Background: Intermediate filaments are tissue-specifically expressed and provide crucial structural support for cells.
Results: Ngs protein assembles into a filamentous network in notochord cells, and its deficiency results in the collapse of these cells.
Conclusion: ngs encodes a new type of intermediate filament protein.
Significance: We identified a new intermediate filament protein essential for maintenance of the notochord.
Keywords: Cell Differentiation, Development, Intermediate Filaments, Stress, Zebra Fish, ngs, Notochord
Abstract
The notochord is an important organ involved in embryonic patterning and locomotion. In zebrafish, the mature notochord consists of a single stack of fully differentiated, large vacuolated cells called chordocytes, surrounded by a single layer of less differentiated notochordal epithelial cells called chordoblasts. Through genetic analysis of zebrafish lines carrying pseudo-typed retroviral insertions, a mutant exhibiting a defective notochord with a granular appearance was isolated, and the corresponding gene was identified as ngs (notochord granular surface), which was specifically expressed in the notochord. In the mutants, the notochord started to degenerate from 32 hours post-fertilization, and the chordocytes were then gradually replaced by smaller cells derived from chordoblasts. The granular notochord phenotype was alleviated by anesthetizing the mutant embryos with tricaine to prevent muscle contraction and locomotion. Phylogenetic analysis showed that ngs encodes a new type of intermediate filament (IF) family protein, which we named chordostatin based on its function. Under the transmission electron microcopy, bundles of 10-nm-thick IF-like filaments were enriched in the chordocytes of wild-type zebrafish embryos, whereas the chordocytes in ngs mutants lacked IF-like structures. Furthermore, chordostatin-enhanced GFP (EGFP) fusion protein assembled into a filamentous network specifically in chordocytes. Taken together, our work demonstrates that ngs encodes a novel type of IF protein and functions to maintain notochord integrity for larval development and locomotion. Our work sheds light on the mechanisms of notochord structural maintenance, as well as the evolution and biological function of IF family proteins.
Introduction
The notochord is a characteristic structure in all members of the phylum Chordata. In lower vertebrates, such as lampreys, sturgeon, and lungfish, the notochord persists throughout life, whereas in higher vertebrates, it occurs transiently and is gradually replaced by the vertebral column (1, 2). In all vertebrates, the notochord plays at least two important roles. First, it acts as the organizer for the patterning of early embryos. Several key signaling molecules required for the proper patterning of adjacent tissues are secreted by the notochord. Through such signals, the notochord induces the formation of the central nervous system, regulates the development of the heart and pancreas, and determines the fate of paraxial mesoderm (3–5). Second, the notochord constitutes the axial skeleton in the early vertebrate embryo (6, 7). If it does not fully differentiate, the elongation of the embryo is affected (8). In addition, the notochord provides the axial skeletal support for many vertebrate larvae, such as fish fry and tadpoles. This support is essential for the larvae to swim, to look for food, and to escape from predators (9).
The notochord arises from chordamesoderm at the early gastrula stage (7, 10). After cell proliferation and cellular rearrangement, the chordamesoderm turns into an elongated stack of cells. The fully differentiated mature notochord consists of many large vacuolated cells and notochordal epithelium, surrounded by one or more sheaths composed of extracellular matrix. The vacuolated cells, also called chordocytes, have a large central vacuole, which gives rise to hydrostatic pressure. The extracellular matrix surrounding the notochord provides a strong and flexible sheath against the pressure, just like a fire hose filled with water. Thus, the notochord becomes a flexible and stiff skeletal rod and provides the axial support for locomotion during embryonic and larval free swimming stages. The notochordal epithelial cells are located just inside the sheath, forming a layer encircling the chordocytes. These cells are not fully differentiated and lack a central vacuole. In certain cases, the notochordal epithelial cells proliferate, vacuolate, and enlarge to eventually differentiate into chordocytes (1). Therefore, notochordal epithelial cells are also called chordoblasts (1, 11–13).
The zebrafish (Danio rerio) is an ideal organism to screen for mutations affecting the morphogenesis of the notochord because its embryo is transparent and the notochord is easy to distinguish from other organs. Many mutations affecting the formation and differentiation of the notochord have been identified in zebrafish, although only few of the corresponding genes have been characterized (8, 14). Mutations in the genes encoding laminin α1, β1, and γ1 result in the loss of notochord sheath and the failure of chordocytes to vacuolate (15). Mutations in the genes encoding the coatomer vesicular coat complex also led to the failure of vacuole formation (16). Most of these mutations affecting the formation and differentiation of the notochord are lethal because of defects in other important organs and embryonic degeneration. Despite these studies, the genetic pathways that govern the morphogenesis and function of the notochord remain elusive. To identify essential genes required for notochord development, we screened phenotypes of the notochord in zebrafish mutant lines that carried pseudo-typed retroviral insertions described in our pilot mutagenesis project (17), and found one mutant that had a defective notochord with a granular surface. We named the corresponding gene ngs, denoting “notochord granular surface.”
Phylogenetic analysis showed that ngs encoded a novel protein of the intermediate filament (IF)3 family, a ubiquitous cytoskeletal scaffold in higher metazoans. So far, six types of IF proteins have been reported and classified according to their tissue specificity: types I and II include keratins, which are expressed in epithelial cells; type III includes desmin (muscle), vimentin (mesenchyme) and GFAP (glial fibrillary acidic protein), expressed in glia; type IV includes the family of neurofilament proteins (neurons); and type V includes lamin A, B, and C, which assemble into the nuclear lamina. Nestin, synemin, and desmuslin belong to type VI IFs. Apart from lamins, all the other IF proteins are located in the cytoplasm and form tissue-specific IFs in cells (18–20). However, no IF genes have been characterized to be specifically and exclusively expressed in the notochord.
All IF proteins share a common structure: a rod domain composed of an α-helix, flanked by a non-α-helical head and a non-α-helical tail. The amino acid sequence of the rod domain is conserved in all IF proteins and is essential for the unique property of self-assembly in this protein family. Keratin, neurofilament proteins and nestin always form heteropolymers, whereas vimentin and desmin form either homo- or heteropolymers (18, 20).
In mice and humans, more than 30 diseases are associated with mutations in the genes encoding IF proteins (19), although IF genes are not essential in yeast, Drosophila, and some mammalian cell lines (21, 22). For example, mutations of keratins 5 and 14 cause mechanical fragility of epithelial cells and result in epidermolysis bullosa simplex (23–25); mutations of GFAP result in neurodegenerative disorders and Alexander disease (26, 27); and mutations of the genes encoding neurofilament light chains cause Charcot-Marie-Tooth disease (28). In contrast, little is known about the developmental and physiological functions of IF proteins.
Here we report the identification of a new gene, ngs, which encodes a novel type of IF protein, chordostatin, and whose expression is restricted to the notochord in zebrafish. Mutation of ngs results in the collapse of notochord chordocytes, which is probably due to a lack of IF structures in their cytoplasm. The notochord in the ngs mutant is ultimately filled with many smaller cells, which are most likely derived from the proliferation of notochord epithelial cells. We conclude that ngs is essential for the assembly of the IF skeleton in chordocytes and is required for the maintenance of notochord structure.
