Background: Studies suggest that lipolysis induces adipose tissue inflammation, commonly associated with type 2 diabetes.
Results: Activation of adipose-resident endothelium is required for β3-adrenergic receptor-mediated but not fasting-induced inflammation.
Conclusion: Both β3-adrenergic receptor stimulation and fasting induce adipose tissue inflammation, but by different mechanisms.
Significance: The study shows heterogeneity of immune cell dynamics in adipose tissue.
Keywords: Adipose Tissue, Adipose Tissue Metabolism, Adrenergic Receptor, Endothelial Cell, Inflammation, Lipolysis, E-Selectin
Abstract
Inflammation induced by wound healing or infection activates local vascular endothelial cells to mediate leukocyte rolling, adhesion, and extravasation by up-regulation of leukocyte adhesion molecules such as E-selectin and P-selectin. Obesity-associated adipose tissue inflammation has been suggested to cause insulin resistance, but weight loss and lipolysis also promote adipose tissue immune responses. While leukocyte-endothelial interactions are required for obesity-induced inflammation of adipose tissue, it is not known whether lipolysis-induced inflammation requires activation of endothelial cells. Here, we show that β3-adrenergic receptor stimulation by CL 316,243 promotes adipose tissue neutrophil infiltration in wild type and P-selectin-null mice but not in E-selectin-null mice. Increased expression of adipose tissue cytokines IL-1β, CCL2, and TNF-α in response to CL 316,243 administration is also dependent upon E-selectin but not P-selectin. In contrast, fasting increases adipose-resident macrophages but not neutrophils, and does not activate adipose-resident endothelium. Thus, two models of lipolysis-induced inflammation induce distinct immune cell populations within adipose tissue and exhibit distinct dependences on endothelial activation. Importantly, our results indicate that β3-adrenergic stimulation acts through up-regulation of E-selectin in adipose tissue endothelial cells to induce neutrophil infiltration.
Introduction
Adipose tissue is a dynamic organ that responds to nutritional cues by synthesizing and storing excess energy in the form of triglycerides during times of abundance, or releasing energy in times of need. Lipolysis, the hydrolysis of adipocyte triglyceride to glycerol and fatty acids, occurs in response to β-adrenergic stimulation by catecholamines during fasting. Conversely, in the fed state, lipolysis is inhibited by insulin, which stimulates lipogenesis (1). Lipolysis is controlled in adipocytes by three main lipases, adipose triglyceride lipase (ATGL),2 hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL) (2–5). Stimulation of β-adrenergic receptors activates adenylyl cyclase, which increases cAMP and activates cAMP-dependent protein kinase A (PKA). PKA phosphorylates the coactivator for ATGL, comparative gene identification-58 (CGI-58) in addition to HSL and the lipid droplet protein perilipin, thus enhancing triglyceride hydrolysis (6–8). This process allows the stored lipid within the adipocyte to be used by other tissues during times of reduced nutrients for such processes as beta-oxidation and adaptive thermogenesis (1, 2).
Adipose tissue homeostasis is maintained in a complex manner through interactions with adipocytes, endothelial cells, immune cells, and others. In obesity, insulin resistance may cause an increase in basal lipolysis associated with an immune response in adipose tissue. Such adipose tissue inflammation is thought to promote systemic glucose intolerance (1). Previous studies have suggested that a similar adipose inflammatory response occurs during weight loss or fasting, states that are also associated with increased adipose tissue lipolysis, as a consequence of β-adrenergic stimulation (9, 10). However, these latter conditions are not associated with glucose intolerance and insulin resistance, raising an interesting paradox.
It is appreciated that inflammatory responses require vascular endothelial cell activation, which increases expression of leukocyte adhesion molecules that are necessary for leukocyte extravasation from the vasculature to areas of inflammation (11, 12). Endothelial cells can become activated in response to lipid stimuli, and this activation model is accepted in the context of atherosclerosis development (13); however, the role that adipose-resident endothelial cells play in recruitment of leukocytes to adipose tissue upon lipolysis is not well understood. Some studies suggest that endothelial-leukocyte interactions are required for adipose tissue inflammation (14–16); however, whether these interactions are mediated by adipose tissue lipolysis is not known.
Here, two model systems of lipolysis induction were utilized; systemic treatment with the β3-adrenergic receptor agonist CL 316,243 or fasting to determine the role of adipose-resident endothelial cell function in the adipose tissue immune response that ensued. Although fasting increased macrophage accumulation within adipose tissue, we surprisingly found that chemical induction of lipolysis caused a robust neutrophil infiltration into adipose tissue. Although fasting did not activate endothelial cells, β3-adrenergic receptor activation-mediated lipolysis robustly increased expression of endothelial leukocyte adhesion molecules intercellular adhesion molecule-1 (ICAM-1), E-selectin, and P-selectin in adipose-resident endothelium. Furthermore, E-selectin was required to mediate the β3-adrenergic receptor activation-induced adipose tissue immune response. Thus, β3-adrenergic receptor-activated lipolysis, but not fasting-induced lipolysis, is associated with an immune response in adipose tissue that correlates with and is mediated by endothelial cell activation.
EXPERIMENTAL PROCEDURES
Animals
Wild type C57Bl/6J animals, B6.129S4-Seletm1Dmil/J and B6.129S7-Selptm1Bay/J animals were purchased from Jackson Laboratories. E-selectin (E-sel) and P-selectin (P-sel) knock out (KO) animals were maintained by homo- and heterozygote breeding at the UMASS animal facility. Male animals were used between 8–12 weeks of age for all of the studies. Mice were injected with 0.5 or 1 mg/kg CL 316,243 (Santa Cruz Biotechnology) or PBS intraperitoneally for the indicated times. Fasting studies were performed after acclimation of the mice to paper bedding for at least 5 days. Mice were fasted for 24 h. The University of Massachusetts Medical School Institutional Animal Care and Use Committee approved all of the animal procedures.
Cell Culture
Human umbilical vein endothelial cell (HUVEC)s (Clonetics) were maintained in EGM-2 media (Lonza) at 37 °C and 5% CO2. Cells were treated with PBS, the indicated amounts of CL 316,243, or 10 ng/ml tumor necrosis factor (TNF)α (Calbiochem) for 6 h.
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated from whole epididymal adipose tissue or HUVECs following the manufacturer's protocol (TriPure, Roche). Precipitated RNA was treated with DNase (DNA-free, Invitrogen) prior to reverse transcription (iScript Reverse transcriptase, Bio-Rad). SYBR green quantitative PCR (iQ SYBR green supermix, Bio-Rad) was performed on the Bio-Rad CFX97. Mice were injected for 30 min, 2 h, 4 h, 6 h, 16 h, or 24 h as indicated. The data obtained from the CL 316,243-injected mice were normalized to that from the PBS-injected mice at each time point to control for injection-induced inflammation. Primer sequences are as follows: Itgam: 5′-ATGGACGCTGATGGCAATACC-3′, 3′-TCCCCATTCACGTCTCCCA-5′; CD68: 5′-CCATCCTTCACGATGACACCT-3′, 3′- GGCAGGGTTATGAGTGACAGTT-5′; Itgax: 5′-CTGGATAGCCTTTCTTCTGCTG-3′, 3′-GCACACTGTGTCCGAACTCA-5′; EMR1: 5′-CCCCAGTGTCCTTACAGAGTG-3′, 3′-GTGCCCAGAGTGGATGTCT-5′; IL1β: 5′-GCAACTGTTCCTGAACTCAACT-3′, 3′-ATCTTTTGGGGTCCGTCAACT-5′; CCL2: 5′-TTAAAAACCTGGATCGGAACCAA-3′, 3′-GCATTAGCTTCAGATTTACGGGT-5′; TNFα: 5′-CAGGCGGTGCCTATGTCTC-3′, 3′-CGATCACCCCGAAGTTCAGTAG-5′; IL-6: 5′-TAGTCCTTCCTACCCCAATTTCC-3′, 3′-TTGGTCCTTAGCCACTCCTTC-5′; ICAM-1: 5′-GTGATGCTCAGGTATCCATCCA-3′, 3′-CACAGTTCTCAAAGCACAGCG-5′; VCAM-1: 5′-AGTTGGGGATTCGGTTGTTCT-3′, 3′-CCCCTCATTCCTTACCACCC-5′; Sele: 5′-ATGAAGCCAGTGCATACTGTC-3′, 3′-CGGTGAATGTTTCAGATTGGAGT-5′; Selp: 5′-CATCTGGTTCAGTGCTTTGATCT-3′, 3′-ACCCGTGAGTTATTCCATGAGT-5′; 36B4: 5′-TCCAGGCTTTGGGCATCA-3′, 3′-CTTTATCAGCTGCACATCACTCAGA-5′; ICAM-1 (human): 5′-TCTGTGTCCCCCTCAAAAGTC-3′, 3′-GGGGTCTCTATGCCCAACAA-5′; VCAM-1 (human): 5′-ATGCCTGGGAAGATGGTCG-3′, 3′-GACGGAGTCACCAATCTGAGC-5′ Sele (human): 5′-GATGAGAGGTGCAGCAAGAAG-3′, 3′-CTCACACTTGAGTCCACTGAAG-5′; RPLP0: 5′-CAGATTGGCTACCCAACTGTT-3′, 3′-GGGAAGGTGTAATCCGTCTCC-5′.
