Background: Pasteurella multocida toxin (PMT) is a highly mitogenic bacterial protein whose detailed mechanism is not well understood.
Results: PMT deamidates and activates Gαq/11 leading to mTORC1 activation.
Conclusion: Gαq/11/PLCβ/PKC-mediated mTORC1 activation is at least partially responsible for PMT-induced cell growth, migration, and proliferation in Swiss 3T3 cells.
Significance: Understanding PMT-induced mTORC1 activation could help elucidate mechanisms of oncogene regulation.
Keywords: mTOR, Phospholipase C, Protein Deamidation, Protein Kinase C (PKC), Signal Transduction, Gαq/11, PLCβ, PMT
Abstract
Pasteurella multocida toxin (PMT) is a potent mitogen known to activate several signaling pathways via deamidation of a conserved glutamine residue in the α subunit of heterotrimeric G-proteins. However, the detailed mechanism behind mitogenic properties of PMT is unknown. Herein, we show that PMT induces protein synthesis, cell migration, and proliferation in serum-starved Swiss 3T3 cells. Concomitantly PMT induces phosphorylation of ribosomal S6 kinase (S6K1) and its substrate, ribosomal S6 protein (rpS6), in quiescent 3T3 cells. The extent of the phosphorylation is time and PMT concentration dependent, and is inhibited by rapamycin and Torin1, the two specific inhibitors of the mammalian target of rapamycin complex 1 (mTORC1). Interestingly, PMT-mediated mTOR signaling activation was observed in MEF WT but not in Gαq/11 knock-out cells. These observations are consistent with the data indicating that PMT-induced mTORC1 activation proceeds via the deamidation of Gαq/11, which leads to the activation of PLCβ to generate diacylglycerol and inositol trisphosphate, two known activators of the PKC pathway. Exogenously added diacylglycerol or phorbol 12-myristate 13-acetate, known activators of PKC, leads to rpS6 phosphorylation in a rapamycin-dependent manner. Furthermore, PMT-induced rpS6 phosphorylation is inhibited by PKC inhibitor, Gö6976. Although PMT induces epidermal growth factor receptor activation, it exerts no effect on PMT-induced rpS6 phosphorylation. Together, our findings reveal for the first time that PMT activates mTORC1 through the Gαq/11/PLCβ/PKC pathway. The fact that PMT-induced protein synthesis and cell migration is partially inhibited by rapamycin indicates that these processes are in part mediated by the mTORC1 pathway.
Introduction
Toxin-producing strains of Pasteurella multocida are known to cause pasteurellosis in humans and animals and atrophic rhinitis in swine (1), a pathology characterized by bone loss in the dorsal and ventral nasal turbinates. The gene (toxA) encoding P. multocida toxin (PMT),3 acquired by horizontal transmission (2), has been cloned and sequenced (3). It is a single polypeptide of 146 kDa whose C-terminal activity domain structure has been solved (4). In addition to its mitogenic properties for certain types of cells, including quiescent fibroblast and osteoclast cells, PMT is a strong inducer of anchorage-independent growth (5–7). Proliferative properties of PMT have been observed in vivo. Injection of purified recombinant toxin into animals induced proliferation in bladder epithelium without evidence of an inflammatory reaction (8). PMT also inhibits in vitro differentiation, e.g. bone cells and preadipocytes, where it prevents the formation of mineralized bone nodules and important adipocyte markers, respectively (9). These properties, growth stimulation and inhibition of cell differentiation, suggest that PMT might have the potential to act as a tumor promoter especially in the case of chronic infections (10).
Recently, PMT has been shown to exert some of its biological effects through the activation of heterotrimeric G-proteins, which involves Gαq-, Gα11-, Gα12/13-, and Gαi-dependent pathways, via the deamidation of a conserved glutamine residue in the α subunit (11, 12). Abnormal G protein signaling induced by bacterial toxins may lead to diverse biological consequences. Through Gαq activation, PMT activates signaling pathways known to be affected by proto-oncogenes, including those associated with phospholipase C, protein kinase C, ERK1/2 MAPKs, calcium mobilization, and STATs (13–18). In addition, PMT has been shown to induce Rho activation, Rho-dependent stress fiber formation, and FAK phosphorylation in a Gα12/13-dependent manner (19, 20). However, the effects of PMT on signaling pathways associated with stimulation of protein synthesis and cell proliferation have not been studied.
The mammalian target of rapamycin (mTOR), a key Ser-Thr kinase highly conserved from yeast to mammals, exists intracellularly in two functionally distinct complexes, mTORC1 and mTORC2 (21–23). mTORC1 consists of the mTOR catalytic subunit and associated proteins raptor, PRAS40, and mLST8/GβL. This complex is involved in the regulation of protein synthesis, cell growth, proliferation, and autophagy in a nutrient- and energy-responsive manner. It has been shown that activation of mTORC1 leads to the rapamycin-sensitive phosphorylation of S6K1, which in turn phosphorylates ribosomal S6 protein (rpS6) (21–25). The mTORC2 is activated by growth factors via a mechanism involving mTOR, rictor, mLST8/GβL, mSin1, and protor. Active mTORC2 activates Akt/PKB, PKCα, and regulates actin cytoskeletal organization.
Here we show that PMT stimulates protein synthesis, ATP production, and cell proliferation and migration in serum-starved Swiss 3T3 fibroblast cells. Concomitantly, PMT also activates mTORC1, monitored by the phosphorylation of rpS6. To elucidate the role of mTORC1 in PMT-induced protein synthesis, we investigated the effect of rapamycin and Torin1, the specific inhibitor of mTORC1, on PMT-induced activation of S6K1 and protein synthesis. Our results reveal that PMT activates mTORC1 via a PKC-mediated pathway, Furthermore, our data also indicate that PMT-induced protein synthesis is mediated in part by the mTORC1 pathway.
EXPERIMENTAL PROCEDURES
Materials
Antibodies directed against phospho-rpS6 (Ser-235/236 and Ser-240/244), rpS6 monoclonal antibody, S6K1 polyclonal antibodies, and rapamycin were from Cell Signaling Technology (Beverly, MA). IRDye 800CW-conjugated affinity-purified anti-rabbit IgG and IRDye 700 CW-conjugated affinity-purified anti-mouse IgG secondary antibodies were from LI-COR Biosciences (Lincoln, NE). PKC and EGFR inhibitors, Gö6976 and AG1478, respectively, were from Calbiochem (San Diego. CA). Torin1 was purchased from Tocris Bioscience (Minneapolis, MN). The RC DC protein assay kit was from Bio-Rad Laboratories (Hercules, CA). Monoclonal antibody, 3G3, developed specifically against the deamidated form of Gαq, and Gαq/11−/− double-deficient fibroblasts were generous gifts from Dr. Shigeki Kamitani, Department of Molecular Bacteriology, Research Institute for Microbial Diseases (Osaka University, Japan), and Professor Stefan Offermanns (Pharmakologisches Institute, Universitat Heidelberg, Germany), respectively. Mouse receptor tyrosine kinase arrays were from RD Systems (Minneapolis, MN). Swiss 3T3 cells and calf fetal serum were obtained from the American Type Culture Collection (Manassas, VA). 1,2-Dioctanoyl-sn-glycerol (8:0) was from Enzo Life Sciences (Farmingdale, NY). ApoSENSOR ATP assay kit was purchased from BioVision (Milpitas, CA). DAPI was from Invitrogen. Culture media and antibiotics were from Invitrogen.
Cell Culture
Swiss-3T3 and mouse embryonic fibroblast cells were cultured in DMEM containing 10% fetal bovine serum (ATCC), l-glutamine, and antibiotics (Invitrogen; 50 units/ml of penicillin, 50 μg/ml of streptomycin) and incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Swiss 3T3 cells were grown in DMEM containing 10% calf fetal serum. An equivalent number of cells was seeded in 10-cm Petri dishes and grown to confluence. Confluent cells were then incubated overnight in serum-free medium.
PMT Treatment and Immunoblot Analysis
Serum-starved cultures of confluent fibroblast cells were treated with PMT at the indicated concentrations and times in the presence or absence of the indicated inhibitors. Cells were then rinsed with ice-cold phosphate-buffered saline and scraped into SDS-PAGE loading buffer. Protein concentrations were determined by the Lowry method using the RC DC protein assay kit according to the manufacturer's recommendations. Equal amounts of proteins were subjected to SDS-polyacrylamide gel electrophoresis. Due to a significant difference in sensitivity between phosphor-rpS6 and rpS6 antibodies, we loaded on the same gel, one-half with 10 μg/well of total protein for detecting phosphor-rpS6 and the other half with 20 μg/well of total protein for detecting rpS6. Proteins were subjected to SDS-polyacrylamide gel electrophoresis. Separated proteins were thereafter transferred onto a nitrocellulose membrane, cut in half, and probed with appropriate antibodies, and detected using the LI-COR Odyssey (Lincoln, NE). The results presented in the figures represent a typical observation from at least three independent experiments.