EXPERIMENTAL PROCEDURES
Zebrafish Husbandry and Mutant Screen
Zebrafish were maintained according to Refs. 35 and 36. Genome-wide mutagenesis by retroviral insertions was carried out previously (17). The founder fish carrying the retroviral insertions were incrossed to get F1, and F1 fish were incrossed to get F2.
Linker-Mediated-PCR and Integration Site Mapping
Linker-Mediated-PCR was performed as described previously (17). To map the mutant sequence, a BLAST search was carried out on the Ensembl genome server (Zv9).
Imaging of Zebrafish Embryos
To compare the notochord between mutant and WT zebrafish, embryos at 72 hours post-fertilization (hpf) were treated with 0.02% tricaine (3-amino benzoic acid ethyl ester, also called ethyl 3-aminobenzoate), positioned in methyl-cellulose, and observed under a stereo-microscope (Stemi 2000-C; Zeiss). EGFP and RFP expression were visualized under a fluorescence microscope (Axioimager Z1; Zeiss) or confocal laser scanning microscopes (LSM-710NLD and Duo Scan).
In Situ Hybridization
Whole mount in situ hybridization was carried out based on a common protocol as described previously (15). The riboprobe was labeled with digoxigenin. To get the probe for in situ hybridization, the cDNA of ngs was amplified by PCR using the primer pairs 5′-CGGAATTCATGAATGAGTGTGAGGATAC-3′ and 5′-GCTCTAGACATCCCAAATATGTACTGTTG-3′ and then cloned into pCS2+ between EcoRI and XbaI. The plasmid was then linearized with HindIII, and in vitro transcription was carried out with T7 RNA polymerase.
Morpholino against ngs and Microinjection
The morpholino sequence against ngs was 5′-CTGTAGCTCATGTTGATACCGGT-3′ (Gene Tools). For knockdown of ngs, 4–6 ng of morpholino were injected into each one-cell stage zebrafish embryo. To visualize the nuclei, 50–100 ng of H2B-RFP mRNA was injected into one-cell stage ngs mutant embryos, and the embryos were observed at 72 hpf under a fluorescence microscope.
In Vivo Assembly of Ngs Protein
To detect the assembly of Ngs protein in vivo, 50 ng of the modified expression vector of pEGFP-N1 containing ngs cDNA fused to the EGFP or ngs-mCherry construct was used for microinjection, and the embryos were observed under confocal laser scanning microscopes.
Tissue Sectioning and Staining
The WT and the mutant zebrafish embryos (with either a normal or a granular notochord) at 48 hpf were collected. After dechorionation, the embryos were anesthetized in 0.02% tricaine and fixed. The paraffin embedding, sectioning, and staining were performed following the standard procedure.
Electron Microscopy
For transmission electron microscopy, the embryos were fixed with 2% glutaraldehyde and 2.5% paraformaldehyde followed by post-fixation with 1.5% OsO4. Ultra-thin sectioning of the notochords was performed as described previously (1, 33). Specimens were examined and recorded in a Hitachi H-7000 (Hitachi, Ltd, Tokyo, Japan).
RESULTS
Isolation of the ngs Mutant and Gene Expression Analysis
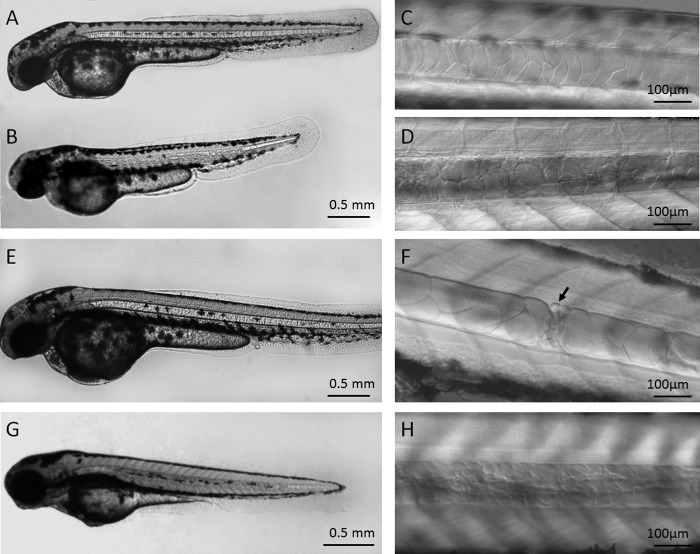
During our retroviral mutagenesis screen, the genomic locations of the proviral integrations were mapped by linker-mediated PCR followed by blasting against the Ensembl zebrafish genomic database (17). The fish carrying the same proviral integrations were inbred, and the embryonic phenotypes were analyzed by microscopic inspection. We found that one mutant, v835-2, displayed specific defects in the notochord. During development, the zebrafish notochord starts to differentiate at the 20-somite stage (19 hpf), in company with vacuolation and swelling in the notochord progenitor cells in an ordered sequence from anterior to posterior. By 42 hpf, the notochord is well developed with a “stack of pennies” appearance under the light microscope (Fig. 1A). In homozygous mutant embryos of v835-2, the notochord defects appeared at 30–35 hpf, when chordoblasts and chordocytes were already evident. At the beginning of the defects, “breaks” emerged at several distinct locations inside the notochord (Fig. 1, E and F), and these breaks gradually spread out through the entire notochord, resulting in many smaller vesicle-like structures, which were smaller cells (see below). Eventually, the smaller vesicles replaced nearly all the well vacuolated large chordocytes inside the notochord. Accordingly, the notochord became granular rather than having the characteristic smooth stack of pennies appearance (Fig. 1, B and D).
FIGURE 1.
The phenotype of ngs mutants and morphants. The notochord of wild-type zebrafish was rod-like (A) with the appearance of a stack of pennies (C), whereas the notochord of the ngs mutant was granular (B) and composed of smaller vesicles (D). This defect emerged at 30–35 hpf, when breaks appeared along the notochord (F). The notochord of the mutant became normal after expression of EF1a-ngs (E). When the embryos were injected with 4 ng of morpholino against ngs, their notochords became granular (G), consisting of many smaller vesicles (H) (left side views).
The corresponding proviral insertion in v835-2 was mapped to the first exon of a novel gene that was predicted by automated computational analysis from Ensembl (zgc:194470, ENSDARG00000054321) (supplemental sequences). When the carriers of this proviral integration were inbred, a quarter of their offspring showed the specific notochord defect, indicating that the mutation is recessive. Interestingly, the homozygous mutants were viable and fertile, giving rise to all their progeny with a granular notochord. Based on the phenotype, we named this gene ngs (notochord granular surface).