Histology
Epididymal adipose tissue was fixed in 10% formalin, paraffin embedded, and stained with hematoxylin and eosin (H&E). Photos were taken with an Axiovert 35 Zeiss microscope (Zeiss, Germany) equipped with an Axiocam CCl camera at 50× or 100× magnification.
Non-esterified Fatty Acid (NEFA) Measurements
EDTA plasma was collected from the retro-orbital sinus after isoflurane anesthesia. NEFA were measured with a colorimetric assay (Wako) using the manual procedure according to the manufacturer's instructions.
Flow Cytometry
The epididymal adipose tissue stromal vascular fraction (SVF) was isolated by digestion in Hanks balanced salt solution, 2.5% BSA and 2 mg/ml collagenase for 45 min and strained through a 70 μm filter followed by red blood cell lysis. Cells were blocked with mouse IgG in FACS buffer (1% BSA/PBS). Cells were stained with antibodies directed toward F4/80 (APC, ABd serotec), CD11b (Percp 5.5, BD), Siglec F (PE, BD), GR-1 (APC-Cy7, BD), Ly6c (PE-Cy7, BD), Galectin 3 (FITC, BioLegend), and CD11c (V450, BD). The data were collected on an LSRII (BD) and were analyzed with FlowJo software. Samples were gated for scatter and single cells. Gates were drawn based on fluorescence minus one (FMO) controls. A total of 100,000 events were recorded.
RESULTS
β-Adrenergic Stimulation in Vivo Induces Adipose Tissue Inflammation
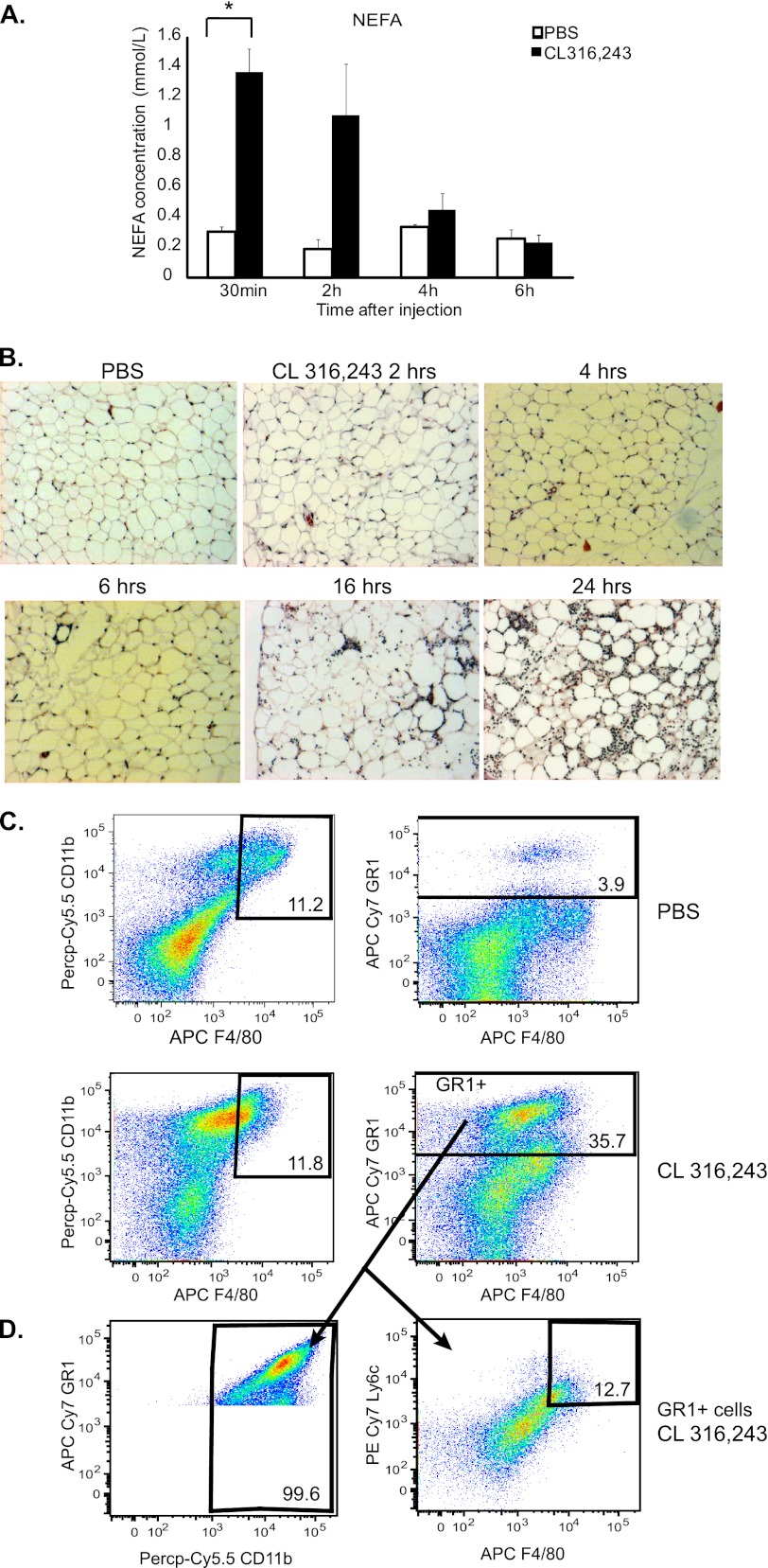
To study the effects of lipolysis on adipose tissue inflammation, we utilized a β3-adrenergic receptor agonist that has been previously documented to robustly induce lipolysis and hence, adipose tissue inflammation, CL 316,243 (CL) (9, 10, 17, 18). 8–12-week old lean male C57Bl/6J mice were injected intraperitoneally once with PBS or 0.5 mg/kg CL 316,243 and plasma and whole epididymal adipose tissue were isolated after 30 min or 2, 4, 6, 16, and 24 h of treatment. As expected, CL 316,243 treatment acutely increased serum non-esterified fatty acids (NEFA) by a maximum of 4-fold at the 30-min time point (Fig. 1A). Adipose tissue samples from these same mice were obtained for histology and the slides stained with H&E. As expected, an increase in adipose tissue inflammatory cells was observed in the CL 316,243-treated mice compared with the PBS-treated mice that peaked between 16 and 24 h after treatment (Fig. 1B).
FIGURE 1.
CL 316,243 administration induces lipolysis and neutrophil accumulation in adipose tissue. Mice were injected with 0.5 mg/kg CL316,243 or PBS intraperitoneally and adipose tissue was harvested 30 min to 24 h later as indicated. A, plasma was drawn from the retroorbital sinus and nonesterified fatty acids (NEFA) were measured (n = 3, *; p < 0.05). B, visceral adipose tissue was isolated, fixed in 10% formalin, embedded in paraffin, and stained with H&E. Slides are representative of 3–6 animals at 100× magnification. C, mice were injected with 1 mg/kg CL 316,243 or PBS. After 18 h, adipose tissue SVF was isolated and FACS analysis was performed. Upper panels, PBS-treated animals. Lower panels, CL-treated animals. Left panels, Siglec F-negative, CD11b- and F4/80-positive cells. Right panels, GR-1-positive cells. Panels are representative of 6–11 animals per group. D, GR-1 positive cells (from 1C, lower right panel) were stained additionally for CD11b (left), Ly6c and F4/80 (right). Panels are representative of 6–11 animals per group.