[35S]Methionine Incorporation Assay
Cells grown to confluence on 10-cm Petri dishes were serum-starved for 24 h then treated with PMT in serum-free DMEM containing 50 μCi/ml of [35S]methionine. Treated cells, thoroughly washed with PBS, were then lysed and equal amounts of protein, determined by BCA reagent, was precipitated with trichloroacetic acid (TCA, 10% final concentration). Samples were centrifuged in a benchtop centrifuge at high speed for 10 min. The supernatant was discarded and the pelleted proteins were dissolved in 6 m guanidine. The 35S incorporation into the protein was determined using a liquid scintillation counter.
ATP Measurement
Cellular ATP levels were determined using the luciferase catalyzed light generation reaction of ATP and luciferin. The light intensity was measured using a luminometer (TD-20/20, Truner Designs, Sunnyvale, CA). Briefly, confluent cells grown in 10-cm Petri dishes were serum starved overnight, then treated with PMT as indicated. Treated cells were harvested, centrifuged, and immediately stored at −70 °C until use. Cell pellets were lysed and the protein concentration was determined using BCA reagent. Equal amounts of protein from each sample were precipitated with picric acid (3.4% final concentration) in the presence of pH indicator for 10 min on ice. The samples were then diluted 2.5-fold with water and 3 m K2CO3 was added dropwise, while vortexing gently, to neutralize the samples. Neutralized samples were centrifuged at 7500 × g (Eppendorf centrifuge 5417R) for 3 min at 4 °C and 10 μl of supernatant was mixed with ATP monitoring solution in a total volume of 100 μl. The luminescence was measured for 10 s and results were expressed as URL/mg of protein.
Mouse Receptor Tyrosine Kinases Array
To study the effect of PMT treatment on membrane receptor tyrosine kinases in serum-starved Swiss 3T3 cells, we used the mouse phosphoreceptor tyrosine kinase (phospho-RTK) array. Each membrane array contains 39 different anti-RTK antibodies, three positive controls, and one negative control printed in duplicate. Cell lysates from untreated control cells and cells treated with PMT for 24 h were diluted and incubated with the membranes according to the manufacturer's recommendation. The membranes were then washed to remove any unbound material and a pan anti-phosphotyrosine antibody conjugated to horseradish peroxidase was used to detect phosphorylated tyrosines on activated receptors by chemiluminescence.
Wound Healing Assay
The scratch wound healing assay is a classic and commonly used method to characterize cell proliferation and migration. In this assay a scratch was made on a serum-starved confluent 3T3 cell monolayer using a 200-μl pipette tip then treated with PMT in the presence or absence of rapamycin. Factors that alter the motility and/or growth of the cell can lead to increased or decreased rate of “healing” of the gap in a process that can be observed over an indicated time period. The cells were then stained with DAPI and monitored with EVOS fluorescence microscope using ×4 lens.
RESULTS AND DISCUSSION
Effect of PMT on Protein Synthesis and ATP Levels
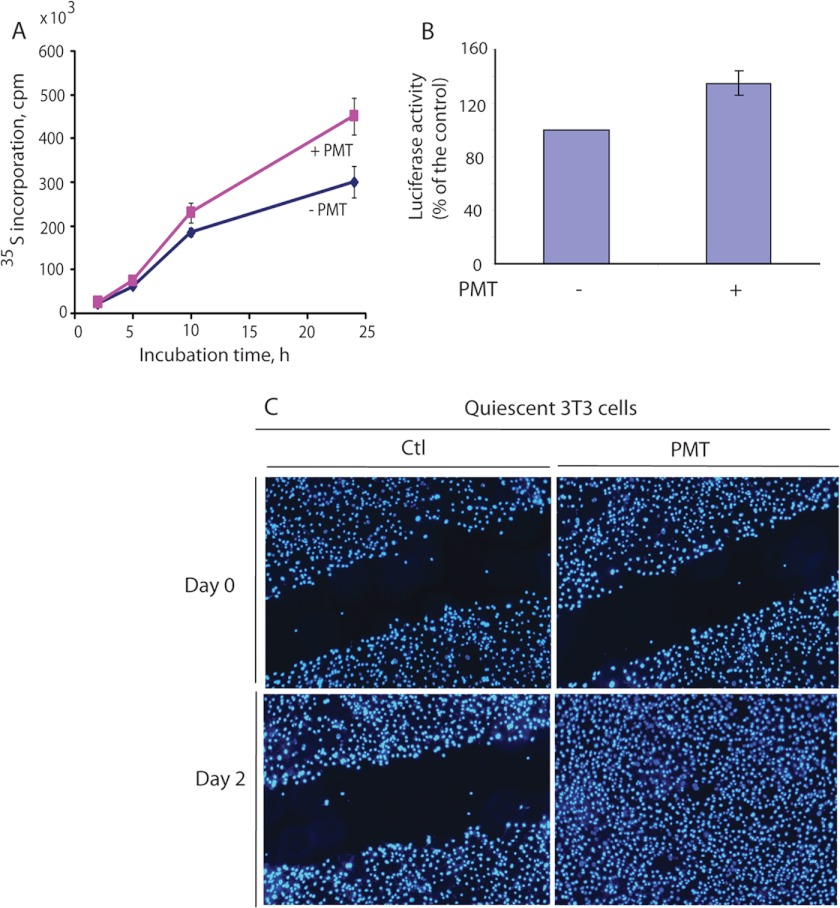
Subjected to serum starvation, Swiss 3T3 cells stop proliferating and enter the quiescent state of the cell cycle. However, such cells remain viable and can be stimulated to reenter the cell cycle by replenishing the medium with serum or appropriate growth factors. The most obvious common point between active growth and quiescence is that actively growing cells activate pathways that are needed for growth, and the machinery for protein synthesis is one of the key contributors to growth. To monitor the effect of PMT on protein synthesis, confluent and serum-starved Swiss 3T3 cells were either left untreated or treated with PMT (50 ng/ml) for the indicated times in serum-free culture media supplemented with [35S]methionine (50 μCi/ml). Fig. 1A shows that despite serum starvation for 24 h, untreated control cells incorporated [35S]methionine over time suggesting that these cells are still metabolically active albeit at a lower level. Compared with these control cells, PMT-treated cells for 2, 5, 10, and 24 h, however, showed an increase in [35S]methonine incorporation by 14, 17, 27, and 46%, respectively. Interestingly, the PMT-mediated increase in protein synthesis levels in adherent cells was also observed in PMT-treated cells kept in suspension on a Petri dish coated with poly(2-hydroxyethylmethacrylate) (result not shown). This result may explain the anchorage-independent growth observed in PMT-treated cells (7).
FIGURE 1.
Effect of PMT on protein synthesis, ATP level, and cell migration and proliferation of serum-starved 3T3 cells. A, protein synthesis was monitored, as a function of time, by [35S]methionine incorporation in serum-starved 3T3 cells either left untreated or exposed to PMT. B, ATP content in nontreated control and PMT-treated cells determined using the luciferase assay method described under “Experimental Procedures.” C, representative images of healing the denuded quiescent 3T3 cell monolayer areas via cell proliferation and migration in control nontreated and PMT (50 ng/ml) treated cells at 0 and 48 h. Complete healing of the scratch wound was observed in treated cells but not in control cells. Here cells were stained with DAPI. The results in A and B represent mean ± S.D. of 3 to 4 independent experiments, and C represents a typical observation from at least three independent experiments.
Protein synthesis is energetically costly, requiring not only ATP and GTP but also the production of ribosomes. Thus we monitored the effect of PMT treatment on cellular ATP levels. Fig. 1B shows that serum-starved cells treated with 50 ng/ml of PMT for 24 h induced a 30% increase in their cellular ATP content in comparison to the control nontreated cells. Note this significant increase in ATP levels were observed despite ATP being consumed due to the PMT-induced elevation of protein synthesis. Besides the effect of PMT on cellular ATP content and protein synthesis, PMT treatment induced migration and proliferation in quiescent 3T3 cells. In an in vitro wound healing assay, addition of PMT for 48 h to serum-starved 3T3 cells resulted in an increase of migration toward the denuded area compared with control nontreated cells (Fig. 1C). This PMT-mediated increase in scratch closure or wound healing observed was attributed to a combination of cell proliferation and migration, a complex phenomenon that requires a coordinated action of multiple pathways including the machinery for protein synthesis and cell adhesion (26). PMT has been shown to stimulate Rho-dependent stress fiber formation and FAK phosphorylation (19, 20), a pathway known to regulate cell migration. The mechanism by which PMT induces ATP production, protein synthesis, and cell migration and proliferation remains to be elucidated.
PMT Induces Ribosomal S6 Protein Phosphorylation
Among cellular regulators of cell growth, mTOR and its immediate upstream and downstream regulators are perhaps the most conserved and appear to have important inputs into growth in Saccharomyces cerevisiae, Drosophila, and mammals. The mTORC1 signaling pathway is a complex regulatory mechanism that can sense and respond to the availability of nutrients, energy, stress, and mitogens to modulate protein synthesis (21, 22, 27). Upon its activation, mTORC1 catalyzes the phosphorylation of S6K1 at its Thr-389 residue leading to its activation, which in turn phosphorylates rpS6. Phosphorylation sites on rpS6 have been mapped to Ser-235, Ser-236, Ser-240, Ser-244, and Ser-247 (28) and has been shown to correlate with an increased rate of protein synthesis (29, 30).