The expression of ngs in the mutant and carrier, as well as WT embryos, was first detected by RT-PCR (supplemental Fig. S1). The results showed that the mRNA level of ngs was dramatically reduced in the mutants. Then we placed the WT cDNA of ngs (the shorter form; details in the supplemental sequences) under the control of Xenopus EF1a (elongation factor 1a) promoter and injected the construct into one-cell stage embryos of the ngs/ngs mutant and generated the transgenic zebrafish line. The notochords of the transgenic fish with the homozygous ngs mutation background appeared normal (Fig. 1E), showing that the ngs cDNA fully rescued the notochord defect caused by the ngs mutation. More importantly, when the translation of ngs was blocked by a specific antisense morpholino oligonucleotide injected into the WT one-cell stage embryos, a phenotype indistinguishable from the ngs mutant was observed (Fig. 1, G and H). These results demonstrate that ngs is responsible for the phenotype in homozygous mutation of this gene.
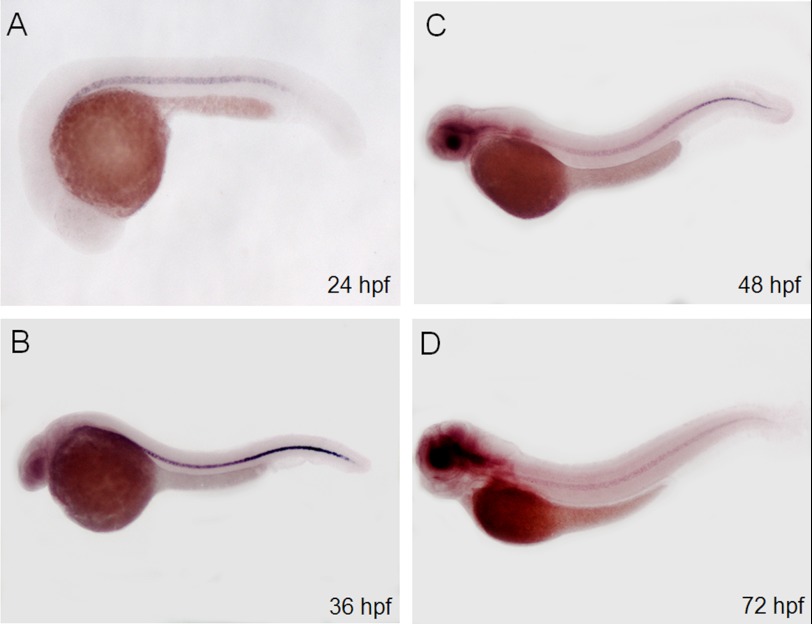
To reveal the spatial expression pattern of ngs, we performed whole mount in situ hybridization. ngs transcription was first detected at 24 hpf specifically in the notochord (Fig. 2A). As the embryo developed, the ngs transcription signal increased until 36 hpf and then decreased. After 72 hpf, the expression of ngs in the notochord remained at a low level (Fig. 2).
FIGURE 2.
Expression pattern of ngs detected by whole mount in situ hybridization. Expression of ngs was detected at 24 hpf specifically in the notochord (A). As the embryo developed, the expression level of ngs increased from 24 to 36 hpf and then decreased (B–D). After 72 hpf, it remained at a low level (left side views).
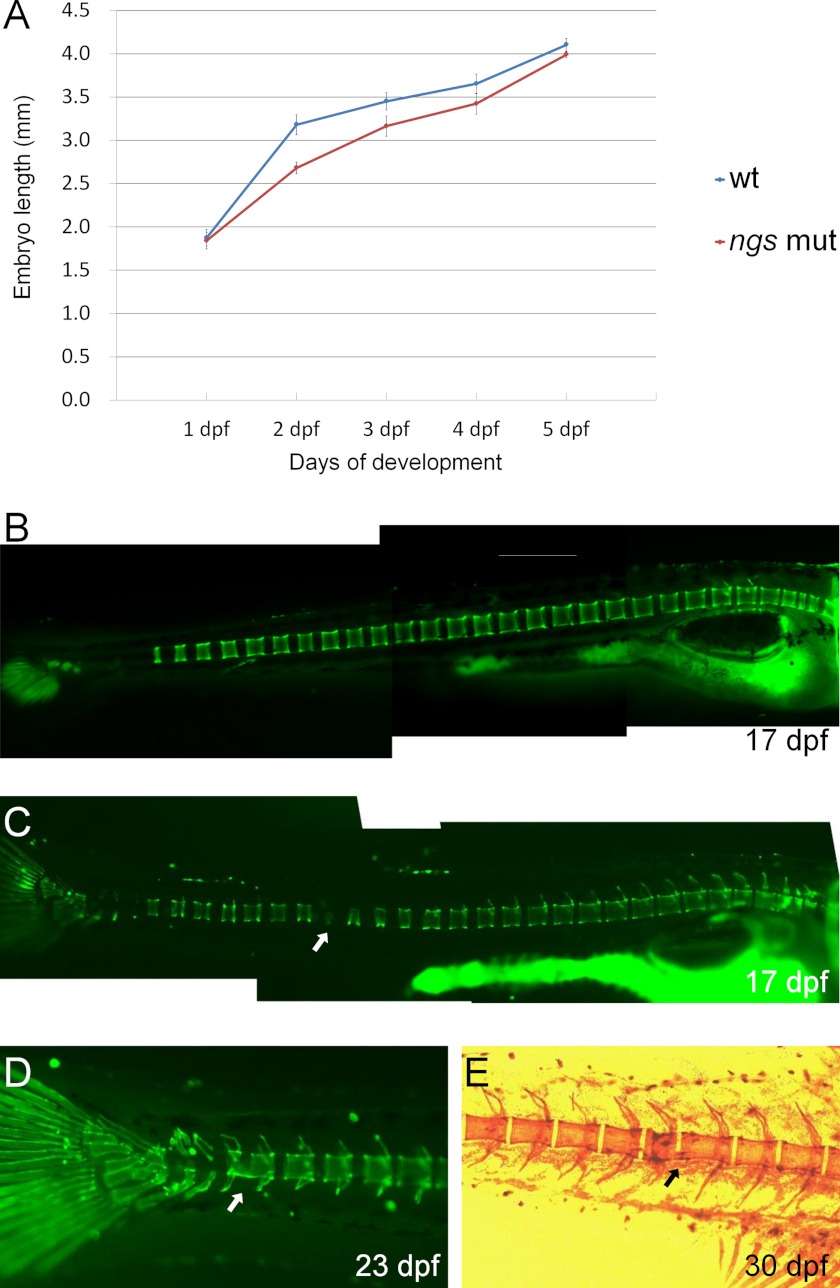
The defects of the notochord affected the elongation of the embryos. The mutant embryos at 2–4 days post-fertilization (dpf) were shorter than the WT embryos (Fig. 3A). In the WT embryos, the development of the vertebraes proceeded in an anterior to posterior sequence (Fig. 3B), whereas in some individuals of the mutant of ngs, the formation of some anterior vertebraes was later than the posterior ones (Fig. 3C, arrow). Moreover, the fusion of some vertebraes was detected in some mutants (Fig. 3, D and E). However, the expression of no tail (ntl) and sonic hedgehog (shh) showed no difference between the WT and the mutant embryos (supplemental Fig. S2). It suggests that the notochord defects in the ngs mutant affect the role of the notochord as the axial skeleton but do not affect the patterning of adjacent tissues.