To identify what cell types were infiltrating into the adipose tissue upon CL 316,243 treatment, we isolated the adipose tissue stromal vascular fraction (SVF) from animals that had been injected with PBS or 1 mg/kg CL 316,243 for 18 h and characterized these cells with flow cytometry. Macrophages were identified as cells that were positive for CD11b and F4/80 antigen, and negative for the eosinophil marker Siglec F (19, 20). In the PBS-treated mice, ∼12% of Siglec F-negative SVF cells were positive for both CD11b and F4/80. Interestingly, no increase in these CD11b & F4/80 double positive cells after CL 316,243 treatment was observed, as was previously reported (Fig. 1C) (9). However, there was a 65% increase in CD11b-positive cells after CL 316,243 treatment (39% in PBS-treated mice versus 64% in CL 316,243-treated mice), which was statistically significant (p < 0.005, data not shown) (Fig. 1C).
Multiple types of immune cells are present within adipose tissue (19, 21–26). In an acute immune response, neutrophil infiltration precedes that of monocytes and macrophages (27). Thus, we hypothesized that these CD11b-positive cells that had infiltrated into the CL 316,243-treated adipose tissue may be of the granulocyte lineage. To assess this, the cells were stained for GR-1, a granulocyte marker that is present on neutrophils. CL 316,243 treatment increased GR-1-positive cells in adipose tissue SVF nearly 10-fold over PBS injection alone (Fig. 1C). These GR-1-positive cells highly expressed CD11b and did not express high levels of F4/80 or Ly6c (Fig. 1D), indicating that they are likely neutrophils rather than myeloid-derived suppressor cells (28). Thus, CL 316,243 administration intraperitoneally is associated with acute adipose tissue inflammation and neutrophil infiltration.
Increased Expression of Cytokines, but Not Macrophage Marker Genes, in Adipose Tissue after CL 316,243 Treatment
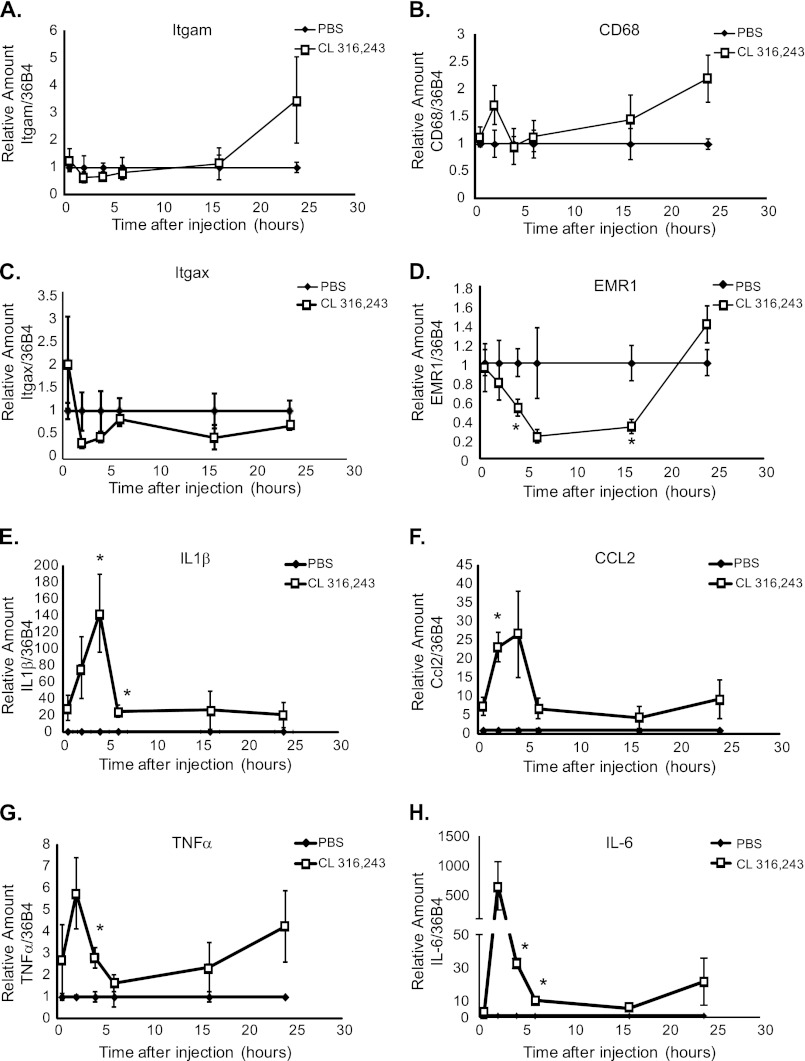
To further characterize the nature of this immune response, RNA was isolated from mice that had been injected intraperitoneally with PBS or 0.5 mg/kg CL 316,243 after 0.5, 2, 4, 6, 16, or 24 h, and the expression of several inflammatory genes was measured by qRT-PCR. Gene expression was normalized to 36B4, and the gene expression in the CL 316,243-treated adipose tissue was compared with that in the PBS-treated mice for each time point. Because CL 316,243 treatment is associated with increased adipose tissue inflammation, macrophage marker genes as well as the expression of several inflammatory cytokines were measured. Consistent with the 65% increase in CD11b-positive cells by FACS (data not shown and Fig. 1C) after CL 316,243 treatment, a 3.47-fold increase in Itgam, which encodes CD11b, was noted 24 h after CL 316,243 treatment (Fig. 2A). A gradual increase in the mRNA levels of an additional leukocyte marker, CD68 was also observed, which peaked at a 2-fold increase in the CL 316,243-treated mice after 24 h (Fig. 2B).
FIGURE 2.
Temporal regulation of inflammatory cell gene expression by CL 316,243. Mice were injected with 0.5 mg/kg CL 316,243 or PBS intraperitoneally for 30 min to 24 h as indicated. Visceral adipose tissue was isolated, RNA was extracted and quantitative RT-PCR was performed for A, Itgam (CD11b), B, CD68, C, Itgax (CD11c), D, EMR1 (F4/80), E, Il1β, F, CCL2 (MCP-1), G, TNFα, and H, IL-6. Data represent average gene expression as compared with 36b4 ± S.E. (n = 3–7, *; p < 0.05).
Although it has been previously documented that CL 316,243 treatment causes adipose tissue macrophage infiltration (9, 10), no change in Itgax mRNA levels, which encodes CD11c protein, could be detected during the CL 316,243 treatment time course (Fig. 2C). Furthermore, an acute 50% reduction in EMR1 expression (Fig. 2D), which encodes the macrophage F4/80 protein, was observed after CL 316,243 treatment compared with PBS-treated mice. These results are consistent with the notion that the CL 316,243-induced infiltrating cells are not macrophages.
Large increases were also noted in the mRNA levels of several inflammatory cytokines within adipose tissue acutely after CL 316,243 treatment. Interleukin (IL)-1β mRNA levels were increased by 140-fold and CCL-2/MCP-1 mRNA levels were increased by 30-fold in CL 316,243-treated compared with PBS-treated adipose tissue. The expression levels of these cytokines peaked 4 h after CL 316,243 treatment, and remained increased for at least 24 h (Fig. 2, E and F).
Cytokines TNFα and IL-6 were also induced in adipose tissue by CL 316,243 treatment, by 6- and 600-fold, respectively (Fig. 2, G and H). The expression of these cytokines peaked two hours after CL 316,243 treatment and also remained elevated over 24 h. Interestingly, the up-regulation of these cytokines within the adipose tissue occurred at a time point that was prior to leukocyte infiltration (Fig. 1B), which suggests that these cytokines may be secreted from adipose-resident cells rather than infiltrating cells. Thus, the up-regulation of these cytokines may be important for induction of leukocyte infiltration into adipose tissue in response to β3-adrenergic receptor activation.
