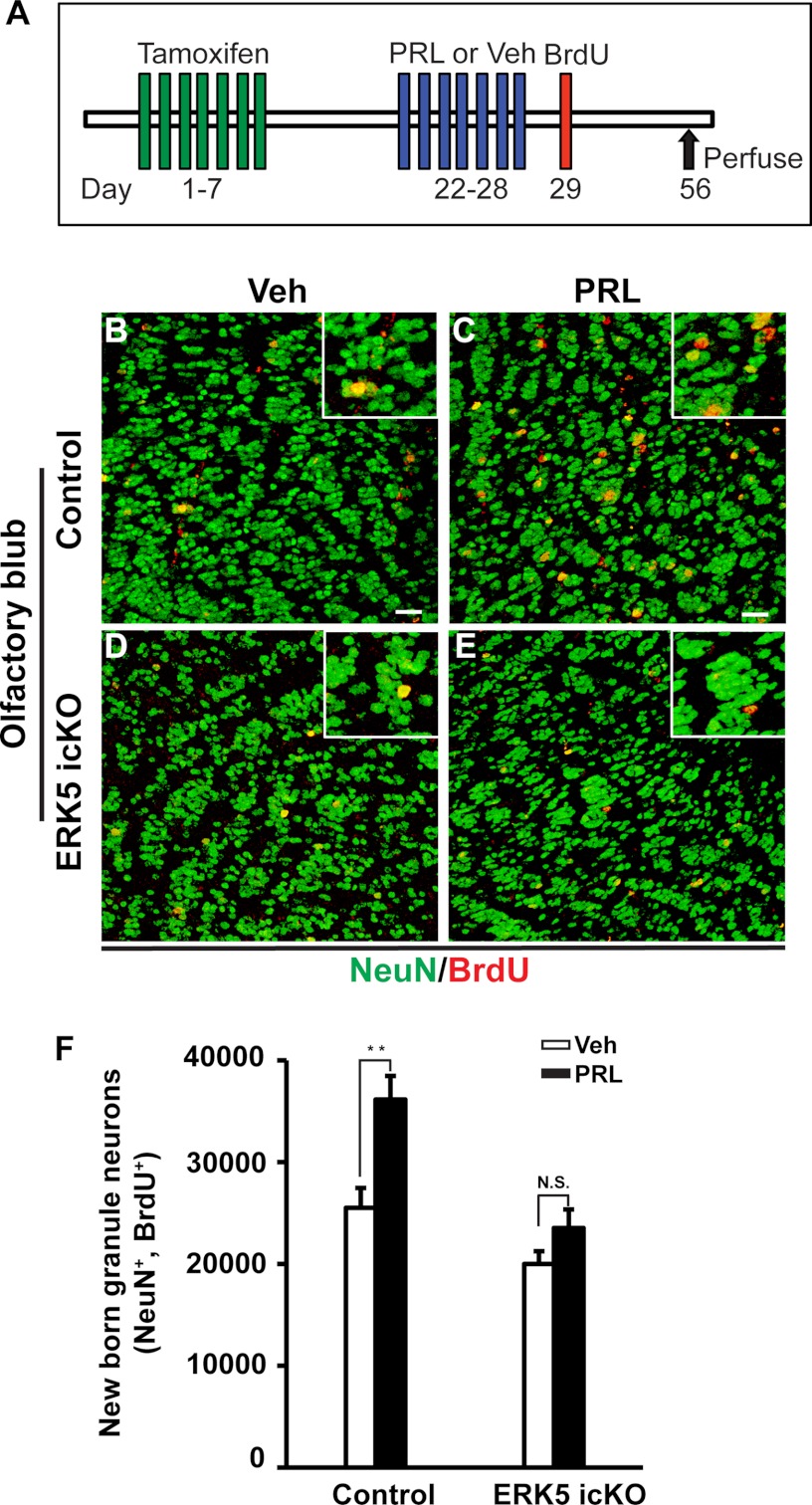
Because the majority of newly generated cells emerging from the SVZ migrate to form new interneurons in the olfactory bulb, we further investigated the role of ERK5 signaling in prolactin-induced olfactory bulb neurogenesis. We infused control and ERK5 icKO mice with prolactin or vehicle, and injected mice with BrdU to label newborn cells. The number of surviving BrdU+ cells in the olfactory bulb was examined 4 weeks after BrdU injection (Fig. 10A). The olfactory bulb slices were co-stained for BrdU and NeuN to label adult-born mature neurons (BrdU+ and NeuN+) (Fig. 10, B–E). Quantification of the data showed that prolactin infusion significantly increased the number of adult-born granule neurons in the OB of control mice (+42%, n = 5 mice/treatment, p < 0.01) but only caused a slight, statistically insignificant increase in ERK5 icKO animals (+17%, n = 5 mice/treatment, p > 0.05) (Fig. 10, B–F). Taken together, these data suggest that ERK5 signaling plays a critical role in prolactin-stimulated SVZ and OB neurogenesis in the adult mouse brain.