FIGURE 3.
The defects of the notochord affect the elongation of the embryos and the formation of the vertebraes. A, the length of 15 embryos of the wild type, and the mutants were measured. The mutant embryos at 2–4 days post-fertilization are shorter than the wild type (the statistical significance is determined using a Student's t test, p < 0.01). B–D, calcein staining of the bone of zebrafish embryos. The formation of the vertebraes in the wild-type embryos proceeds in an anterior to posterior sequence (B), whereas in the mutant, some middle vertebrae is still forming when the posterior vertebraes formed (C, arrow). The fusion of some vertebraes is detected in some mutants (D and E, arrows). In E, the bone was stained with alizarin red.
The Granular Notochord in ngs Mutant Was Filled with Smaller Cells
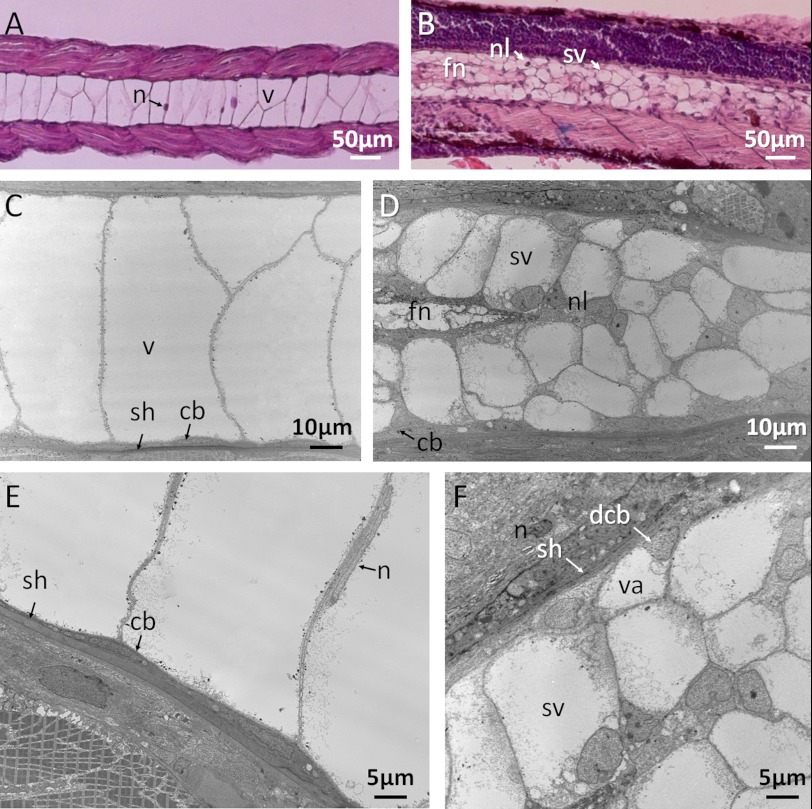
Several experiments were carried out to determine the components and structure of the granular notochord in the ngs mutant. First, the structure of the notochords of both the WT and the mutant zebrafish were investigated in paraffin-embedded tissue sections. At 48 hpf, the chordocytes in the WT were fully differentiated and vacuolated. They had a large central vacuole and very little cytoplasm. Their nuclei lay adjacent to the plasma membrane. The vacuolated cells were so large that most of the medulla of the notochord was composed of only a single row of the cells (Fig. 4A). In contrast, in the granular region of the notochord in the mutant embryos, there were no large vacuolated cells, and the medulla was filled with smaller and cell-like structures each with a nucleus-like dot adjacent to the plasma membrane. There was a thickened dark-stained region in the center of the mutant notochord (Fig. 4B). This structure resembles the “funiculus,” which is found in the notochord of several other species, such as sturgeon and lungfish, but not in the WT zebrafish.
FIGURE 4.
The structure of the notochords of wild-type and mutant zebrafish. A and B, hematoxylin and eosin staining of longitudinal sections of the notochords of the wild type (A) and the ngs mutant (B), viewed under a light microscope. The notochord in the wild type was mainly composed of large vacuolated cells (v), with the nucleus (n) adjacent to the cytoplasmic membrane. In the mutant, the notochord was composed of smaller vesicle-like structures (sv). Each vesicle contained a nucleus-like structure (nl). A funiculus was also seen in the notochord of the mutant (fn). C and D, the ultra-structure of the notochords in the wild type (C) and the ngs mutant (D). C–E, the notochord of the wild-type fish consisted of an outer sheath (sh), a single-layer thin epithelium of chordoblasts (cb), and a single-cell-wide medulla of large vacuolated chordocytes (v) with disc-shaped nuclei (E, indicated by n). In the ngs mutant, the medulla was filled by many smaller vesicle-like structures (sv). Each of the smaller cells contained a nucleus-like structure (nl), a relatively smaller central vacuole and more cytoplasm. F, many chordoblasts in the mutant started to differentiate (indicated by dcb), the cells expanded, and vacuoles appeared (va).
Transmission electron microscopy showed that the notochord of the WT zebrafish consisted of an outer sheath, a single-layered thin chordoblast epithelium and a single-cell-wide medulla composed of large vacuolated chordocytes (Fig. 4, C and E). In contrast, the large mature chordocytes were evidently replaced by smaller cells in the ngs mutant embryos. Each of the smaller cells contained a nucleus, a smaller central vacuole, and relatively more cytoplasm than the large vacuolated chordocytes in the WT embryos (Fig. 4D). The nucleus of the smaller cells, unlike the disc-shaped nucleus of the chordocytes in the WT embryos, was round and less condensed. The nucleolus was easily seen in the nucleus of the smaller cells. In addition, some epithelial cells were swelling, and vacuoles were also clearly seen (Fig. 4, D and F). All of these structural features suggest that the smaller cells were derived from the notochord epithelium (chordoblasts), and they were less differentiated than the mature chordocytes.
The existence of the nucleus was also confirmed by the expression of histone H2B fused with RFP (H2B-RFP) through injection of the corresponding expression vector into one-cell stage embryos of the mutants (Fig. 5, A and B). Next, the DNA content of the smaller cells was assessed. Through a large scale enhancer trap screen,4 we established the transgenic zebrafish line mp398b whose notochord was specifically labeled by EGFP (Fig. 5C). This line was crossed with the ngs mutant to obtain ngs mutants with a EGFP-positive notochord. In the embryos from this transgenic mutant, the smaller cells inside the granular notochord were labeled with EGFP (Fig. 5D). The EGFP-positive cells (the small cells in the notochord) and the GFP-negative cells (cells of other tissues) were sorted out by FACS, and the DNA content of these cells was analyzed. The results showed that most of the smaller cells contained the same amount of DNA as the cells from other tissues (Fig. 5, E and F).