β3-Adrenergic Stimulation Does Not Up-regulate Leukocyte Adhesion Molecules in Endothelial Cells in Vitro
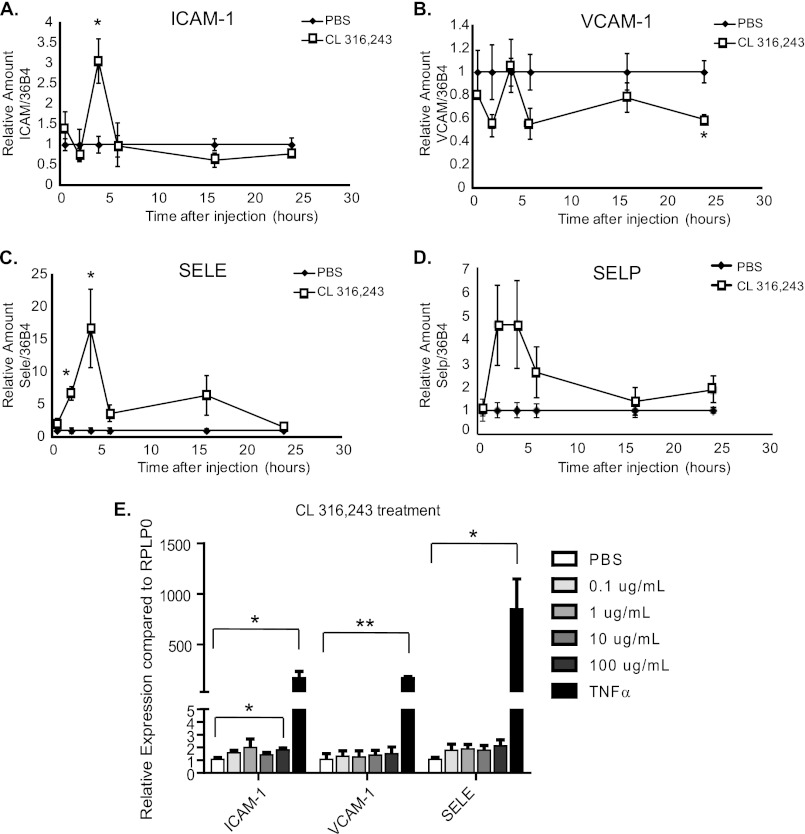
We hypothesized that the immune cells that were infiltrating adipose tissue after CL 316,243 treatment derived from the circulation, and if this were the case, that these immune cells were being recruited to the adipose tissue through activation of adipose-resident endothelium. Inflammatory stimuli cause transcriptional up-regulation of endothelial genes ICAM-1, vascular-cell adhesion molecule (VCAM)-1, E-selectin, and P-selectin, and this up-regulation is necessary for leukocyte recruitment into the inflamed tissue (11, 29). Indeed, CL 316,243-treated mice displayed an increase in the mRNA expression of these genes compared with PBS-treated mice. In the CL 316,243-treated mice, there was a 3-fold increase in ICAM-1 expression, a 17-fold increase in E-selectin expression, and a 4.5-fold increase in P-selectin expression that peaked 4 h after CL 316,243 treatment (Fig. 3, A–D). This peak in the expression of these leukocyte adhesion molecule genes preceded the immune cell infiltration into adipose tissue (Fig. 1), suggesting that up-regulation of these molecules may be necessary for lipolysis-induced leukocyte infiltration. However, VCAM-1 expression was not induced after CL 316,243 treatment (Fig. 3B). As VCAM-1 is important for firm adhesion of monocytes/macrophages and T cells to the vessel wall, rather than neutrophils (12), it is not surprising that this adhesion molecule was not up-regulated, as macrophages were not the primary adipose tissue infiltrating cell type (Figs. 1 and 2).
FIGURE 3.
Endothelial cell activation by β3-adrenergic stimulation of adipose tissue in vivo, but not in vitro CL 316,243 treatment. Mice were injected with 0.5 mg/kg CL 316,243 or PBS intraperitoneally for 30 min to 24 h as indicated. Visceral adipose tissue was isolated, mRNA was extracted, and qRT-PCR was performed for A, Icam-1, B, Vcam-1, C, Sele (E-selectin), and D, Selp (P-selectin). Data represent average gene expression as compared with 36b4 ± S.E. (n = 3–10, *; p < 0.05). E, human umbilical vein endothelial cells (HUVEC) were treated for 6 h with various doses of CL316,243 or 10 ng/ml TNFα. mRNA was extracted and qRT-PCR was performed for ICAM-1, VCAM-1, and SELE (E-selectin). Data represent average gene expression as compared with RPLP0 ± S.E. (n = 3–5, *; p < 0.05, **; p < 0.005).
To test whether endothelial cells could be directly activated by CL 316,243 in the absence of other cell types, primary endothelial cells derived from human umbilical veins (HUVECs) were treated with increasing doses of CL 316,243 for 6 h, at which time RNA was extracted and qRT-PCR was performed for ICAM-1, VCAM-1, and E-selectin. Importantly, no up-regulation of the adhesion molecules was noted after CL 316,243 treatment at doses that are comparable to the in vivo dose employed (0.1–1 μg/ml), and only at the highest dose of CL 316,243 treatment (100 μg/ml) was a very small up-regulation of ICAM-1 expression noted. In contrast, stimulation of HUVECs with TNFα, a known activator of endothelial cells, caused significant up-regulation of ICAM-1, VCAM-1, and E selectin (Fig. 3E). We also performed similar experiments in which HUVECs were treated with 1 μg/ml CL 316,243 in combination with 100 μm palmitate or oleate for 4 h, and we found that these fatty acids were not sufficient to up-regulate leukocyte adhesion molecule expression in the presence or absence of CL 316,243 (data not shown). These results suggest that CL 316,243 treatment itself causes little or no endothelial cell activation, but suggest that other adipose-resident cells activate the endothelial cells in a paracrine manner.
Differences between Fasting-induced and CL 316,243-induced Adipose Tissue Inflammation
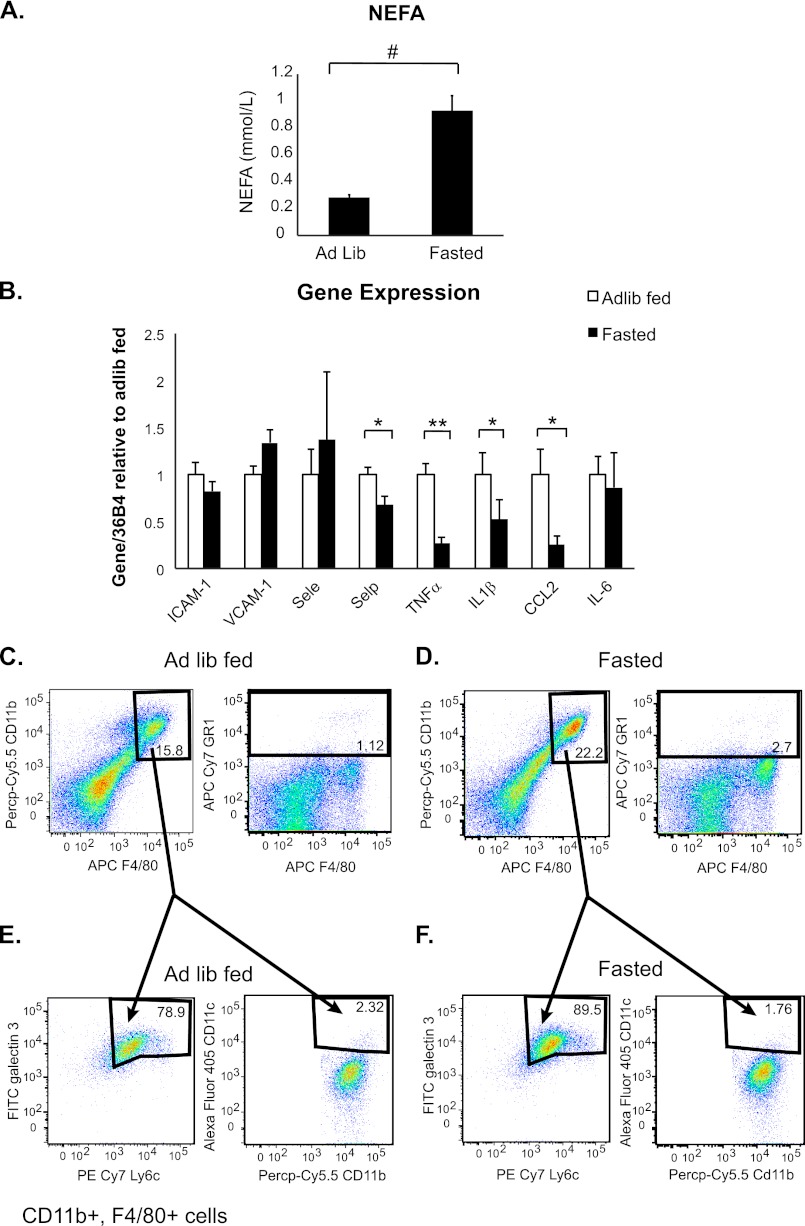
The results obtained after CL 316,243 injection suggested that the adipose tissue lipolysis that ensues upon β3-adrenergic stimulation causes adipose tissue inflammation and endothelial cell activation. To test this hypothesis, we extended our study to a different physiological system that would also increase adipocyte lipolysis. Recent studies have suggested that extended fasting increases adipose tissue macrophage infiltration because of enhanced lipolysis (9, 30); therefore, we subjected lean 8–12 week old C57BL/6J mice to a 24-h fast, isolated plasma, and assessed NEFA levels as an indication of lipolysis. As expected, a 3-fold increase in plasma NEFA was observed in the fasted animals compared with those that had been ad lib fed (Fig. 4A). Furthermore, plasma NEFA levels in the fasted animals were comparable to those seen in the mice 30 min after injection with 0.5 mg/kg CL 316,243 (Fig. 1A).