FIGURE 10.
ERK5 is required in prolactin-induced olfactory bulb neurogenesis in vivo. A, schematic illustration of experimental design. Tamoxifen was orally administered to female control (ERK5loxP/loxP) or ERK5 icKO mice (Nestin-CreERTM/ERK5loxP/loxP) for 7 consecutive days. Two weeks after the last dose of tamoxifen treatment, mice were infused with either vehicle control or prolactin for 7 days followed by BrdU injection to label proliferating cells. Animals were perfused 4 weeks after the last dose of BrdU for immunostaining to identify adult-born mature neurons. B—E, confocal images of BrdU (red) and NeuN (green) staining in the granule cell layer of the OB of control mice (B, C) and ERK5 icKO (D, E) infused with either vehicle (B, D) or prolactin (C, E). Insets are enlarged views for better visualization of BrdU+/NeuN+ cells. Scale bar: 25 μm. F, quantification of adult-born granule neurons (BrdU+ and NeuN+) in the OB. n = 5 mice/genotype/treatment. **, p < 0.01; n.s. not statistically significant.
ERK5 Is Required for Adult Neurogenesis in the SVZ after Mating
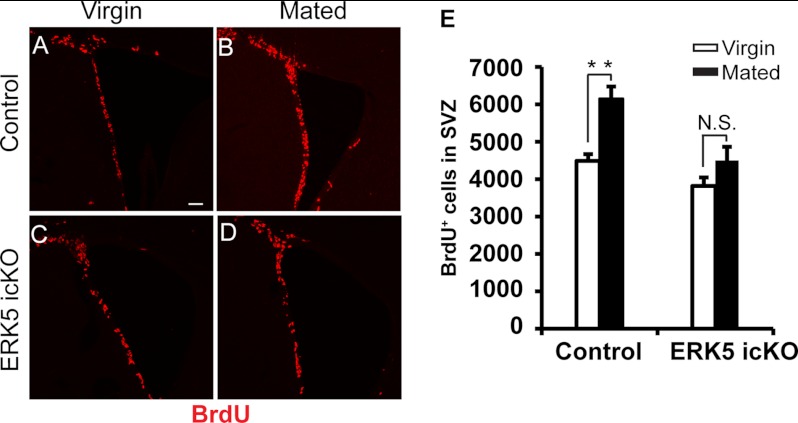
Since prolactin is implicated in pregnancy or pseudopregnancy-induced adult neurogenesis in the SVZ following mating (2), we evaluated whether ERK5 signaling is also required for this process. Two weeks after tamoxifen treatment, control or ERK5 icKO female mice were paired with males overnight, and successful mating was confirmed by the appearance of a vaginal plug the next morning, a time designated as gestation day (GD) 0.5. Females without plugs after pairing were excluded from this experiment. Virgin female littermates were used as controls. BrdU was administered to mice on GD7, a time point of peak BrdU incorporation after mating (2). Mated control mice showed a significantly increased number of BrdU+ cells in the SVZ at GD7 over virgin control mice (Fig. 11), consistent with previous reports (2, 10). However, mated ERK5 icKO mice at GD7 did not show a statistically significant increase in the number of BrdU+ cells over virgin ERK5 icKO mice.
FIGURE 11.
Cell proliferation in the SVZ is increased after mating in control but not ERK5 icKO mice. Two weeks after a 7-day tamoxifen administration, female mice were mated with male mice and BrdU incorporation was examined at GD7. Virgin littermates that were not paired with males were used as controls. A–D, confocal images of BrdU staining in the SVZ of virgin (A, C) or mated (B, D) mice that are either of control (A, B) or ERK5 icKO (C, D). Scale bar: 50 μm. E, quantification of the total number of BrdU+ cells in the SVZ of control and ERK5 icKO mice. n = 4/genotype/treatment. **, p < 0.01; n.s. not statistically significant.