FIGURE 5.
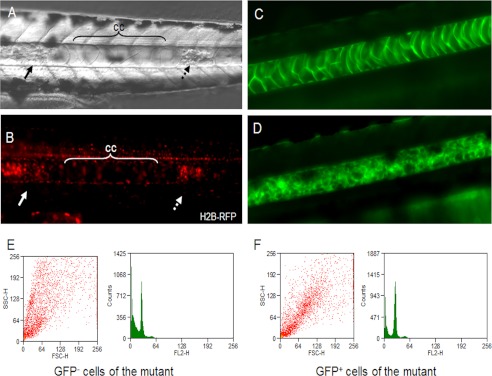
Identification of the small vesicles in the notochord of the ngs mutant. Embryos injected with H2B-RFP were examined under a differential interference contrast microscope (A) and a fluorescence microscope (B) at 40 hpf. In the region where the chordocytes were still morphologically normal (cc), there were very few nuclei. In contrast, in the region where the chordocytes were replaced by small vesicles, many nuclei were observed (indicated by arrow). C, in transgenic zebrafish line mp398b, the notochord was labeled with EGFP. D, the ngs mutant under the background of mp398b. E and F, the EGFP-positive small vesicles were separated from the EGFP-negative cells, and the DNA contents in both groups were determined by FACS.
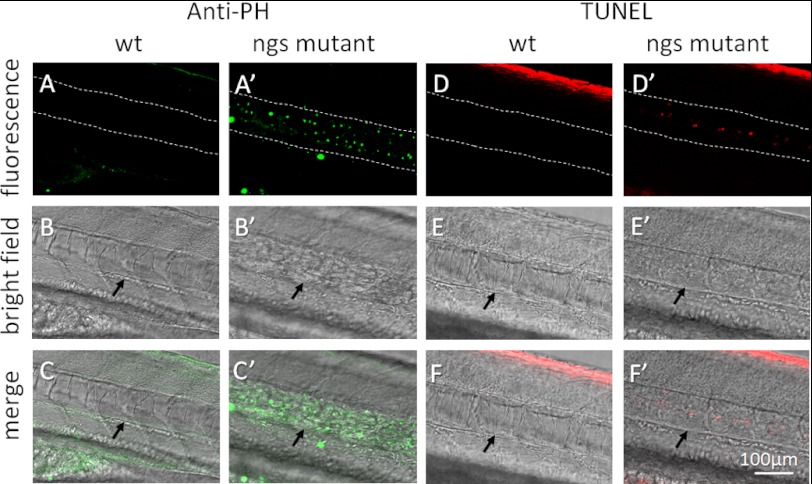
We also investigated the proliferation of the cells in the notochord with antibody against phosphorylated histone H3. Many cells were undergoing mitosis in the notochord of ngs mutants but not in WT embryos (Fig. 6, A–C′). The terminal TUNEL assay revealed that only the funiculus located in the center of the mutant notochord underwent apoptosis, but no small cells were labeled (Fig. 6, D′–F′). It is well known that the funiculus is composed of the debris of apoptotic chordocytes, and the existence of a funiculus means that the chordocytes renew very quickly (1). These data reveal that the small cells in the notochord of the ngs mutant are not the apoptotic bodies of chordocytes. On the contrary, these cells proliferate very quickly.
FIGURE 6.
Proliferation and apoptosis of cells in the notochord. Mitosis was detected by immunofluorescence with antibody against phosphorylated histone H3. Many nuclei were labeled in the notochord of the mutant (A′, B′, and C′), whereas no mitosis was detected in the notochord of wild-type embryos (A, B, and C). Apoptosis was not detected in the notochord of wild-type embryos by TUNEL assay (D, E, and F). In the mutant, apoptosis was detected in the funiculus located in the center of the notochord (D′, E′, and F′). Dashed lines and arrows indicate the location of the notochord.
Furthermore, we randomly labeled notochord epithelial cells (the chordoblasts) in ngs mutant embryos by injecting the pEGFP-N1 vector and found that, along with the replacement of chordocytes by the smaller cells, some of the smaller cells were EGFP-positive, indicating that the epithelial cells are the source of the smaller cells (supplemental Fig. S3, A, A′, B, and B′). Taken together, we conclude that the granular notochord in the ngs mutant is composed of smaller cells that contain a normal amount of DNA in their genome and may be less differentiated.
Muscle Contraction and Locomotion Promote the Replacement of Chordocytes by the Smaller Cells
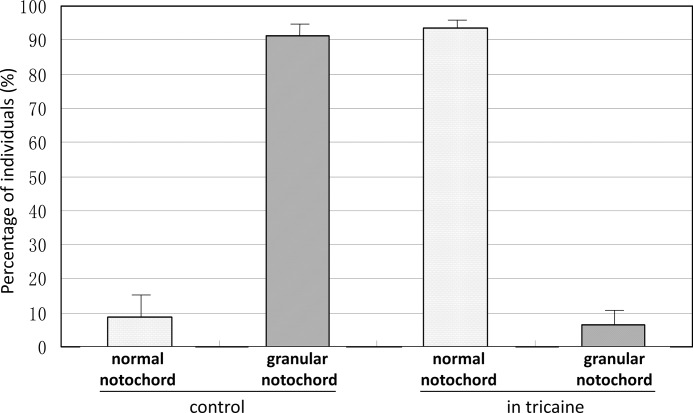
We noted that the replacement of chordocytes by the smaller cells always started from the middle region in the trunk and then spread to both ends. Zebrafish larvae become able to move their tail within 24 hpf and swim freely within 3 dpf, and the notochord plays an important role in locomotion. When a larva swims, its trunk is more active and strained than its head and tail. To determine whether the degeneration of notochord structure and the replacement of chordocytes by smaller cells were related to muscle contraction during locomotion, we anesthetized the mutant larvae with tricaine, a voltage-gated Na+ channel blocker, to inhibit the contraction of their muscles during 24–48 hpf. After this treatment, the notochord defect appeared in <10% of the homozygous mutant larvae. In contrast, >90% of mutant larvae had an abnormal notochord when cultured in the absence of tricaine (Fig. 7). This result suggests that muscle contraction promotes the replacement of chordocytes by the smaller cells as a result of damage to vacuolated chordocytes caused by local pressure.
FIGURE 7.
Formation of granular notochord is related to locomotion. ngs mutants at 24 hpf were maintained in either standard fish water or water containing 0.17 mg/ml tricaine. At 48 hpf, 8.8 ± 8.0% of larvae maintained in standard medium had a normal notochord, and 91.2 ± 8.0% had a granular notochord, whereas in the larvae maintained in tricaine, 93.7 ± 2.2% had a normal notochord, and 6.3 ± 2.2% had a defective notochord.