FIGURE 4.
Fasting-induced lipolysis causes macrophage infiltration and reduces cytokine expression in adipose tissue. Mice were fed ad libidum or fasted as indicated. A, plasma was drawn from the retroorbital sinus and NEFA were measured (n = 6, #; p < 0.0005). B, visceral adipose tissue was isolated, RNA was extracted, and quantitative RT-PCR was performed for the indicated genes. Data represent average gene expression as compared with 36b4 ± S.E. (n = 6–7, *; p < 0.05, **; p < 0.005). C–F, adipose tissue SVF was isolated and FACS analysis was performed. C, ad lib fed animals. D, fasted animals. Left panels, Siglec F-negative, CD11b- and F4/80-positive cells (p < 0.005). Right panels, GR-1-positive cells. Panels are representative of 9–11 animals per group. E and F, CD11b, F4/80-positive cells from C-D were stained additionally for Galectin 3, Ly6c, and CD11c. E, ad lib fed animals. F, fasted animals. Left, Galectin 3, Ly6c-positive cells. Right, CD11b, CD11c-positive cells. Panels are representative of 9–11 animals per group.
Next mRNA was extracted from visceral adipose tissue of the fed and fasted animals and qRT-PCR was performed for the same inflammatory genes measured after CL 316,243 treatment (Figs. 2 and 3). Surprisingly, no correlation of gene expression between CL 316,243-induced inflammation and fasting-induced inflammation was observed. Though significant up-regulation of ICAM-1 and E selectin after CL 316,243 treatment was noted, no up-regulation of either of these genes in adipose tissue after fasting (Fig. 4B) could be detected. Furthermore, though we had observed several-fold increases in TNFα, IL1β, CCL-2, and IL-6 after CL 316,243 treatment (Fig. 2, E–H), fasting actually induced a significant reduction in TNFα, IL1β, and CCL-2 mRNA levels (Fig. 4B), consistent with a previous report (30).
Finally, we assessed what types of cells were infiltrating adipose tissue after fasting. Mice were fasted for 24 h, SVF was isolated from the epididymal adipose tissue and flow cytometry was performed. In contrast to CL 316,243 treatment, a 50% increase (15% to 22%, p = 0.002) in macrophage cells that were Siglec F negative, CD11b and F4/80 positive was observed in fasted animals. Furthermore, fasting did not induce a GR1-positive cell population (Fig. 4D). The infiltrated cells in both ad lib fed and fasted animals expressed high levels of Ly6c and Galectin 3 and did not express CD11c (Fig. 4, E and F), suggesting that this cell population is an M2-like “anti-inflammatory” cell population (23) and is similar to the type of macrophages that normally reside in lean adipose tissue, which is consistent with a previous report (30). Therefore, although fasting and CL 316,243 treatment each induce adipose tissue lipolysis, and thus, plasma NEFA levels to a similar extent (Figs. 1A and 4A), these two model systems induce different adipose tissue phenotypes. CL 316,243 treatment induces a more inflammatory, neutrophil-mediated infiltration, whereas fasting reduces inflammatory cytokine expression while increasing M2-type macrophages in adipose tissue.
E-selectin Is Required for β3-Adrenergic Receptor-mediated Inflammation
E- and P-selectin are required for leukocyte recruitment to sites of inflammation in animal models, and are necessary for neutrophil rolling along vessels within inflamed tissue. It has also been demonstrated that E- and P-selectin have redundant and unique functions (12, 31–34). E-selectin expression is low until it is transcriptionally up-regulated in response to stimuli. Conversely, P-selectin is not as transcriptionally regulated, and is stored preformed in Weibel-Palade bodies within the endothelial cell (11). We hypothesized that because neutrophil infiltration was associated with CL 316,243-mediated inflammation, and E-selectin was the leukocyte adhesion molecule that was most highly induced by CL 316,243 treatment in adipose tissue, that E-selectin would be required for CL 316,243-induced adipose tissue inflammation. However, though it was not transcriptionally up-regulated to the same extent as E-selectin, we could not exclude the possibility that P-selectin may also be important for CL 316,243-induced neutrophil recruitment to adipose tissue. Conversely, because none of the leukocyte adhesion molecules were induced by fasting, and neutrophil infiltration was not associated with fasting-induced inflammation, we hypothesized that neither E- nor P-selectin would be required for fasting-induced inflammation.
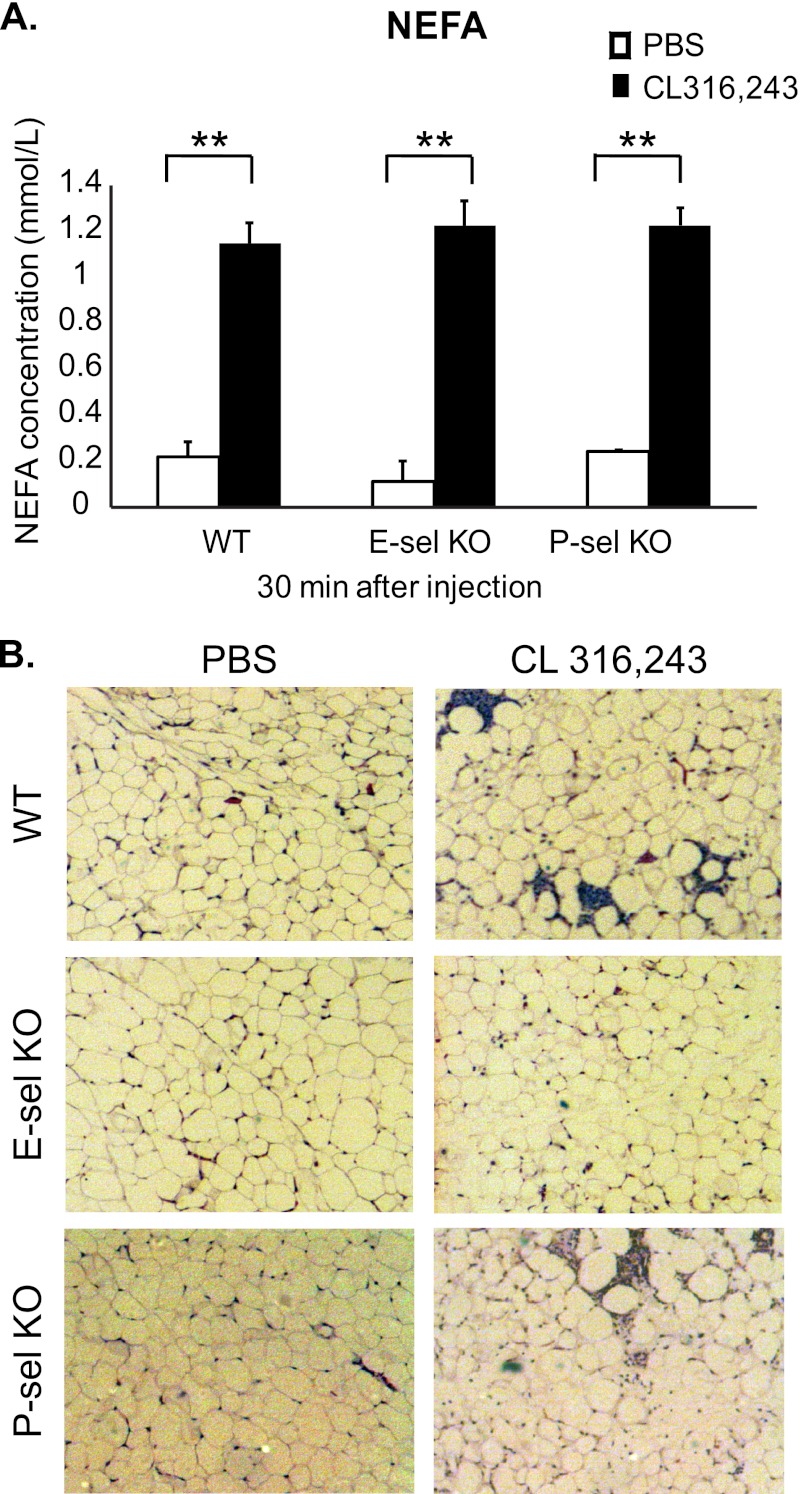
To test this hypothesis, we utilized mice lacking E-selectin (E-sel KO) or P-selectin (P-sel KO), which have been extensively described (31–33, 35). These mice were injected with 0.5 mg/kg CL 316,243 or PBS, and plasma was collected for NEFA analysis. Importantly, 30 min following injection of 0.5 mg/kg CL 316,243, NEFA levels were increased 4–5 fold in the plasma of E-sel and P-sel KO mice, similar to wild type animals (Fig. 5A), suggesting that β3-adrenergic receptor stimulation-mediated lipolysis occurs normally in these animals.