DISCUSSION
It has been almost a decade since the discovery of prolactin-stimulated adult neurogenesis in the SVZ and OB during early stages of pregnancy/pseudopregnancy after mating (2). Subsequent studies suggest that prolactin also mediates adult neurogenesis in the SVZ and OB induced by dominant male pheromones and paternal recognition of offspring (8, 12). However, downstream signaling mechanisms underlying prolactin-induced adult neurogenesis remain completely undefined.
ERK5 protein expression is high during early embryonic development and gradually declines as the brain matures (14). We recently discovered that, in the adult brain, ERK5 expression is restricted to the neurogenic regions including the SGZ and SVZ (21). In this study, we further characterized ERK5 expression in the SVZ of the adult mouse brain. ERK5 is expressed in stem/progenitor cells, transiently amplifying progenitors and/or newborn neurons, but not in mature neurons along the SVZ. The majority of the transiently amplifying progenitors and/or newborn neurons (DCX+ or PSA-NCAM+) express ERK5. This expression pattern suggests an important function for ERK5 in adult SVZ neurogenesis, particularly in regulating neuronal fate specification and neuronal differentiation. As progenitor cells and immature neurons differentiate into mature neurons, ERK5 expression declines.
Because ERK5 is activated by a variety of receptors including those for growth factors, neurotrophic factors, cytokines, and G-protein-coupled receptors (22, 34, 35), and prolactin stimulation of SVZ neurogenesis requires signaling through prolactin receptors that are abundant in transiently amplifying progenitors (8, 12), we hypothesized that ERK5 may be a downstream signaling pathway mediating the adult neurogenic effect of prolactin.
We report here that prolactin receptor is indeed expressed in cells that also express ERK5. Prolactin receptor is expressed along the SVZ and co-localized to many ERK5+ cells in vivo. Furthermore, almost all prolactin receptor-expressing cells also express ERK5 in cultured SVZ-aNPCs. Moreover, BrdU incorporation and DNA synthesis occurs in cells expressing prolactin receptor in culture. Prolactin activation of ERK5 peaks at 60 min and persists for at least 3 h, which should be long enough to trigger long-term processes such as DNA synthesis, cell proliferation, and neuronal differentiation. In fact, prolactin activation of ERK5 is more persistent than its activation of the canonical STAT5, which returned to baseline level at 3 h. Furthermore, EGF stimulation of HeLa cell proliferation requires ERK5 activation even though EGF stimulation of ERK5 lasts only 20 min in these cells (36). Indeed, we demonstrate in this study that prolactin stimulates BrdU incorporation and neuronal differentiation both in vitro and in vivo. These processes are blocked by shRNA in vitro and by conditional deletion of erk5 gene in vivo. These data support the hypothesis that prolactin stimulates adult SVZ neurogenesis through prolactin receptor activation of ERK5 in a cell-autonomous manner.
Prolactin activates ERK5 but not the closely related ERK1/2 in SVZ-derived aNPCs. This finding is significant because these cells express abundant ERK1/2 and there has been no report where an extracellular ligand such as a peptide hormone or growth factor selectively actives ERK5 but not ERK1/2 in other cells. In contrast to SVZ-aNPCs, prolactin does not activate ERK5 in SGZ-derived aNPCs. Because data presented here and several in vivo studies have reported that prolactin stimulates adult neurogenesis in the SVZ but leaves the SGZ unaffected (2, 3, 8), our data further suggest that ERK5 signaling may be a physiologically relevant mediator of prolactin-induced SVZ neurogenesis.
STAT5 is the canonical signaling pathway implicated in prolactin signaling (29). Our data also show that prolactin induces STAT5 phosphorylation, and presumably activation in SVZ-aNPCs. However, prolactin activation of ERK5 and STAT5 has different kinetics with ERK5 activation being more persistent than that of STAT5. Furthermore, studies using pharmacological inhibitors of MEK5/ERK5 and STAT5 activation suggest that prolactin activation of the STAT5 and ERK5 signaling pathways are independent of each other, indicating that there is no crosstalk between the two pathways. These data provide new insights into prolactin signaling.
We demonstrate that shRNA knockdown of ERK5 attenuates prolactin-stimulated proliferation and neuronal differentiation of SVZ-derived aNPCs in culture. Inducible erk5 deletion in adult neural stem cells of transgenic mice inhibits proliferation in the SVZ following prolactin infusion in vivo or after mating. It also abrogates prolactin induction of adult-born neurons in the OB. This is the first demonstration of a role for the ERK5 MAP kinase in prolactin regulation of adult neurogenesis in the SVZ.