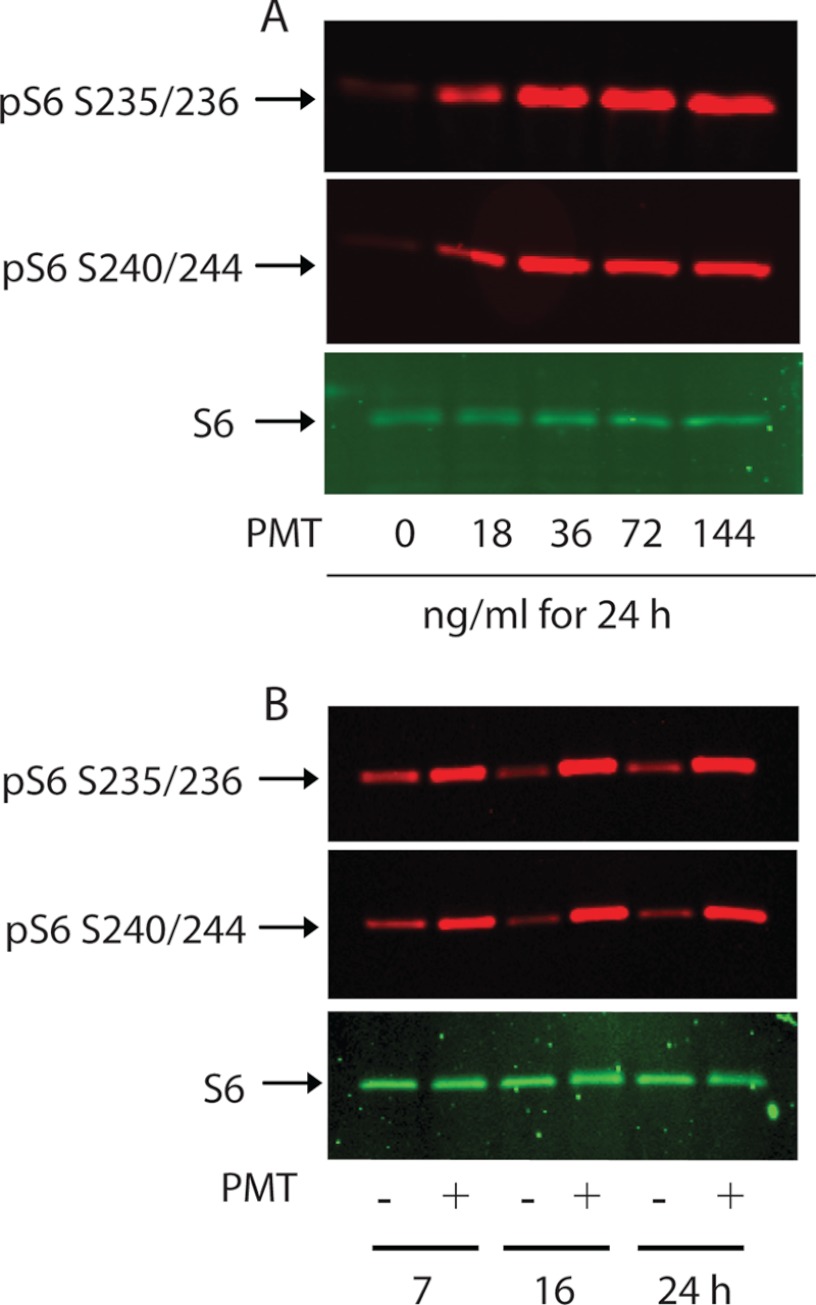
We sought to determine whether the mTOR signaling pathway is involved in the observed proliferative response induced by PMT in quiescent Swiss 3T3 cells. To this end, rpS6 phosphorylation in serum-starved Swiss 3T3 cells was investigated as a readout of mTOR activation. Fig. 2, A and B, show a substantial and sustained increase in rpS6 phosphorylation on both Ser-235/Ser-236 and Ser-240/Ser-244 in quiescent Swiss 3T3 cells treated with PMT in a concentration- and time-dependent manner. Relative to PMT-treated 3T3 cells, the level of rpS6 phosphorylation in the nontreated control cells was weak and remained unchanged over the indicated time. Furthermore, the increase in the rpS6 phosphorylation levels in PMT-treated cells is not due to the increase in rpS6 protein whose levels remain comparable between all samples.
FIGURE 2.
PMT induces ribosomal rpS6 protein phosphorylation in Swiss 3T3 cells. Confluent serum-starved Swiss 3T3 cells were treated with the indicated concentrations of PMT for 24 h (A) or with 36 ng/ml of PMT for the indicated times (B). Immunoblot analysis was carried out as described under ”Experimental Procedures.“ Immunoblots were developed with antibodies that recognize either phospho-S6 Ser-235/236, phosphor-S6 Ser-240/244, or nonphosphorylated S6. In this and the following figures when applicable, S6 represents rpS6.
PMT-induced rpS6 phosphorylation was observed after the cells were exposed to toxin (20 ng/ml) for longer than 1 h (data not shown). This suggests that PMT does not act through an extracellular receptor as do classical growth factors whose action is mediated within seconds. In addition, contrary to the classical growth factors whose action are transient, PMT-mediated rpS6 phosphorylation was sustained over a long period of time, exceeding 24 h, again ruling out the possibility that PMT-induced rpS6 phosphorylation is mediated by a cell membrane receptor. The protracted effect of PMT is consistent with a recent observation showing that PMT catalyzes the irreversible deamidation of the Gα subunit of heterotrimeric G proteins (11). These properties make PMT an excellent pharmacological tool to study the molecular mechanism of the activation of PMT targeted signaling pathways, including mTOR. Furthermore, the observed lag phase in the action of recombinant P. multocida toxin may reflect its binding to the plasma membrane (31), followed by cellular entry and possible processing and activation. When 3T3 cells were treated with the same concentration of PMT as above, followed with 30 min treatment with either 2-deoxyglucose (25 mm) or rotenone (10 μm) to lower cellular ATP levels, a significant decrease in PMT-induced rpS6 phosphorylation was observed (result not shown). This result is consistent with the notion that the PMT-mediated mTOR signaling pathway is sensitive to the availability of nutrients and energy (22).
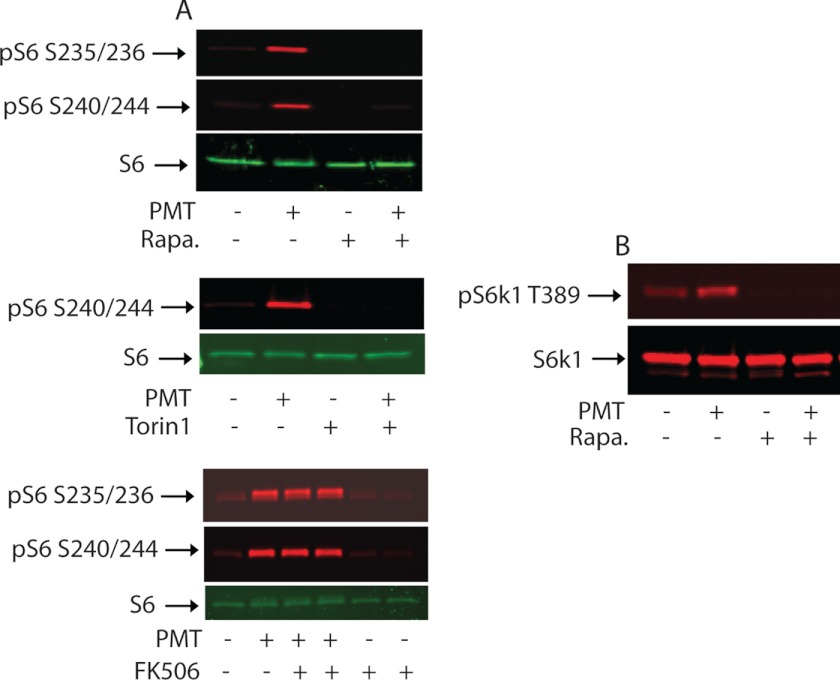
To demonstrate that rpS6 phosphorylation induced by PMT treatment can be attributed to the catalytic action of mTORC1, we investigated the effect of rapamycin, an established specific allosteric inhibitor of mTORC1 that was originally identified as an immunosuppressant and later as an anticancer agent. When serum-deprived 3T3 cells were pretreated with or without 30 nm rapamycin for 30 min prior to treatment with 50 ng/ml of PMT, we found that pretreatment with rapamycin eliminated both basal and PMT-induced rpS6 phosphorylation (data not shown). A similar result was obtained when cells were first treated with PMT for 24 h and then exposed to rapamycin for just 90 min (Fig. 3A, upper panel). Furthermore, when 150 nm Torin1, a recently developed ATP competitive inhibitor for both mTORC1 and mTORC2 (32), was added for 90 min to cells that had been exposed to the toxin for 24 h, both basal and PMT-induced rpS6 phosphorylation were totally abolished (Fig. 3A, middle panel). As a control, the immunosuppressant, FK506, which binds FKBP12 protein, had no effect on PMT-mediated rpS6 phophorylation (Fig. 3A, lower panel). Taken together, these results indicate that PMT treatment induces rpS6 phosphorylation via the mTOR-mediated pathway and its dephosphorylation occurs at a relatively fast rate in the presence of either rapamycin or Torin1. It should be pointed out that both S6K1 and the 90-kDa ribosomal S6 kinase (RSK) are known to phosphorylate rpS6 in vivo. However, the fact that PMT-induced rpS6 phosphorylation is completely blocked by rapamycin or Torin1 ruled out the involvement of RSK because RSK is insensitive to inhibition by rapamycin (33).