FIGURE 5.
E-selectin KO mice are protected from CL 316,243-mediated adipose tissue immune cell infiltration. Wild type, E-sel KO, or P-sel KO mice were injected with 0.5 mg/kg CL 316,243 or PBS intraperitoneally for 30 min to 24 h as indicated. A, plasma was drawn from the retroorbital sinus and NEFA were measured (n = 3 - 7, **; p < 0.005). B, visceral adipose tissue was isolated, fixed in 10% formalin, embedded in paraffin, and stained with H&E. Slides are representative of 3–6 animals at 50× magnification.
Because maximal leukocyte infiltration of adipose tissue is between 16–24 h after CL 316,243 injection, tissues were isolated from these animals 18 h post-injection. Visceral adipose tissue was excised from the injected mice and histological sections were prepared and stained with H&E. No obvious histological changes in the adipose tissue from either genotype that had been injected with PBS were detected. As expected, enhanced immune cell infiltration in wild type animals was observed. In P-sel KO mice, adipose tissue inflammation was induced to a similar extent as in the wild type mice (Fig. 5B). However, the E-sel KO mice were remarkably protected from the leukocyte infiltration that ensued upon CL 316,243 injection, thus suggesting that E-selectin is required for β3-adrenergic receptor-induced neutrophil infiltration (Fig. 5B).
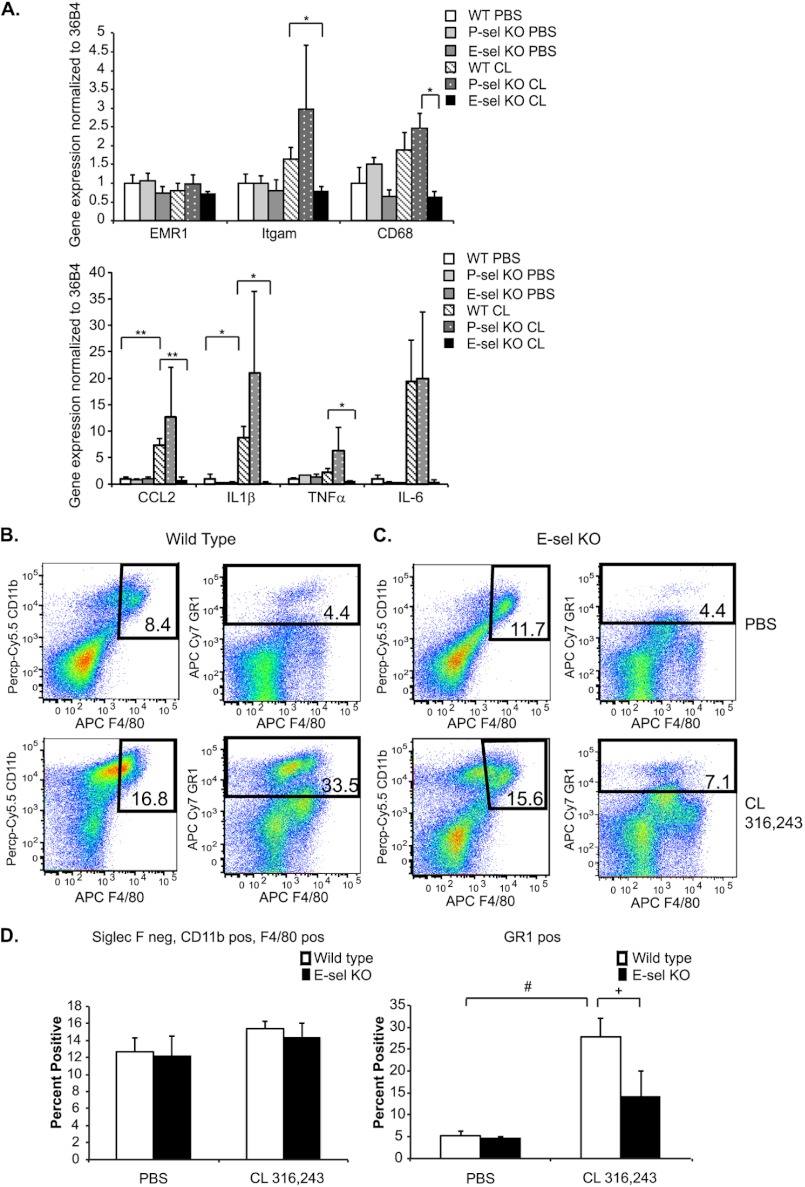
We next determined whether E-selectin was also required for CL 316,243-induced inflammatory gene expression in visceral adipose tissue. To assess this, mRNA was extracted from visceral adipose tissue 18 h after injection with PBS or 0.5 mg/kg CL 316,243, and real time qRT-PCR was performed on several inflammatory genes. EMR1 (F4/80) mRNA levels exhibited no increase after CL 316,243 treatment in any of the genotypes, which correlates with the notion that the infiltrating cells in this model are not macrophages. However, there was a 60% increase in Itgam (CD11b) expression in wild type animals after CL treatment that was not seen in the E-sel KO animals. CL injection increased CD68 expression in wild type and P-sel KO mice by 88% and 2-fold, respectively. However, E-sel KO mice were refractory to this increase and also displayed a significant reduction in CD68 expression after CL injection compared with P-sel KO animals (Fig. 6A).
FIGURE 6.
E-selectin KO mice are protected from CL 316,243-mediated adipose tissue inflammation. A, wild type, E-sel KO, or P-sel KO mice were injected with 0.5 mg/kg CL 316,243 or PBS intraperitoneally for 18 h. Visceral adipose tissue was isolated, mRNA was extracted, and qRT-PCR was performed for the indicated genes. Data represent average gene expression as compared with 36b4 ± S.E. (n = 3–9, *; p < 0.05, **; p < 0.005). B–D, wild type or E-sel KO mice were injected with 1 mg/kg CL 316,243 or PBS for 18 h. Adipose tissue SVF was isolated and FACS analysis was performed. B, wild type mice. C, E-sel KO mice. Upper panels, PBS-treated animals. Lower panels, CL-treated animals. Left panels, Siglec F-negative, CD11b and F4/80-positive cells. Right panels, GR-1-positive cells. Panels are representative of 5–14 animals per group. D, quantitation of B-C. Left panel, Siglec F-negative, CD11b and F4/80-positive cells. Right panel, GR-1-positive cells. (n = 5–14, +; p = 0.06, #; p < 0.0005).