To test this hypothesis, we manually disrupted some vacuolated chordocytes in the WT zebrafish embryos at 35–40 hpf to produce a gap inside the notochord. After incubation overnight, the gap was filled with smaller cells similar to those found in the ngs mutant embryos (supplemental Fig. S3, C and D).
Taken together, these experiments revealed that ngs mutation makes the notochord fragile, and the vacuolated chordocytes collapse in ngs mutant larvae because of the forces generated by muscle contraction during movement and swimming. The gaps produced because of the chordocyte collapse trigger the notochord epithelial cells to proliferate, migrate into the gaps, differentiate to fill the gaps, and eventually lead to the granular appearance of the notochord.
Ngs Encodes a Novel Type of IF Family Protein That Assembles into Intermediate Filaments in the Zebrafish Notochord
According to phylogenetic analysis, ngs is localized to chromosome 4 and predicted to encode a new type of IF protein (supplemental Fig. S4). The amino acid sequence of the rod domain deduced from ngs mRNA is ∼40% identical to vimentin and desmin in zebrafish, mice, and humans (supplemental figures). Because the protein product of the ngs gene is involved in the maintenance of chordocyte integrity, we named this new protein chordostatin.
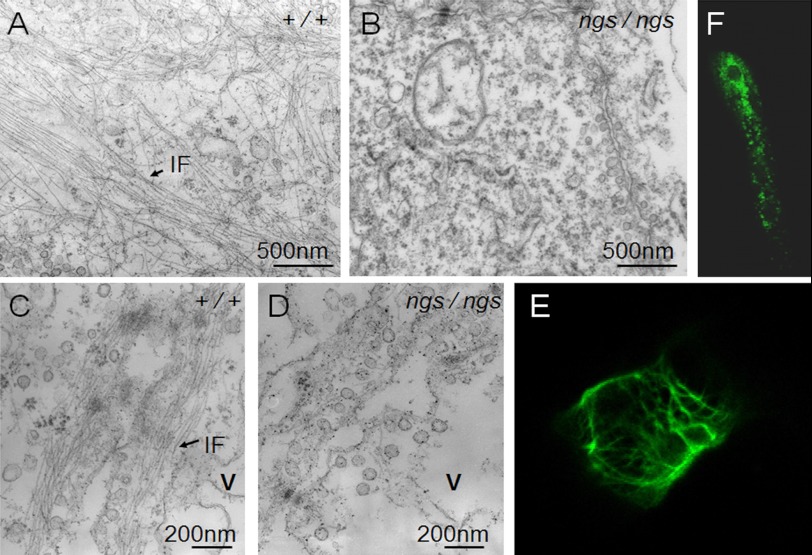
Transmission electron microscopy revealed the existence of bundles of 10-nm-thick IF-like filaments in the chordocytes of WT embryos at 48 hpf. These filaments were located in the thin layer of the cytoplasm between the vacuole and the cytoplasmic membrane. They formed a meshwork around the central vacuole (Fig. 8A) or were oriented nearly parallel to the membrane (Fig. 8C). In contrast, very few filaments along the membranes or around the vacuoles were found in the smaller cells of the ngs mutant (Fig. 8B) or in the mutant chordocytes (Fig. 8D) that had not yet been replaced by the smaller cells at 48 hpf.
FIGURE 8.
Transmission electron micrographs of notochords and the assembly of ngs-encoded protein. In sections of notochord from a wild-type fish, 10-nm-thick filaments were clearly observed (A and C). Many filaments were located in the thin layer of cytoplasm between the vacuolar membrane and the cytoplasmic membranes (C). In the ngs mutant, very few filaments were found in either the smaller cells (B) or the normal chordocytes, which had not yet broken (D). The ngs-encoded protein (chordostatin) fused to EGFP formed a filamentous network in chordocytes (E), whereas in other cells (muscle cell shown here as an example), no filamentous structures but only aggregations of protein were observed (F). v, vacuole.
To test whether it was the product of the ngs gene that assembled into the IF-like filaments in the chordocytes, ngs cDNA was cloned into pEGFP-N1 vector and injected into WT zebrafish embryos to express the chordostatin-EGFP fusion protein. Under the fluorescence microscope, some chordocytes were found to be EGFP-positive. By confocal microscopy, a green filamentous network was clearly visible in the cortex of these EGFP-positive chordocytes (Fig. 8E). In EGFP-expressing cells other than chordocytes, no filamentous bundles but only protein aggregates were observed (Fig. 8F). This result demonstrated that the ngs gene does encode a novel IF protein, which assembles into IF-like filaments specifically in the chordocytes of zebrafish.
Expression of ngs Confers Physical Strength to the Chordocytes
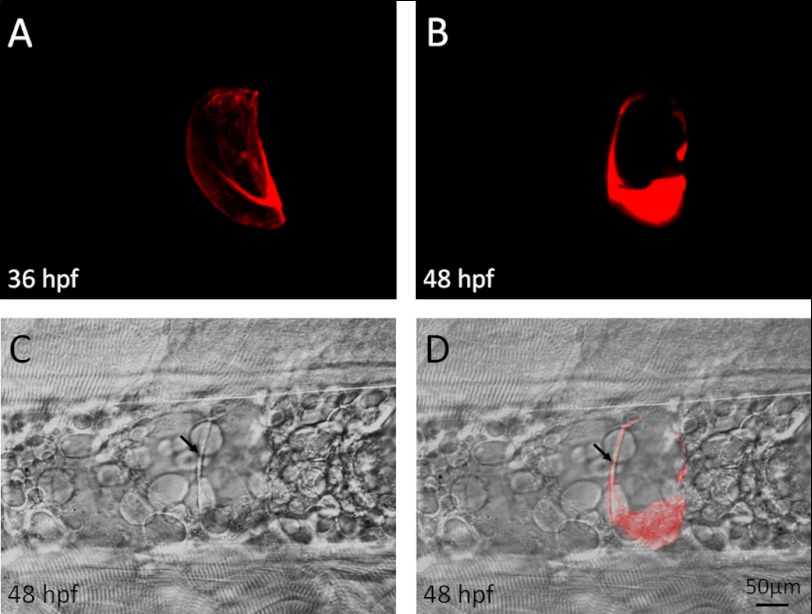
The construct ngs-mCherry, containing ngs fused to mCherry, was injected into mutant embryos at the one-cell stage. Filamentous bundles were detected inside the mCherry-positive chordocytes (Fig. 9A). At 48 hpf, when nearly all the mCherry-negative chordocytes underwent collapse and were replaced by the small cells, the mCherry-positive chordocytes remained intact (Fig. 9, B–D). These data show that the Ngs protein provides mechanical support to the chordocytes.
FIGURE 9.
Ngs protein confers physical strength on chordocytes. A, after injection of ngs-mCherry into the ngs mutant embryos, some chordocytes were labeled with mCherry at 36 hpf. B and C, at 48 hpf, these cells remained intact (arrow), whereas the other chordocytes were replaced by small cells. D, merge of B and C.