FIGURE 3.
Effect of rapamycin, Torin1, and FK506 on PMT-induced rpS6 and S6K1 phosphorylation. A, serum-deprived Swiss 3T3 cells were left untreated or treated with 50 ng/ml of PMT for 24 h followed by treatment with rapamycin (30 nm) (upper panel), Torin1 (150 nm) (middle panel), or FK 506 (200 nm) (lower panel) for 90 min. Protein extracts were subjected to immunoblotting with antibodies against rpS6 and pS6 Ser-235/236 and pS6 Ser-240/244. B, serum-starved cells were treated with PMT for 6 h followed by rapamycin treatment as above and equal amounts of protein were separated by SDS-PAGE. Immunoblots were developed using S6K1 and pS6K1 Thr-389 antibodies as indicated.
PMT Induces Phosphorylation of Ribosomal S6 Protein Kinase
Activation of S6K1, a downstream target of mTOR, requires a complex sequence of Ser/Thr phosphorylation events. Among them, phosphorylation of Thr-389 was shown to be essential for S6K1 activity (34, 35). Thr-389 is the phosphorylation site of kinases highly sensitive to rapamycin (36). To monitor PMT-induced S6K1 activation in fibroblast cells, a polyclonal antibody that recognizes phosphorylated Thr-389 was used. Fig. 3B shows that Thr-389 phosphorylation was elevated after the 3T3 cells were treated with 50 ng/ml of PMT for 6 h. However, when 3T3 cells were pretreated with 30 nm rapamycin, the observed basal and PMT-induced phosphorylation at Thr-389 of S6K1 was totally abolished. These results indicate that the observed PMT-induced S6K1 phosphorylation/activation is mediated by mTORC1.
Akt is a positive regulator upstream of the mTORC1, known to stimulate mTORC1 by phosphorylating PRAS40, a suppressor of mTORC1, leading to its dissociation from mTORC1, and/or by phosphorylating TSC2 and relieving its negative inhibition of Rheb, a GTPase that regulates the activity of mTORC1. The full activation of Akt involves its phosphatidylinositol 3,4,5-trisphosphate-dependent plasma membrane translocation, where it is first phosphorylated at Ser-473 by mTORC2 (37) and then at Thr-308 by phosphoinositide-dependent kinase (PDK1) (38, 39). Akt has also been shown to regulate cellular energy metabolism (40). Thus, we checked whether PMT also activates Akt. Despite the fact that PMT activates mTOR and stimulates ATP production, we did not observe any increase in Akt phosphorylation at either Ser-473 or Thr-308. These results together with the observation that wortmannin, an inhibitor of PI3K, did not show any effect on PMT-induced S6 phosphorylation (data not shown), suggest that PMT did not activate PI3K/Akt nor did it activate mTORC2 pathways as do growth factors like insulin.
PMT-mediated RTK Phosphorylation Exerts No Effect on mTOR Activation
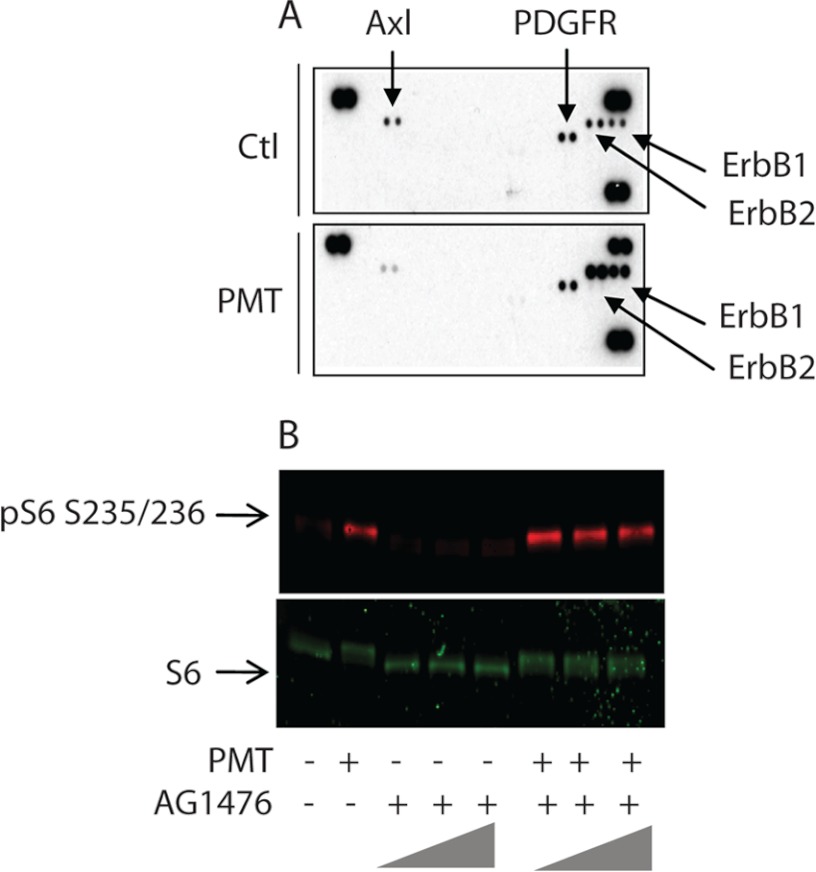
Receptor tyrosine kinases (RTK) are major activators of the mTOR pathway in mammalian cells, but not in yeast. To study the effect of PMT treatment on membrane receptor tyrosine kinases that may in turn activate the mTOR signaling pathway in serum-starved Swiss 3T3 cells, we used the mouse phosphor-receptor tyrosine kinase (phosphor-RTK) array. Each membrane array contains 39 different anti-RTK antibodies, three positive controls, and one negative control printed in duplicate. Cell lysates from control nontreated cells and cells treated with PMT for 24 h were diluted and incubated with the membranes. The membranes were then washed to remove any unbound material and a pan anti-phosphotyrosine antibody conjugated to horseradish peroxidase was used to detect phosphorylated tyrosines on activated receptors by chemiluminescence. Fig. 4A shows that phosphorylation of the PDGF receptor remains unchanged in both control and PMT-treated cells, whereas phosphorylation of the Axl receptor tyrosine kinase was reduced by PMT. However, PMT treatment induced a significant increase in the phosphorylation of both EGFR and Erb2. These receptors belong to the ErbB protein family, also known as epidermal growth factor receptor (EGFR) family, which consists of four members; namely, ErbB1, (also known as EGFR), ErbB2, ErbB3, and ErbB4. They play critical roles in the proliferation, migration, survival, and differentiation of cells. Dysregulation of signaling by ErbBs has been implicated in the pathogenesis and progression of human cancers. ErbB2 is similar in overall structure to the EGFR (41). Furthermore, in contrast to the EGFR, Erb2 is not a genuine receptor because it has no known extracellular ligand. However, it does possess a catalytically active PTK subdomain, and functions as a co-receptor that forms heterodimers with other activated ErbB family members.
FIGURE 4.
Receptor tyrosine kinase activation by PMT. A, serum-starved Swiss 3T3 cells were either left untreated or treated with 50 ng/ml of PMT for 24 h. Cells were lysed according to the manufacturer's recommendations and 100 μg of protein were applied to the receptor tyrosine kinase array that contained 39 different anti-RTK antibodies, three positive controls, located at three corners, and one negative control, located at the lower left corner, printed in duplicate. The membranes were then washed and revealed using an anti-phosphotyrosine antibody conjugated to horseradish peroxidase. B, quiescent Swiss 3T3 cells were treated with PMT for 24 h in the absence or presence of 1.0, 5.0, and 10.0 μm AG1476. After the incubation, cells were lysed and immunoblot analysis was carried out as described under ”Experimental Procedures.“ The immunoblots were developed using antibody against phosphor-S6 and unphosphorylated S6 protein. Ctl, control.