Inflammatory cytokine expression was induced (CCL-2, 7-fold; IL1β, 9-fold; TNFα, 2-fold; and IL-6, 19-fold) after CL 316,243 treatment in wild type mice, with equivalent or enhanced induction of these genes in P-sel KO mice. However, E-sel KO mice were completely protected from the increased adipose tissue inflammatory cytokine response that ensued after CL 316,243 injection. Furthermore, significant reductions in CCL-2, IL1β, and TNFα were seen in CL 316,243-injected E-sel KO mice compared with wild type mice (Fig. 6A).
Finally, we evaluated whether E-sel KO mice were protected from neutrophil infiltration that ensued after CL 316,243 injection. To test this hypothesis, wild type and E-sel KO mice were injected with 1 mg/kg CL 316,243, and visceral adipose tissue was isolated 18 h later to obtain the SVF for FACS analysis. Though a 5-fold, significant increase was noted in GR1-positive cells in wild type animals after CL 316,243 injection, no significant increase in GR1-positive cells was found in E-sel KO mice (Fig. 6, B–D). Furthermore, there were no differences in fasting-induced accumulation of Siglec F negative, CD11b- and F4/80-positive cells in E-sel or P-sel KO mice compared with wild type mice as measured by flow cytometry (data not shown). These data suggest that E-selectin, but not P-selectin, is required for the adipose tissue inflammation that ensues upon β3-adrenergic receptor stimulation, which is mediated by neutrophil recruitment. This is in contrast to fasting-induced adipose tissue inflammation, which is mediated by macrophages.
DISCUSSION
Much effort has been expended in recent years to understand the immune response that ensues in adipose tissue in obesity. Though many hypotheses have been brought forth to explain why this immune response occurs, recent studies suggest that lipids released by adipose tissue lipolysis may mediate this response (9, 10). To that end, increased adipose tissue leukocyte infiltration was observed in response to either fasting or β-adrenergic stimuli that cause lipid release from adipocytes (9, 10). The findings we present here confirm that immune cell infiltration into adipose tissue occurs in both chemical and fasting-induced models of lipolysis. Importantly, however, our studies revealed that these two modes of stimulation initiate quite different immune responses.
First, whereas CL 316,243-mediated inflammation is associated with neutrophil recruitment (Fig. 1), fasting-mediated inflammation is associated with macrophage accumulation (Fig. 4). These data are in contrast to a previous report that suggested that the infiltrating cells in both scenarios were macrophages (9). Second, CL 316,243-mediated inflammation is associated with acute increases in inflammatory cytokines (Figs. 2 and 6), while fasting-induced inflammation is associated with decreases in these same cytokines (Fig. 4). Third, and most strikingly, the CL 316,243-mediated immune response is associated with increased adipose-resident endothelial cell leukocyte adhesion molecule up-regulation, and requires E-selectin (Figs. 3, 5, 6). Conversely, fasting-induced inflammation did not cause adhesion molecule up-regulation, and did not require E-selectin (Fig. 4). Thus, the data presented herein suggest that though lipolysis triggers adipose tissue immune cell infiltration, there must be other factors in addition to lipolysis that determines what type of inflammation will ensue.
In immune responses, there is an ordered pattern to leukocyte rolling within the blood vessel and transmigration into inflamed tissues (12). Indeed, leukocyte rolling velocity is decreased in adipose tissue from obese mice (14). Furthermore, recent studies have suggested that adipose tissue inflammation in obesity is mediated by leukocyte recruitment from the blood (36). Leukocyte adhesion molecules E- and P- selectin mediate neutrophil rolling along blood vessels (11, 12), which slows down the leukocytes and allows them to firmly adhere, a process mediated by ICAM-1. Finally the cells can leave the vessel through integrin-mediated interactions (11, 12, 37).
Though E- and P-selectin have been shown in many contexts to have overlapping functions (32, 33, 38, 39), the experiments described here clearly show that E-selectin, but not P-selectin, is required for CL 316,243-induced adipose tissue neutrophil infiltration (Figs. 5 and 6). This is also supported by the fact that among the leukocyte adhesion molecules that were up-regulated after β3-adrenergic receptor stimulation in adipose tissue, E-selectin expression was increased in an acute manner and to the greatest extent (Fig. 3). Two groups recently demonstrated that P-selectin-mediated interactions are necessary for obesity-induced insulin resistance and glucose intolerance (15, 16). However, no studies have been performed to determine whether E-selectin plays a role in this process.
It was recently shown that adrenergic impulses activate endothelial cells in the bone marrow and skeletal muscle, and that these impulses were necessary for circadian oscillations that govern basal leukocyte recruitment to peripheral tissues (40). The data presented here also suggest that β-adrenergic stimuli activate adipose-resident endothelium; thus, basal adipose tissue inflammation, particularly neutrophil infiltration, may have a circadian pattern. Little is known about neutrophil function in adipose tissue; however, recent studies suggest that these cells may have a pro-inflammatory function and contribute to tissue dysfunction in obesity (22, 26, 41). Because E-selectin null animals are protected from neutrophil infiltration in response to CL 316,243 administration, these mice may also be protected from high fat diet-induced neutrophil infiltration (Figs. 5 and 6). Therefore, these animals may be useful for studying the long-term effects of acute neutrophil infiltration on adipose tissue function and insulin sensitivity.
As long-term, low-dose CL-treatment is associated with improved glucose tolerance and insulin sensitivity (42, 43), it is difficult to envision how neutrophil infiltration, which is pro-inflammatory, may be associated with the beneficial phenotypes of CL-treatment. We suggest that perhaps chronic adrenergic stimulation, as with therapeutic CL-infusion, promotes a desensitized state in which no further activation can occur. Indeed, the endothelium enters a period that is refractory to activation after a prior stimulation to control excessive inflammation (44).
There are several possible reasons why CL 316,243 treatment but not fasting activates adipose-resident endothelium (Figs. 3 and 4). First, different lipid species may be released by chemically induced β3-adrenergic receptor stimulation and fasting that may affect the endothelium differently. Several observations suggest that fatty acids released from adipocytes contribute to this phenomenon. ATGL-deficient mice are resistant to fasting-induced macrophage infiltration (9) and HSL-deficient mice are resistant to CL 316,243-mediated CCL-2/MCP-1 up-regulation (17), suggesting that lipolysis is necessary for inducing inflammation in both systems. However, mice lacking HSL have increased basal adipose tissue inflammation in the context of reduced lipolysis (17), further supporting the idea that other factors also contribute to adipose tissue inflammation.
Second, fasting is associated with hormonal changes such as reduced leptin secretion (45), which may modulate pathways that are dominant over lipolysis-driven signaling mechanisms. Finally, CL 316,243 treatment of mice, shown here to be associated with elevated adipose tissue inflammatory cytokine levels (Figs. 2 and 3), was also reported to greatly increase serum insulin levels (46, 47) in a manner that is dependent on β3-adrenergic receptor expression in white adipose tissue (47). At high concentrations, insulin can stimulate expression of adhesion molecules on the endothelium and promote leukocyte adhesion (48, 49). Therefore, elevated cytokine or insulin levels may act alone or synergistically to activate the endothelium. This hypothesis may explain why incubation of HUVECs with CL 316,243 alone had no effect on adhesion molecule expression (Fig. 3). Furthermore, adipose tissue inflammatory responses to CL 316,243 versus fasting may differ in part because fasting stimulates lipolysis more slowly and without the rise in insulin levels observed upon CL 316,243 treatment.
Taken together, these results suggest that the immune response in adipose tissue is complex and likely is stimulus-specific. Furthermore, different stimuli may enact distinct patterns of endothelial cell activation that enable infiltration by specific populations of immune cells. The data presented herein suggest that lipolysis in and of itself is not sufficient to induce adipose tissue immune cell infiltration, because fasting and CL-treatment induced lipolysis to a similar extent, as measured by plasma NEFA levels, yet induced very different inflammatory responses. Thus, there are additional factors that not only contribute to inflammation, but also contribute to the type of inflammation that occurs. Finally, for these reasons it is inappropriate to use CL-treatment and fasting interchangeably as a means to study acute adipose tissue inflammation. It will be important to determine the mechanistic differences in inflammatory responses under these various physiological conditions to fully understand their functional roles in adipose biology and systemic glucose tolerance.