DISCUSSION
The IF is one of the three major cytoskeletal elements in most vertebrate cells. Unlike microfilaments and microtubules, the expression and distribution of IFs are tissue-specific (18–20). More than 65 genes have been identified to encode the IF family of proteins in human. The IF network is a relatively stable architecture in the cell. So far, no chemicals have been found that efficiently block or disrupt IF assembly. Thus, it is more difficult to investigate the functions of this component than other cytoskeleton proteins. Currently, it is believed that the IF provides a crucial structural support for cells. This notion is partially derived from the data from IF-related diseases in humans and gene-targeting results in mice. For example, dominant negative mutations in the genes encoding keratins 5 and 14 were described in patients with epidermolysis bullosa simplex. The mutant protein destroys the keratin network in the basal (progenitor) and suprabasal (differentiating) keratinocytes of the epidermis, resulting in blister emergence at the epidermal basal cell compartment after mild frictional trauma (29). Keratin 17-null mice suffer from alopecia, whereas Keratin 8 knock-out mice have colorectal hyperplasia and inflammation (30, 31).
In this work, we described a novel notochord-specific IF family protein, chordostatin, encoded by ngs in zebrafish. Proviral insertion led to down-regulation of the ngs gene and a dramatic decrease or absence of the IF-like network in chordocytes.
As previously shown, the notochord acts as the axial skeleton before the vertebrae appear and plays an important role in supporting locomotion in the larval and tadpole stages during individual development. The stiffness of the notochord comes from the hydrostatic pressure from vacuolated cells, whereas the strong and flexible sheath acts against this pressure (1, 14, 15). Because the vacuolated cells have to endure high pressure, they must be rigid enough, especially in the larval fish and tadpole, to resist this hydrostatic pressure, as well as the mechanical pressure generated by muscle contraction during swimming. The sheath plays an important role in protecting the cells from collapse (14, 15). Our present work revealed that an IF-like network around the central vacuole formed by chordostatin also provides a pivotal structural support for the chordocytes.
It has been reported that lamprey, sturgeon, and lungfish contain an IF network around the vacuoles in chordocytes (1, 32). However, the protein components of these IFs are not well investigated so far. They have only been shown to be immunologically similar to keratins (33). During human development, keratin has also been detected in the notochord (34). Our data indicated that chordostatin is an essential component of the IFs in zebrafish notochord cells. Without this protein, the IF network in the chordocyte did not form. On the other hand, chordostatin was only able to assemble into a filamentous network in chordocytes but not in other cells. Chordostatin neither co-assembled into filamentous structures with keratin, vimentin, or desmin nor assembled into filaments by itself in cultured cells (data not shown). Purified chordostatin also failed to assemble into filaments in vitro (data not shown). It is very likely that some protein(s) specifically expressed in chordocytes aid chordostatin to assemble into filaments. How this occurs remains unclear, and the putative partner(s) needs to be identified and verified.
Unlike mutants defective in the perinotochordal basement membrane, the collapse of the chordocytes in the ngs−/− mutant does not cause degeneration of the embryo. This might be due to the fact that the sheath is intact in mutant embryos and chordoblasts can proliferate quickly within the sheath to fill the medulla. Although the smaller cells are not fully vacuolated, they could provide enough support for normal function of the notochord. The funiculus, which we observed in the ngs−/− mutant, is a typical structure in the notochord of sturgeon and lungfish (1). Unlike zebrafish, the notochords of these fish are composed of several layers of vacuolated cells, just like the ngs−/− mutant. The chordoblasts in the epithelium of their notochords continuously proliferate and differentiate into vacuolated chordocytes, whereas the chordocytes in the center collapse gradually. The debris of the apoptotic chordocytes then forms the funiculus (1). We also noted that ngs mutant embryos were clearly shorter than the WT from 36 hpf to about 7 days post-fertilization (Figs. 1, A and B, and 2A). This may be due to the reduced mechanical stiffness of the notochord affecting elongation of the embryos (6). Interestingly, the mutant embryos recovered from this defect in about 10 days.
The protein deduced from ngs mRNA only shares 40–45% or less identity in its rod domain with the other members of the IF family, such as desmin or vimentin in zebrafish, Xenopus, mice, and humans. Bioinformatics analysis revealed no orthologous genes of ngs in amniotes such as chickens, mice, or humans, whereas in fugu (Takifugu rubripes), medaka (Oryzias latipes), Tetraodon nigroviridis, and Xenopus, ngs has an orthologous gene (supplemental figures). Considering that the function of Ngs protein is to mechanically support the chordocytes during larval swimming, it is reasonable to speculate that ngs was lost in amniotes during evolution because they do not need it to mechanically support the chordocytes, because the early embryos of amniotes do not need to move at all. When the embryo is able to move actively, the notochord has already been replaced by the vertebral column.
In conclusion, we have identified a novel gene, ngs, which encodes a new type of IF protein and is specifically expressed in the notochord of zebrafish. This novel IF protein assembles into a filamentous network, so as to perform its function of mechanical support in chordocytes during the locomotion of the embryos. It confers plasticity on the notochord to resist mechanical damage and maintains the integrity of fully differentiated chordocytes during development. Our work has important implications for understanding vertebrate development, as well as the evolution and function of IF family proteins.
Acknowledgments
We thank Dr. I. C. Bruce for critical reading of the manuscript; the Tol2 enhancer trap team in our laboratory (especially Lu Wen, Wei Wei, Yulin Xue, Naizhong Zheng, Juan Du, Fei Qi, and Wei Dong) for the contribution to the initial Tol2-mediated enhancer trap screening; Xinxing Liu, Yuying Gao, Jiao Zhang, and Qian Zhang for lab management and technical support; and Yingdi Jia, Jingliang Chen, and Houhua Cui for zebrafish husbandry.
This work was supported by National Natural Science Foundation of China Grants 30871418, 31110103904, and 30730056 and 973 Program Grant 2012CB945101.

This article contains supplemental text, sequences, and Figs. S1–S4.
Y. Xue, L. Wen, S. Lin, and B. Zhang, unpublished data.
- IF
- intermediate filament
- EGFP
- enhanced GFP
- hpf
- hours post-fertilization.