To determine the role of PMT-mediated EGFR activation on the mTOR signaling pathway we investigated the effect of a known EGFR-specific inhibitor, AG1476. In comparison to the control nontreated cells, PMT-treated cells showed, as above, a huge increase in the level of rpS6 phosphorylation. However, when serum-starved 3T3 cells were treated with PMT in the presence of 1.0, 5.0, or 10.0 μm AG1476, we did not observe any decrease in the levels of phosphorylated rpS6 (Fig. 4B). These results suggest that whereas PMT induces transactivation of EGFR (16) it appears to have no role in PMT-induced mTORC1 activation. However, it should be pointed out that transactivation of EGFR induced by PMT has been shown to stimulate other signaling pathways including the ERK cascade in cardiac fibroblasts but not in cardiomyocytes (42). Thus PMT-mediated EGFR and ERK activation may well participate, along with mTOR, in PMT-induced cell proliferation.
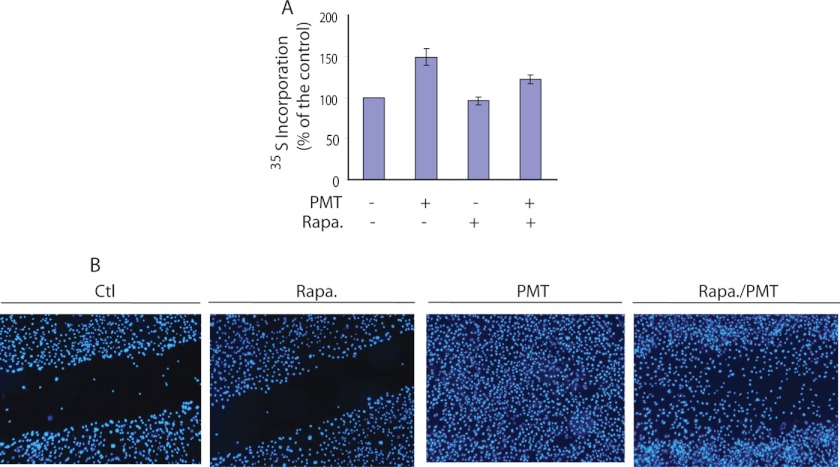
Rapamycin Partially Inhibits PMT-induced Protein Synthesis
We have shown that PMT activates the mTOR signaling pathway in serum-starved fibroblast cells. To investigate the relationship between the observed PMT-induced mTORC1 signaling activation and protein synthesis, we studied the effect of rapamycin on PMT-induced protein synthesis in Swiss 3T3 cells. The results shown in Fig. 5A reveal that protein synthesis in Swiss 3T3 cells was elevated by 48 ± 9% following 24 h treatment with PMT (50 ng/ml) in comparison to the control nontreated cells. However, when cells were treated with 30 nm rapamycin, a concentration that completely eliminated the rpS6 and S6K1 phosphorylation induced by PMT, [35S]methionine incorporation was reduced from 48 ± 9 to 22 ± 4%, whereas rapamycin exerted no effect in the absence of PMT. This observation reveals that PMT-induced protein synthesis is partially regulated by the S6K1-mediated pathway. The mTORC1 pathway is known to regulate cell growth and protein synthesis through its two downstream substrates, 4E-BP1 and S6K1. It should be pointed out that although much attention has been focused on the correlation between S6K1 activation/rpS6 phosphorylation and the initiation of protein synthesis in response to mitogens (28, 43–45), the mechanism by which S6K1 and rpS6 phosphorylation activate the translation machinery remains obscure. In fact, this notion has been challenged by the observation that a higher rate of protein synthesis occurred in knock-in mice whose ribosomal S6 protein contained alanine substitutions at all five phosphorylatable serine residues (46) and the translation of 5′ terminal oligopyrimidine tract mRNAs was not impaired in S6K1 knock-out mice (47). The observed partial inhibitory effect of rapamycin on PMT-induced protein synthesis is also consistent with findings that whereas mTORC1 phosphorylates both S6K1 and 4E-BP1, rapamycin efficiently inhibits S6K1 phosphorylation but only partially inhibits the phosphorylation of 4E-BP1 (23, 48, 49).
FIGURE 5.
Effect of rapamycin on PMT-induced protein synthesis and cell migration. A, [35S]methionine incorporated into proteins in serum-starved cells left untreated, treated with 30 nm rapamycin alone, 50 ng/ml of PMT alone, or with rapamycin in the presence or absence of PMT for 24 h in serum-free medium as described under ”Experimental Procedures.“ B, representative images of wound healing of quiescent Swiss 3T3 cells due to cell proliferation and migration in control nontreated and in rapamycin (30 nm)-treated and PMT (50 ng/ml)-treated in the presence or absence of rapamycin (30 nm) and incubated for 48 h. Cells were stained with DAPI. The PMT-treated cells showed a robust healing, whereas rapamycin plus PMT exhibited partial wound healing. Ctl, control.
To investigate the role of mTOR in PMT-induced cell proliferation and migration, we studied the effect of rapamycin on PMT-induced wound healing. The results shown in Fig. 5B reveal a partial healing of scratch wounds occurred in quiescent Swiss 3T3 cells treated with both PMT (50 ng/ml) and rapamycin (30 nm) for 48 h, whereas quiescent cells treated with PMT (50 ng/ml) alone for the same period of time exhibited a robust wound healing capacity. Control nontreated cells and rapamycin-treated cells did not show any sign of wound healing. Together these results show that rapamycin partially inhibited PMT-induced protein synthesis, cell proliferation, and migration. However, we cannot exclude the possibility that rapamycin could also exert an inhibitory effect on the PMT-induced activation of focal adhesion kinase (10).
Role of Gαq/11 in PMT-induced S6 Phosphorylation and Protein Synthesis
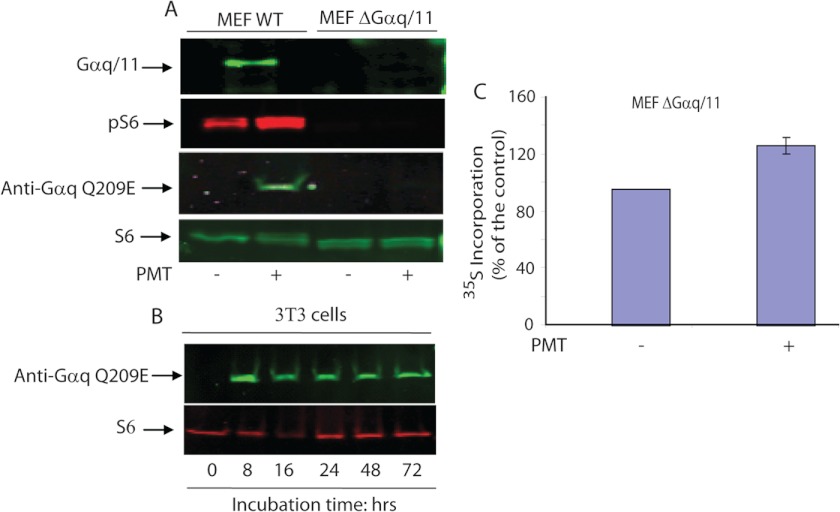
It has been reported that PMT-induced pleiotropic cellular effects are mediated via Gαq but not by its highly related Gα11 protein (17). In fact, PMT-mediated Gαq deamidation causes inhibition of its GTPase activity and subsequently leads to the activation of the Gαq-mediated signaling pathway. Using mouse embryonic fibroblast (MEF) cell lines derived from mouse embryos deficient in both Gαq/11 subunits (upper band of Fig. 6A), we studied its effects on PMT-induced rpS6 phosphorylation. When WT MEF cells were subjected to 48 h serum deprivation, and then treated with 100 ng/ml of PMT for 24 h, a robust increase in the rpS6 phosphorylation was observed (Fig. 6A). In contrast, exposure of Gαq/11 double-deficient MEF cells to the same concentration of PMT and incubation time as those used for the wild type MEF did not result in any significant increase in the phosphorylation of rpS6. In addition, Western blot analysis using the 3G3 monoclonal antibody that recognizes the deamidated form of Gαq (Q209E) shows that the deamidated Gαq was detected only in PMT-treated wild type MEF cells but not in Gαq/11 knock-out or untreated MEF cells. Although 3G3 has been shown to cross-react with the deamidated form of other heterotrimeric G proteins, such as Gα11, Gα12, and Gα13 (12), under our experimental conditions, we did not observe any detectable level of deamidated Gα12, and Gα13 in PMT-treated Gαq/11 knock-out MEF cells. However, we cannot exclude the possibility that the immunoreactive band observed using the 3G3 antibody shown in Fig. 6A may contain the deamidated form of both Gαq and Gα11, because these two Gα subunits have similar molecular weight. Fig. 6B shows that when Swiss 3T3 cells were treated with 50 ng/ml of PMT, a substantial increase in the deamidated form of Gαq was also observed following 8 h treatment with PMT. In contrast to PMT-treated cells, the level of the deamidated form of Gαq in control nontreated cells is undetectable. Furthermore, the level of deamidated Gαq remains nearly constant up to 72 h following PMT treatment. This observation, in conjunction with the prolonged effect of PMT, is consistent with recent findings showing that PMT induces the deamidation of Gln-209 in Gαq of the heterotrimeric G protein leading to its persistent activation (11, 12). Together, these results show that the observed PMT-induced rpS6 phosphorylation is Gαq/11 dependent, a process likely involving the PMT-catalyzed deamidation of Gαq/11.