Acknowledgments
We thank Matthieu Prot for help with dissections, Joseph Virbasius for helpful discussion, and the UMASS morphology and flow cytometry cores for assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant DK030898 and a grant from the International Research Alliance at Novo Nordisk Foundation Center for Metabolic Research (to M. P. C.), NIH Training Grant 5T32HD007312-25, and American Heart Association postdoctoral fellowship AHA 11POST704009 (to R. J. R. F.), and support for core facilities by the UMASS Diabetes and Endocrinology Research Center (DK032520).
- ATGL
- adipose triglyceride lipase
- NEFA
- non-esterified fatty acid
- HSL
- hormone-sensitive lipase
- MGL
- monoacylglycerol lipase
- ICAM
- intercellular adhesion molecule-1
- H&E
- hematoxylin and eosin
- SVF
- stromal vascular fraction.
REFERENCES
- 1. Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., Madeo F. (2012) FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281, 40236–40241 [DOI] [PubMed] [Google Scholar]
- 4. Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 5. Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 6. Granneman J. G., Moore H. P., Krishnamoorthy R., Rathod M. (2009) Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J. Biol. Chem. 284, 34538–34544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strålfors P., Belfrage P. (1983) Phosphorylation of hormone-sensitive lipase by cyclic AMP-dependent protein kinase. J. Biol. Chem. 258, 15146–15152 [PubMed] [Google Scholar]
- 8. Brasaemle D. L., Levin D. M., Adler-Wailes D. C., Londos C. (2000) The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta 1483, 251–262 [DOI] [PubMed] [Google Scholar]
- 9. Kosteli A., Sugaru E., Haemmerle G., Martin J. F., Lei J., Zechner R., Ferrante A. W., Jr. (2010) Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granneman J. G., Li P., Zhu Z., Lu Y. (2005) Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 289, E608–E616 [DOI] [PubMed] [Google Scholar]
- 11. Pober J. S., Sessa W. C. (2007) Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 [DOI] [PubMed] [Google Scholar]
- 12. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 13. Ringseis R., Eder K. (2010) Fatty acids and signalling in endothelial cells. Prostaglandins, Leukotrienes, Essential Fatty Acids 82, 189–198 [DOI] [PubMed] [Google Scholar]
- 14. Nishimura S., Manabe I., Nagasaki M., Seo K., Yamashita H., Hosoya Y., Ohsugi M., Tobe K., Kadowaki T., Nagai R., Sugiura S. (2008) In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J. Clin. Invest. 118, 710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato C., Shikata K., Hirota D., Sasaki M., Nishishita S., Miyamoto S., Kodera R., Ogawa D., Tone A., Kataoka H. U., Wada J., Kajitani N., Makino H. (2011) P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance. Diabetes 60, 189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russo H. M., Wickenheiser K. J., Luo W., Ohman M. K., Franchi L., Wright A. P., Bodary P. F., Nuñez G., Gabriel N., Eitzman D. T. (2010) P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ. Res. 107, 388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mottillo E. P., Shen X. J., Granneman J. G. (2007) Role of hormone-sensitive lipase in β-adrenergic remodeling of white adipose tissue. Am. J. Physiol. Endocrinol. Metab. 293, E1188–E1197 [DOI] [PubMed] [Google Scholar]
- 18. Mottillo E. P., Shen X. J., Granneman J. G. (2010) beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim. Biophys. Acta 1801, 1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., Locksley R. M. (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun K., Kusminski C. M., Scherer P. E. (2011) Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 [DOI] [PubMed] [Google Scholar]
- 22. Elgazar-Carmon V., Rudich A., Hadad N., Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 23. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Talukdar S., Oh da Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., Ofrecio J., Lin M., Brenner M. B., Olefsky J. M. (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 [DOI] [PubMed] [Google Scholar]
- 28. Xia S., Sha H., Yang L., Ji Y., Ostrand-Rosenberg S., Qi L. (2011) Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 286, 23591–23599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pober J. S. (2002) Endothelial activation: intracellular signaling pathways. Arthritis Res. 4, Suppl. 3, S109–S116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asterholm I. W., McDonald J., Blanchard P. G., Sinha M., Xiao Q., Mistry J., Rutkowski J. M., Deshaies Y., Brekken R. A., Scherer P. E. (2012) Lack of “immunological fitness” during fasting in metabolically challenged animals. J. Lipid Res. 53, 1254–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunkel E. J., Ley K. (1996) Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 79, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 32. Labow M. A., Norton C. R., Rumberger J. M., Lombard-Gillooly K. M., Shuster D. J., Hubbard J., Bertko R., Knaack P. A., Terry R. W., Harbison M. L. (1994) Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1, 709–720 [DOI] [PubMed] [Google Scholar]
- 33. Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. (1993) Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 74, 541–554 [DOI] [PubMed] [Google Scholar]
- 34. Frenette P. S., Mayadas T. N., Rayburn H., Hynes R. O., Wagner D. D. (1996) Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 84, 563–574 [DOI] [PubMed] [Google Scholar]
- 35. Ley K., Allietta M., Bullard D. C., Morgan S. (1998) Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 83, 287–294 [DOI] [PubMed] [Google Scholar]
- 36. Oh D. Y., Morinaga H., Talukdar S., Bae E. J., Olefsky J. M. (2012) Increased macrophage migration into adipose tissue in obese mice. Diabetes 61, 346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sengenès C., Miranville A., Lolmède K., Curat C. A., Bouloumié A. (2007) The role of endothelial cells in inflamed adipose tissue. J. Int. Med. 262, 415–421 [DOI] [PubMed] [Google Scholar]
- 38. Jung U., Ley K. (1999) Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin. J. Immunol. 162, 6755–6762 [PubMed] [Google Scholar]
- 39. Robinson S. D., Frenette P. S., Rayburn H., Cummiskey M., Ullman-Culleré M., Wagner D. D., Hynes R. O. (1999) Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc. Natl. Acad. Sci. U.S.A. 96, 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheiermann C., Kunisaki Y., Lucas D., Chow A., Jang J. E., Zhang D., Hashimoto D., Merad M., Frenette P. S. (2012) Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deleted in proof
- 42. Weyer C., Tataranni P. A., Snitker S., Danforth E., Jr., Ravussin E. (1998) Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective β3-adrenoceptor agonist in humans. Diabetes 47, 1555–1561 [DOI] [PubMed] [Google Scholar]
- 43. de Souza C. J., Hirshman M. F., Horton E. S. (1997) CL-316,243, a β3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes 46, 1257–1263 [DOI] [PubMed] [Google Scholar]
- 44. Karmann K., Min W., Fanslow W. C., Pober J. S. (1996) Activation and homologous desensitization of human endothelial cells by CD40 ligand, tumor necrosis factor, and interleukin 1. J. Exp. Med. 184, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahima R. S., Lazar M. A. (2008) Adipokines and the peripheral and neural control of energy balance. Mol. Endocrinol. 22, 1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Susulic V. S., Frederich R. C., Lawitts J., Tozzo E., Kahn B. B., Harper M. E., Himms-Hagen J., Flier J. S., Lowell B. B. (1995) Targeted disruption of the beta 3-adrenergic receptor gene. J. Biol. Chem. 270, 29483–29492 [DOI] [PubMed] [Google Scholar]
- 47. Grujic D., Susulic V. S., Harper M. E., Himms-Hagen J., Cunningham B. A., Corkey B. E., Lowell B. B. (1997) β3-adrenergic receptors on white and brown adipocytes mediate β3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J. Biol. Chem. 272, 17686–17693 [DOI] [PubMed] [Google Scholar]
- 48. Giri H., Muthuramu I., Dhar M., Rathnakumar K., Ram U., Dixit M. (2012) Protein tyrosine phosphatase SHP2 mediates chronic insulin-induced endothelial inflammation. Arteriosclerosis, Thrombosis, Vascular Biol. 32, 1943–1950 [DOI] [PubMed] [Google Scholar]
- 49. Li G., Barrett E. J., Ko S. H., Cao W., Liu Z. (2009) Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology 150, 3475–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]