REFERENCES
- 1. Schmitz R. J. (1998) Comparative ultrastructure of the cellular components of the unconstricted notochord in the sturgeon and the lungfish. J. Morphol. 236, 75–104 [DOI] [PubMed] [Google Scholar]
- 2. Stemple D. L. (2004) The notochord. Curr. Biol. 14, R873-R874 [DOI] [PubMed] [Google Scholar]
- 3. Blagden C. S., Currie P. D., Ingham P. W., Hughes S. M. (1997) Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 11, 2163–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fouquet B., Weinstein B. M., Serluca F. C., Fishman M. C. (1997) Vessel patterning in the embryo of the zebrafish. Guidance by notochord. Dev. Biol. 183, 37–48 [DOI] [PubMed] [Google Scholar]
- 5. Goldstein A. M., Fishman M. C. (1998) Notochord regulates cardiac lineage in zebrafish embryos. Dev. Biol. 201, 247–252 [DOI] [PubMed] [Google Scholar]
- 6. Adams D. S., Keller R., Koehl M. A. (1990) The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115–130 [DOI] [PubMed] [Google Scholar]
- 7. Glickman N. S., Kimmel C. B., Jones M. A., Adams R. J. (2003) Shaping the zebrafish notochord. Development 130, 873–887 [DOI] [PubMed] [Google Scholar]
- 8. Odenthal J., Haffter P., Vogelsang E., Brand M., van Eeden F. J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., Kane D. A., Kelsh R. N., Mullins M. C., Warga R. M., Allende M. L., Weinberg E. S., Nüsslein-Volhard C. (1996) Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development 123, 103–115 [DOI] [PubMed] [Google Scholar]
- 9. Stehr C. M., Linbo T. L., Incardona J. P., Scholz N. L. (2006) The developmental neurotoxicity of fipronil. Notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol. Sci. 92, 270–278 [DOI] [PubMed] [Google Scholar]
- 10. Tada M. (2005) Notochord morphogenesis. A prickly subject for ascidians. Curr. Biol. 15, R14-R16 [DOI] [PubMed] [Google Scholar]
- 11. Grotmol S., Nordvik K., Kryvi H., Totland G. K. (2005) A segmental pattern of alkaline phosphatase activity within the notochord coincides with the initial formation of the vertebral bodies. J. Anat. 206, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grotmol S., Kryvi H., Keynes R., Krossøy C., Nordvik K., Totland G. K. (2006) Stepwise enforcement of the notochord and its intersection with the myoseptum. An evolutionary path leading to development of the vertebra? J. Anat. 209, 339–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Platz F. (2006) Structural and experimental investigations of the functional anatomy and the turgor of the notochord in the larval tail of anuran tadpoles. Ann. Anat. 188, 289–302 [DOI] [PubMed] [Google Scholar]
- 14. Stemple D. L., Solnica-Krezel L., Zwartkruis F., Neuhauss S. C., Schier A. F., Malicki J., Stainier D. Y., Abdelilah S., Rangini Z., Mountcastle-Shah E., Driever W. (1996) Mutations affecting development of the notochord in zebrafish. Development 123, 117–128 [DOI] [PubMed] [Google Scholar]
- 15. Parsons M. J., Pollard S. M., Saúde L., Feldman B., Coutinho P., Hirst E. M., Stemple D. L. (2002) Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137–3146 [DOI] [PubMed] [Google Scholar]
- 16. Coutinho P., Parsons M. J., Thomas K. A., Hirst E. M., Saúde L., Campos I., Williams P. H., Stemple D. L. (2004) Differential requirements for COPI transport during vertebrate early development. Dev. Cell 7, 547–558 [DOI] [PubMed] [Google Scholar]
- 17. Wang D., Jao L. E., Zheng N., Dolan K., Ivey J., Zonies S., Wu X., Wu K., Yang H., Meng Q., Zhu Z., Zhang B., Lin S., Burgess S. M. (2007) Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc. Natl. Acad. Sci. U.S.A. 104, 12428–12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim S., Coulombe P. A. (2007) Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 21, 1581–1597 [DOI] [PubMed] [Google Scholar]
- 19. Omary M. B., Coulombe P. A., McLean W. H. (2004) Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087–2100 [DOI] [PubMed] [Google Scholar]
- 20. Parry D. A., Strelkov S. V., Burkhard P., Aebi U., Herrmann H. (2007) Towards a molecular description of intermediate filament structure and assembly. Exp. Cell Res. 313, 2204–2216 [DOI] [PubMed] [Google Scholar]
- 21. Erber A., Riemer D., Bovenschulte M., Weber K. (1998) Molecular phylogeny of metazoan intermediate filament proteins. J. Mol. Evol. 47, 751–762 [DOI] [PubMed] [Google Scholar]
- 22. Venetianer A., Schiller D. L., Magin T., Franke W. W. (1983) Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature 305, 730–733 [DOI] [PubMed] [Google Scholar]
- 23. Bonifas J. M., Rothman A. L., Epstein E. H., Jr. (1991) Epidermolysisbullosa simplex. Evidence in two families for keratin gene abnormalities. Science 254, 1202–1205 [DOI] [PubMed] [Google Scholar]
- 24. Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. (1991) Point mutations in human keratin 14 genes of epidermolysisbullosa simplex patients. Genetic and functional analyses. Cell 66, 1301–1311 [DOI] [PubMed] [Google Scholar]
- 25. Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. (1992) A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature 356, 244–246 [DOI] [PubMed] [Google Scholar]
- 26. Li R., Messing A., Goldman J. E., Brenner M. (2002) GFAP mutations in Alexander disease. Int. J. Dev. Neurosci. 20, 259–268 [DOI] [PubMed] [Google Scholar]
- 27. Brenner M., Johnson A. B., Boespflug-Tanguy O., Rodriguez D., Goldman J. E., Messing A. (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120 [DOI] [PubMed] [Google Scholar]
- 28. Mersiyanova I. V., Perepelov A. V., Polyakov A. V., Sitnikov V. F., Dadali E. L., Oparin R. B., Petrin A. N., Evgrafov O. V. (2000) A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am. J. Hum. Genet. 67, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao T., Longley M. A., Wang X. J., Roop D. R. (2001) An inducible mouse model for epidermolysisbullosa simplex. Implications for gene therapy. J. Cell Biol. 152, 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baribault H., Penner J., Iozzo R. V., Wilson-Heiner M. (1994) Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8, 2964–2973 [DOI] [PubMed] [Google Scholar]
- 31. McGowan K. M., Tong X., Colucci-Guyon E., Langa F., Babinet C., Coulombe P. A. (2002) Keratin 17 null mice exhibit age- and strain- dependent alopecia. Genes Dev. 16, 1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bartels H., Potter I. C. (1998) Membrane structure of the cells of the lamprey notochord. J. Electron. Microsc. 47, 627–636 [Google Scholar]
- 33. Schmitz R. J. (1998) Immunohistochemical identification of the cytoskeletal elements in the notochord cells of bony fishes. J. Morphol. 236, 105–116 [DOI] [PubMed] [Google Scholar]
- 34. Götz W., Kasper M., Fischer G., Herken R. (1995) Intermediate filament typing of the human embryonic and fetal notochord. Cell Tissue Res. 280, 455–462 [DOI] [PubMed] [Google Scholar]
- 35. Nüssslein-Volhard C., Dahm R. (2002) Zebrafish: A Practical Approach, pp. 7–58, Oxford University Press, New York [Google Scholar]
- 36. Westerfield M. (2007) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th Ed., Chapter 2–4, University of Oregon Press, Eugene, OR [Google Scholar]