In summary, we discovered that ERK5 is a novel signaling pathway responsible for prolactin- and mating-stimulated adult neurogenesis in the SVZ and OB. These findings identify prolactin, an endogenous hormone, as an upstream activator of ERK5 signaling in the regulation of adult neurogenesis in the SVZ. Results in this study implicate ERK5-regulated adult neurogenesis in the SVZ/OB for physiologically important processes, including mating. Because plasma levels of prolactin are elevated following orgasm in humans (6, 7), results in this study may have implications for adult neurogenesis in humans.
Acknowledgments
We thank Drs. Cathy Tournier from the University of Manchester (Manchester, UK) and Bradford C. Berk from the University of Rochester for the transfer of ERK5loxP/loxP mice, Dr. Hongjun Song from the Johns Hopkins University School of Medicine for pXIE and pSIE retroviral vectors, Boehringer Ingelheim Pharmaceuticals for providing BIX02188, Dr. Daniel Storm and members of the Xia laboratory for critical reading of the manuscript, and Glen MacDonald for technical assistance on confocal imaging.
This work was supported, in whole or in part, by the National Institutes of Health (MH095840, to Z. X., T32HD007183 and F1DC011216, to Y. W. P.).
- SVZ
- subventricular zone
- ERK5
- extracellular signal-regulated kinase 5
- PRL
- prolactin
- SGZ
- subgranular zone
- Ob
- olfactory bulb
- aNPC
- adult neural progenitor cell
- icKO
- induced-conditional knockout.
REFERENCES
- 1. Breton-Provencher V., Lemasson M., Peralta M. R., 3rd, Saghatelyan A. (2009) Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 29, 15245–15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J. C., Weiss S. (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120 [DOI] [PubMed] [Google Scholar]
- 3. Torner L., Karg S., Blume A., Kandasamy M., Kuhn H. G., Winkler J., Aigner L., Neumann I. D. (2009) Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J. Neurosci. 29, 1826–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barkley M. S., Bradford G. E., Geschwind II. (1978) The pattern of plasma prolactin concentration during the first half of mouse gestation. Biol. Reprod. 19, 291–296 [DOI] [PubMed] [Google Scholar]
- 5. Freeman M. E., Kanyicska B., Lerant A., Nagy G. (2000) Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631 [DOI] [PubMed] [Google Scholar]
- 6. Exton M. S., Krüger T. H., Koch M., Paulson E., Knapp W., Hartmann U., Schedlowski M. (2001) Coitus-induced orgasm stimulates prolactin secretion in healthy subjects. Psychoneuroendocrinology 26, 287–294 [DOI] [PubMed] [Google Scholar]
- 7. Krüger T. H., Haake P., Hartmann U., Schedlowski M., Exton M. S. (2002) Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci. Biobehav. Rev. 26, 31–44 [DOI] [PubMed] [Google Scholar]
- 8. Mak G. K., Enwere E. K., Gregg C., Pakarainen T., Poutanen M., Huhtaniemi I., Weiss S. (2007) Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci. 10, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 9. Larsen C. M., Kokay I. C., Grattan D. R. (2008) Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm. Behav. 53, 509–517 [DOI] [PubMed] [Google Scholar]
- 10. Larsen C. M., Grattan D. R. (2010) Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151, 3805–3814 [DOI] [PubMed] [Google Scholar]
- 11. Larsen C. M., Grattan D. R. (2012) Prolactin, neurogenesis, and maternal behaviors. Brain Behav. Immun. 26, 201–209 [DOI] [PubMed] [Google Scholar]
- 12. Mak G. K., Weiss S. (2010) Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 13, 753–758 [DOI] [PubMed] [Google Scholar]
- 13. Lee J. D., Ulevitch R. J., Han J. (1995) Primary structure of BMK1: a new mammalian map kinase. Biochem. Biophys. Res. Commun. 213, 715–724 [DOI] [PubMed] [Google Scholar]
- 14. Liu L., Cavanaugh J. E., Wang Y., Sakagami H., Mao Z., Xia Z. (2003) ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 100, 8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shalizi A., Lehtinen M., Gaudilliere B., Donovan N., Han J., Konishi Y., Bonni A. (2003) Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J. Neurosci. 23, 7326–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watson F. L., Heerssen H. M., Bhattacharyya A., Klesse L., Lin M. Z., Segal R. A. (2001) Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 4, 981–988 [DOI] [PubMed] [Google Scholar]