FIGURE 6.
Role of Gαq/11 in PMT-induced rpS6 phosphorylation and protein synthesis. A, wild type and Gαq/11-deficient MEF cells were serum-starved for 48 h and then either left untreated or treated with 100 ng/ml of PMT for 24 h. Immunoblots were developed using antibodies that recognize phospho-S6 Ser-235/236, or phosphor-S6 Ser-240/244, Gαq/11, nonphosphorylated S6, and Gαq(Q209E), as indicated under ”Experimental Procedures.“ S6 represents rpS6. Top panel of A represents a separate experiment to show that MEF ΔGαq/11 does not contain Gαq/11 protein. B, quiescent 3T3 cells were treated with PMT (50 ng/ml) for the indicated period of time. As described above, the immunoblot was probed with monoclonal 3G3 antibodies that recognize the deamidated form of Gαq (Gαq(Q209E)) and with antibodies against S6 protein. C, Gαq/11-deficient MEF cells were serum-starved for 48 h and either left untreated or treated with 100 ng/ml of PMT for 24 h in DMEM containing [35S]methionine. The quantity of [35S]methionine incorporation was measured as described under ”Experimental Procedures.“ The error bars indicate the S.D. from the mean of 3 independent experiments.
Despite the fact that PMT failed to activate mTOR in Gαq/11-deficient cells, it did induce a 30 ± 6% increase in [35S]methionine incorporation in Gαq/11 double knock-out MEF cells (Fig. 6C). This observation is in line with previous results showing a partial inhibitory effect of rapamycin on PMT-induced protein synthesis, cell proliferation, and migration. These observations suggest that PMT may induce a yet to be identified mTORC1-independent protein synthesis pathway(s).
Diacylglycerol and PMA Activate mTORC1 through PKC in a Rapamycin-dependent Manner
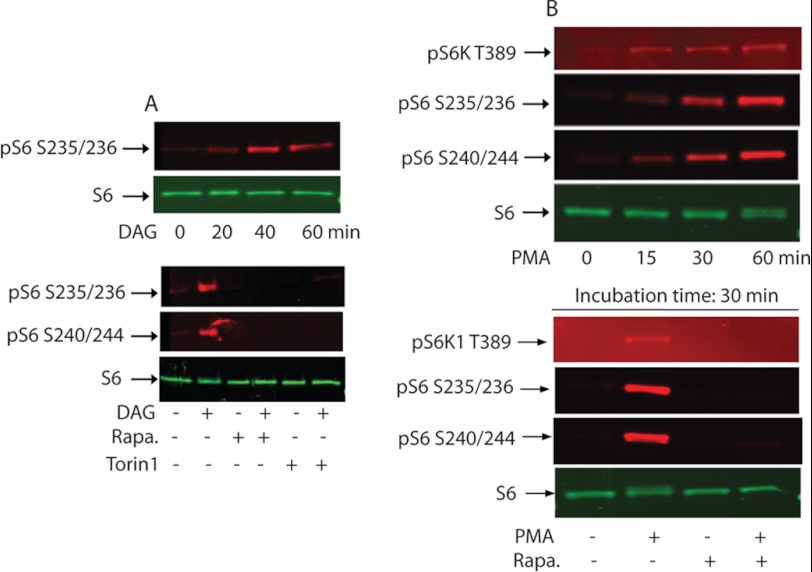
The Gαq family of Gα subunits is the class of heterotrimeric G proteins that are known to mediate the activation of phospholipase Cβ (PLCβ) signaling pathways. The PMT-catalyzed deamidation of Gαq/11 has been shown to induce a persistent activation of the G-protein (11, 12). The activated Gαq is then dissociated from the heterotrimeric G protein to form an active Gαq-PLCβ complex, which in turn catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacylglycerol (DAG) (50). Furthermore, PMT treatment has been shown to exhibit a Gαq-dependent elevation of cellular content of Ins(1,4,5)P3, Ins(1,3,4,5)P4, and/or Ins(1,3,4)P3, products of PtdIns(4,5)P2 hydrolysis, in 3T3 cells (5, 14) and in MEF cells (17, 51). Kamitani et al. (12) also reported recently that PMT induces PLCβ activation in Swiss 3T3 cells as well as MEF cells in a Gαq/11-dependent manner. These observations are consistent with the notion that PMT induces Gαq/11-dependent activation of PLCβ to produce IP3 and DAG. We next checked whether DAG alone when added exogenously to the serum-starved 3T3 cells can recapitulate the effect of PMT in terms of mTOR activation. Fig. 7A, upper panel, shows that addition of DAG alone at a concentration of 2 μg/ml for different periods of time up to 60 min leads to a substantial increase in the phosphorylation of rpS6 at Ser-235/Ser-236 in comparison to the control nontreated cells. However, DAG-induced rpS6 phosphorylation was totally abolished when the cells were treated with the same concentration of DAG for 30 min in the presence of either 100 nm rapamycin or 150 nm Torin1 (Fig. 7A, lower panel).
FIGURE 7.
rpS6 phosphorylation by exogenously added DAG or PMA. A, confluent and serum-starved Swiss 3T3 cells were treated with 2 μg/ml of DAG for the indicated period of time (upper panel) or treated with DAG in the absence or presence of 100 nm rapamycin or 150 nm Torin1 (lower panel). As described above, immunoblots were developed using antibodies that recognize phospho-S6 Ser-235/236, or phosphor-S6 Ser-240/244, and nonphosphorylated S6. B, serum-starved 3T3 cells were treated as above with 100 nm PMA for the indicated period of times (upper panel) or with 100 nm PMA in the presence or absence of 30 nm rapamycin for 30 min. Western blot analysis was carried out as described above.
DAG and tumor-promoting phorbol esters have been shown to activate protein kinase C (PKC) (52). Because DAG induces phosphorylation of rpS6 in serum-starved cells, we considered whether another PKC activator, PMA, would do the same. Results depicted in Fig. 7B shows that PMA indeed activates the mTORC1 signaling pathway in confluent and serum-starved 3T3 cells. The upper panel reveals a time-dependent phosphorylation of S6K1(Thr-389) and rpS6(Ser-235, -236, -240, and -244) stimulated by 100 nm PMA. The lower panel depicts that PMA-stimulated S6K1 and rpS6 phosphorylation were abolished when the reactions were carried out in the presence of 30 nm rapamycin, a level that totally inhibited the PMT-induced S6K1 and rpS6 phosphorylation. These results support the notion that PKC plays a role in activating mTORC1.
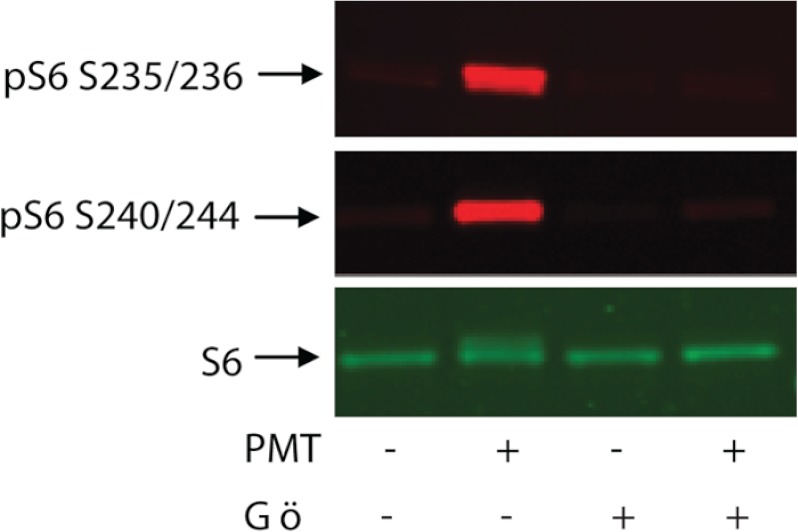
To further demonstrate the role of PKC in PMT-induced rpS6 phosphorylation, we exposed quiescent 3T3 cells to PMT in the presence or absence of a well known PKC inhibitor, Gö6976. The results shown in Fig. 8 reveal that PMT-induced rpS6 phosphorylation is effectively inhibited by 1 μm Gö6976. It should be pointed out that PMT has been reported to activate PKCμ (also known as PKD), which in turn activates cAMP response element-binding protein in cardiomyocytes (53). In view of the fact that Gö6976 can effectively inhibit several PKC isozymes including PKCμ (54), we are currently trying to identify the PKC isozyme that is responsible in activating the mTORC1 pathway. Nevertheless, these results indicate that the observed PMT-induced mTORC1 activation is mediated by a PKC-dependent pathway. Consistent with these observations, Herbert et al. (55) have shown that when serum-starved HEK 293 cells were treated with 12-O-tetradecanoylphorbol 13-acetate, it resulted in an increase of the phosphorylation of S6K1. Using the same cell type, Ballif et al. (56) reported that PMA induced phosphorylation at Ser-1364 of TSC2, a tumor suppressor upstream of mTORC1, was independent of ERK because the ERK inhibitor, U0126, had no effect. However, whether this phosphorylation will lead to the activation of mTOR was not demonstrated.