- 17. Finegan K. G., Wang X., Lee E. J., Robinson A. C., Tournier C. (2009) Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 16, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L., Cundiff P., Abel G., Wang Y., Faigle R., Sakagami H., Xu M., Xia Z. (2006) Extracellular signal-regulated kinase (ERK) 5 is necessary and sufficient to specify cortical neuronal fate. Proc. Natl. Acad. Sci. U.S.A. 103, 9697–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cundiff P., Liu L., Wang Y., Zou J., Pan Y. W., Abel G., Duan X., Ming G. L., Englund C., Hevner R., Xia Z. (2009) ERK5 MAP kinase regulates neurogenin1 during cortical neurogenesis. PLoS ONE 4, e5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou J., Pan Y. W., Wang Z., Chang S. Y., Wang W., Wang X., Tournier C., Storm D. R., Xia Z. (2012) Targeted deletion of ERK5 MAP kinase in the developing nervous system impairs development of GABAergic interneurons in the main olfactory bulb and behavioral discrimination between structurally similar odorants. J. Neurosci. 32, 4118–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan Y. W., Chan G. C., Kuo C. T., Storm D. R., Xia Z. (2012) Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J. Neurosci. 32, 6444–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obara Y., Nakahata N. (2010) The signaling pathway leading to extracellular signal-regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol. Pharmacol. 77, 10–16 [DOI] [PubMed] [Google Scholar]
- 23. Tatake R. J., O'Neill M. M., Kennedy C. A., Wayne A. L., Jakes S., Wu D., Kugler S. Z., Jr., Kashem M. A., Kaplita P., Snow R. J. (2008) Biochem. Biophys. Res. Commun. 377, 120–125 [DOI] [PubMed] [Google Scholar]
- 24. Müller J., Sperl B., Reindl W., Kiessling A., Berg T. (2008) Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem. 9, 723–727 [DOI] [PubMed] [Google Scholar]
- 25. Cavanaugh J. E., Ham J., Hetman M., Poser S., Yan C., Xia Z. (2001) Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J. Neurosci. 21, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y. W., Zou J., Wang W., Sakagami H., Garelick M. G., Abel G., Kuo C. T., Storm D. R., Xia Z. (2012) Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis. J. Biol. Chem. 287, 23306–23317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rietze R. L., Reynolds B. A. (2006) Neural stem cell isolation and characterization. Methods Enzymol. 419, 3–23 [DOI] [PubMed] [Google Scholar]
- 28. Wang Y., Su B., Xia Z. (2006) Brain-derived neurotrophic factor activates ERK5 in cortical neurons via a Rap1-MEKK2 signaling cascade. J. Biol. Chem. 281, 35965–35974 [DOI] [PubMed] [Google Scholar]
- 29. Liu X., Robinson G. W., Wagner K. U., Garrett L., Wynshaw-Boris A., Hennighausen L. (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186 [DOI] [PubMed] [Google Scholar]
- 30. English J. M., Vanderbilt C. A., Xu S., Marcus S., Cobb M. H. (1995) Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem. 270, 28897–28902 [DOI] [PubMed] [Google Scholar]
- 31. Zhou G., Bao Z. Q., Dixon J. E. (1995) Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 270, 12665–12669 [DOI] [PubMed] [Google Scholar]
- 32. Brown J. P., Couillard-Després S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G. (2003) Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 [DOI] [PubMed] [Google Scholar]
- 33. Kronenberg G., Reuter K., Steiner B., Brandt M. D., Jessberger S., Yamaguchi M., Kempermann G. (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463 [DOI] [PubMed] [Google Scholar]
- 34. Marinissen M. J., Chiariello M., Pallante M., Gutkind J. S. (1999) A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol. Cell Biol. 19, 4289–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kendrick T. S., Bogoyevitch M. A. (2007) Activation of mitogen-activated protein kinase pathways by the granulocyte colony-stimulating factor receptor: mechanisms and functional consequences. Front Biosci. 12, 591–607 [DOI] [PubMed] [Google Scholar]
- 36. Kato Y., Tapping R. I., Huang S., Watson M. H., Ulevitch R. J., Lee J. D. (1998) Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395, 713–716 [DOI] [PubMed] [Google Scholar]