FIGURE 8.
PKC inhibitor, Gö6976, inhibits PMT-induced rpS6 phosphorylation. Quiescent Swiss 3T3 cells were treated with PMT (50 ng/ml) for 24 h then exposed to 1 μm Gö6976 for 90 min. Cell were lysed, and the immunoblot was developed, as described above, using antibodies against phosphor-S6 or unphosphorylated S6 protein. S6 represents rpS6.
Concluding Remarks
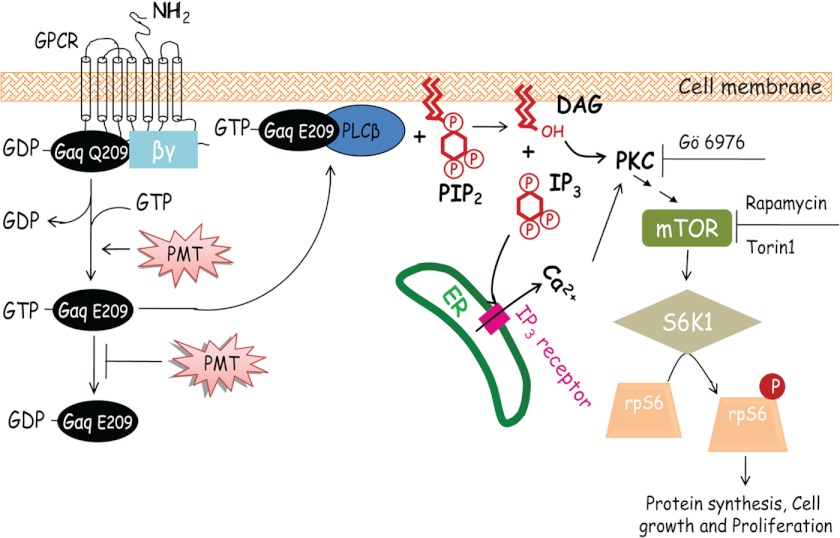
In this study, using Swiss 3T3 cells, we revealed for the first time that PMT activates mTORC1 in a Gαq/11-dependent manner, monitored by the rapamycin and Torin1-sensitive S6K1 and rpS6 phosphorylation. It is well established that mTORC1 functions as a key regulator in cell growth and division mediated by the availability of nutrients and energy. Based on our study and those reported in the literatures, we propose a mechanistic scheme (Fig. 9) to depict a potential mechanism by which PMT mediates protein synthesis and cellular proliferation. As shown in this scheme, PMT catalyzes the deamidation of the conserved Gln-209 in Gαq and/or Gα11 subunit of the heterotrimeric G protein leading to the dissociation of the active GTP-bound Gαq, and/or Gα11, which in turn binds to PLCβ to form an active complex. The active PLCβ catalyzes the hydrolysis of PIP2 to generate DAG and IP3. The IP3 can bind to the IP3 receptor and trigger the release of Ca2+ from the ER. Both DAG and Ca2+ are activators of PKC, which in turn activates mTORC1 to facilitate cellular protein synthesis, cell proliferation, and migration. The fact that the PMT-induced mTORC1 activation involves an irreversible deamidation step suggests that its effect on mTORC1 activation would be long lasting, unlike the transient nature of a growth factor-induced signaling.
FIGURE 9.
A proposed mechanistic scheme depicting PMT-induced mTORC1 activation. Here PMT was shown to activate the heterotrimeric G protein by catalyzing the deamidation of Gln-209 in Gαq and leading to its activation. The activated GTP-bound Gαq(Glu-209) subunit then forms a complex with PLCβ to activate its phospholipase activity. The active PLCβ catalyzes the hydrolysis of PIP2 to generate DAG and IP3. IP3 binds to the IP3 receptor to trigger the release of Ca2+ from the ER. Both DAG and Ca2+ can activate PKC and leading to mTORC1 activation, which in turn activates protein synthesis and cell proliferation. Inhibition of PKC by Gö6976, or inhibition of mTOR by rapamycin or Torin1 leads to the inhibition of rpS6 phosphorylation.
Furthermore, our data also show that PMT induces protein and ATP synthesis, and cell migration and proliferation in serum-starved Swiss 3T3 cells. The activated mTORC1 catalyzes the phosphorylation of S6K1 at its Thr-389 residue and leads to its activation. The active S6K1 in turn catalyzes the phosphorylation of rpS6. The fact that the observed PMT-induced protein synthesis is partially sensitive to rapamycin (Fig. 5A) is consistent with the notion that PMT-induced protein synthesis is at least in part mediated by the mTORC1 pathway.
Acknowledgments
We thank Dr. Shigeki Kamitani and Prof. Stefan Offermanns for providing the 3G3 monoclonal antibody that recognizes the deamidated form of Gαq and MEF wild type and Gαq/11-deficient cells, respectively.
This work is supported, in whole or in part, by the intramural Research Program of the National Heart, Lung, and Blood Institute and by National Institutes of Health and Grant AI038396 (to B. A. W.) from the NIAID.
- PMT
- Pasteurella multocida toxin
- mTORC1
- mammalian target of rapamycin complex 1
- S6K1
- ribosomal S6 kinase
- DAG
- diacylglycerol
- IP3
- inositol trisphosphate
- RTK
- receptor tyrosine kinase
- RSK
- ribosomal S6 kinase
- EGFR
- epidermal growth factor receptor
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- rpS6
- ribosomal protein S6
- PLCβ
- phospholipase C β
- 4E-BP1
- eIF4E-binding protein 1
- PI3K
- phosphatidylinositol 3-kinase
- PMA
- phorbol 12-myristate 13-acetate
- rpS6
- ribosomal S6 protein.
REFERENCES
- 1. Wilson B. A., Ho M. (2011) Cellular and molecular action of the mitogenic protein-deamidating toxin from Pasteurella multocida. FEBS J. 278, 4616–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pullinger G. D., Bevir T., Lax A. J. (2004) The Pasteurella multocida toxin is encoded within a lysogenic bacteriophage. Mol. Microbiol. 51, 255–269 [DOI] [PubMed] [Google Scholar]
- 3. Buys W. E., Smith H. E., Kamps A. M., Kamp E. M., Smits M. A. (1990) Sequence of the dermonecrotic toxin of Pasteurella multocida ssp. multocida. Nucleic Acids Res. 18, 2815–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kitadokoro K., Kamitani S., Miyazawa M., Hanajima-Ozawa M., Fukui A., Miyake M., Horiguchi Y. (2007) Crystal structures reveal a thiol protease-like catalytic triad in the C-terminal region of Pasteurella multocida toxin. Proc. Natl. Acad. Sci. U.S.A. 104, 5139–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rozengurt E., Higgins T., Chanter N., Lax A. J., Staddon J. M. (1990) Pasteurella multocida toxin. Potent mitogen for cultured fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 87, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lax A. J., Grigoriadis A. E. (2001) Pasteurella multocida toxin. The mitogenic toxin that stimulates signalling cascades to regulate growth and differentiation. Int. J. Med. Microbiol. 291, 261–268 [DOI] [PubMed] [Google Scholar]
- 7. Higgins T. E., Murphy A. C., Staddon J. M., Lax A. J., Rozengurt E. (1992) Pasteurella multocida toxin is a potent inducer of anchorage-independent cell growth. Proc. Natl. Acad. Sci. U.S.A. 89, 4240–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoskins I. C., Thomas L. H., Lax A. J. (1997) Nasal infection with Pasteurella multocida causes proliferation of bladder epithelium in gnotobiotic pigs. Vet. Rec. 140, 22. [DOI] [PubMed] [Google Scholar]
- 9. Aminova L. R., Wilson B. A. (2007) Calcineurin-independent inhibition of 3T3-L1 adipogenesis by Pasteurella multocida toxin. Suppression of Notch1, stabilization of β-catenin and pre-adipocyte factor 1. Cell Microbiol. 9, 2485–2496 [DOI] [PubMed] [Google Scholar]
- 10. Lax A. J. (2005) Opinion. Bacterial toxins and cancer. A case to answer? Nat. Rev. Microbiol. 3, 343–349 [DOI] [PubMed] [Google Scholar]
- 11. Orth J. H., Preuss I., Fester I., Schlosser A., Wilson B. A., Aktories K. (2009) Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc. Natl. Acad. Sci. U.S.A. 106, 7179–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamitani S., Ao S., Toshima H., Tachibana T., Hashimoto M., Kitadokoro K., Fukui-Miyazaki A., Abe H., Horiguchi Y. (2011) Enzymatic actions of Pasteurella multocida toxin detected by monoclonal antibodies recognizing the deamidated α subunit of the heterotrimeric GTPase Gq. FEBS J 278, 2702–2712 [DOI] [PubMed] [Google Scholar]
- 13. Staddon J. M., Chanter N., Lax A. J., Higgins T. E., Rozengurt E. (1990) Pasteurella multocida toxin, a potent mitogen, stimulates protein kinase C-dependent and -independent protein phosphorylation in Swiss 3T3 cells. J. Biol. Chem. 265, 11841–11848 [PubMed] [Google Scholar]
- 14. Staddon J. M., Barker C. J., Murphy A. C., Chanter N., Lax A. J., Michell R. H., Rozengurt E. (1991) Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-trisphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J. Biol. Chem. 266, 4840–4847 [PubMed] [Google Scholar]
- 15. Murphy A. C., Rozengurt E. (1992) Pasteurella multocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. Requirement for a functional G protein. J. Biol. Chem. 267, 25296–25303 [PubMed] [Google Scholar]
- 16. Seo B., Choy E. W., Maudsley S., Miller W. E., Wilson B. A., Luttrell L. M. (2000) Pasteurella multocida toxin stimulates mitogen-activated protein kinase via Gq/11-dependent transactivation of the epidermal growth factor receptor. J. Biol. Chem. 275, 2239–2245 [DOI] [PubMed] [Google Scholar]
- 17. Zywietz A., Gohla A., Schmelz M., Schultz G., Offermanns S. (2001) Pleiotropic effects of Pasteurella multocida toxin are mediated by Gq-dependent and -independent mechanisms. involvement of Gq but not G11. J. Biol. Chem. 276, 3840–3845 [DOI] [PubMed] [Google Scholar]
- 18. Wilson B. A., Aminova L. R., Ponferrada V. G., Ho M. (2000) Differential modulation and subsequent blockade of mitogenic signaling and cell cycle progression by Pasteurella multocida toxin. Infect. Immun. 68, 4531–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas W., Pullinger G. D., Lax A. J., Rozengurt E. (2001) Escherichia coli cytotoxic necrotizing factor and Pasteurella multocida toxin induce focal adhesion kinase autophosphorylation and Src association. Infect. Immun. 69, 5931–5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orth J. H., Lang S., Taniguchi M., Aktories K. (2005) Pasteurella multocida toxin-induced activation of RhoA is mediated via two families of Gα proteins, Gαq, and Gα12/13. J. Biol. Chem. 280, 36701–36707 [DOI] [PubMed] [Google Scholar]
- 21. Jacinto E. (2008) What controls TOR? IUBMB Life 60, 483–496 [DOI] [PubMed] [Google Scholar]
- 22. Ma X. M., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 23. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR. From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pullen N., Thomas G. (1997) The modular phosphorylation and activation of p70S6k. FEBS Lett. 410, 78–82 [DOI] [PubMed] [Google Scholar]
- 25. Choo A. Y., Yoon S. O., Kim S. G., Roux P. P., Blenis J. (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U.S.A. 105, 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zahm J. M., Kaplan H., Hérard A. L., Doriot F., Pierrot D., Somelette P., Puchelle E. (1997) Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil. Cytoskeleton 37, 33–43 [DOI] [PubMed] [Google Scholar]
- 27. Schieke S. M., Phillips D., McCoy J. P., Jr., Aponte A. M., Shen R. F., Balaban R. S., Finkel T. (2006) The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 281, 27643–27652 [DOI] [PubMed] [Google Scholar]
- 28. Jefferies H. B., Fumagalli S., Dennis P. B., Reinhard C., Pearson R. B., Thomas G. (1997) Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70S6k. EMBO J. 16, 3693–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsen A., Sypherd P. S. (1980) Physiological control of phosphorylation ribosomal protein S6 in Mucor racemosus. J. Bacteriol. 141, 20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gressner A. M., Wool I. G. (1974) The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J. Biol. Chem. 249, 6917–6925 [PubMed] [Google Scholar]
- 31. Brothers M. C., Ho M., Maharjan R., Clemons N. C., Bannai Y., Waites M. A., Faulkner M. J., Kuhlenschmidt T. B., Kuhlenschmidt M. S., Blanke S. R., Rienstra C. M., Wilson B. A. (2011) Membrane interaction of Pasteurella multocida toxin involves sphingomyelin. FEBS J. 278, 4633–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romeo Y., Zhang X., Roux P. P. (2012) Regulation and function of the RSK family of protein kinases. Biochem. J. 441, 553–569 [DOI] [PubMed] [Google Scholar]
- 34. Carling D. (2004) The AMP-activated protein kinase cascade. A unifying system for energy control. Trends Biochem. Sci. 29, 18–24 [DOI] [PubMed] [Google Scholar]
- 35. Inoki K., Zhu T., Guan K. L. (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 36. Kimura N., Tokunaga C., Dalal S., Richardson C., Yoshino K., Hara K., Kemp B. E., Witters L. A., Mimura O., Yonezawa K. (2003) A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8, 65–79 [DOI] [PubMed] [Google Scholar]
- 37. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 38. Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714 [DOI] [PubMed] [Google Scholar]
- 39. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 40. Hahn-Windgassen A., Nogueira V., Chen C. C., Skeen J. E., Sonenberg N., Hay N. (2005) Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 280, 32081–32089 [DOI] [PubMed] [Google Scholar]
- 41. Bublil E. M., Yarden Y. (2007) The EGF receptor family. Spearheading a merger of signaling and therapeutics. Curr. Opin. Cell Biol. 19, 124–134 [DOI] [PubMed] [Google Scholar]
- 42. Sabri A., Wilson B. A., Steinberg S. F. (2002) Dual actions of the Gαq agonist Pasteurella multocida toxin to promote cardiomyocyte hypertrophy and enhance apoptosis susceptibility. Circ. Res. 90, 850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duncan R., McConkey E. H. (1982) Preferential utilization of phosphorylated 40-S ribosomal subunits during initiation complex formation. Eur. J. Biochem. 123, 535–538 [DOI] [PubMed] [Google Scholar]
- 44. Schwab M. S., Kim S. H., Terada N., Edfjäll C., Kozma S. C., Thomas G., Maller J. L. (1999) p70(S6K) controls selective mRNA translation during oocyte maturation and early embryogenesis in Xenopus laevis. Mol. Cell Biol. 19, 2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bandi H. R., Ferrari S., Krieg J., Meyer H. E., Thomas G. (1993) Identification of 40 S ribosomal protein S6 phosphorylation sites in Swiss mouse 3T3 fibroblasts stimulated with serum. J. Biol. Chem. 268, 4530–4533 [PubMed] [Google Scholar]
- 46. Ruvinsky I., Sharon N., Lerer T., Cohen H., Stolovich-Rain M., Nir T., Dor Y., Zisman P., Meyuhas O. (2005) Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 19, 2199–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pende M., Um S. H., Mieulet V., Sticker M., Goss V. L., Mestan J., Mueller M., Fumagalli S., Kozma S. C., Thomas G. (2004) S6K1−/−/S6K2−/− mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell Biol. 24, 3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choo A. Y., Blenis J. (2009) Not all substrates are treated equally. Implications for mTOR, rapamycin resistance and cancer therapy. Cell Cycle 8, 567–572 [DOI] [PubMed] [Google Scholar]
- 49. Thoreen C. C., Sabatini D. M. (2009) Rapamycin inhibits mTORC1, but not completely. Autophagy 5, 725–726 [DOI] [PubMed] [Google Scholar]
- 50. Rhee S. G. (2001) Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orth J. H., Lang S., Aktories K. (2004) Action of Pasteurella multocida toxin depends on the helical domain of Gαq. J. Biol. Chem. 279, 34150–34155 [DOI] [PubMed] [Google Scholar]
- 52. Nishizuka Y. (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308, 693–698 [DOI] [PubMed] [Google Scholar]
- 53. Ozgen N., Obreztchikova M., Guo J., Elouardighi H., Dorn G. W., 2nd, Wilson B. A., Steinberg S. F. (2008) Protein kinase D links Gq-coupled receptors to cAMP response element-binding protein (CREB)-Ser133 phosphorylation in the heart. J. Biol. Chem. 283, 17009–17019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chung Y. W., Kim H. K., Kim I. Y., Yim M. B., Chock P. B. (2011) Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced manganese superoxide dismutase (MnSOD) expression. Activation of CREB and FOXO3a by PKC-α phosphorylation and by PKC-mediated inactivation of Akt, respectively. J. Biol. Chem. 286, 29681–29690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herbert T. P., Kilhams G. R., Batty I. H., Proud C. G. (2000) Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J. Biol. Chem. 275, 11249–11256 [DOI] [PubMed] [Google Scholar]
- 56. Ballif B. A., Roux P. P., Gerber S. A., MacKeigan J. P., Blenis J., Gygi S. P. (2005) Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc. Natl. Acad. Sci. U.S.A. 102, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]