Abstract
Background:
Alzheimer's disease (AD), a progressive brain disorder, is the most common cause of dementia among the elderly. Donepezil hydrochloride is a potent, reversible, and highly selective inhibitor of acetylcholinesterase (AChE). It is chemically distinct from other cholinesterase (ChE) inhibitors which are effective in the treatment of AD.
Objectives:
To evaluate the safety and efficacy of donepezil hydrochloride therapy over a 12 weeks period in patients with mild to moderate AD in Indian population.
Materials and Methods:
In this post-marketing study, patients with mild to moderate AD received oral donepezil hydrochloride 5 mg/day for 4 weeks followed by 10 mg/day for 8 weeks. Patients were assessed 4 times weekly for cognition on ‘Mini Mental Status Examination (MMSE) scale’, and function on ‘Activities of Daily Living (ADL) index’. Clinicians and caregivers assessment of safety and efficacy was assessed on a 5-point rating scale.
Results:
One hundred and seventy two of one hundred and eighty two patients completed 12 weeks of study period. MMSE score significantly improved (P<0.0001) from 16.72 at baseline to 19.77 after 12 weeks, and there was significant improvement (P<0.05) in ADL index in 13 of 17 domains after 12 weeks. Caregivers and clinicians rated the therapy as very good to good in >80% and >90% patients, respectively. Adverse events were consistent with the known pharmacological and safety profile of donepezil.
Conclusions:
Donepezil is well tolerated in Indian patients with mild to moderate AD with significant improvement in cognition and function.
Keywords: Alzheimer's, cognition, dementia, donepezil
INTRODUCTION
Dementia is a health care problem of epidemic proportions and has tremendous consequences for patients, families, and society.[1]
Alzheimer's disease (AD), a progressive brain disorder and the most common cause of dementia among the elderly, is characterized by a progressive decline of memory and intellectual abilities, which eventually becomes severe enough to interfere with functioning in daily living, the overall quality of life, and ultimately leads to death.[2] Prevalence is 1% to 2% at age of 65 years, but increases markedly to 35% or greater by age 85 years. Because of a demographic shift toward a more aged population, the percentage of affected individuals is rapidly increasing. Therefore, accurate and timely diagnosis, and effective treatments are critical to optimal outcomes over the 8- to 10-year course of the illness.[3]
Donepezil hydrochloride is a potent, reversible, and highly selective inhibitor of acetylcholinesterase (AChE), and, as a piperidine-based agent, it is chemically distinct from other cholinesterase (ChE) inhibitors.[4–7] The safety and efficacy of donepezil has been reported in the clinical trials,[8–10] but the data on safety in Indian population is lacking. The present post-marketing surveillance study was conducted with an objective of evaluating the safety and efficacy of donepezil hydrochloride in patients suffering from mild to moderate AD in Indian population.
MATERIALS AND METHODS
This post-marketing observational study was conducted at 11 participating sites across India. A written permission to conduct the said study was obtained from the Indian drug regulatory authority (The Drug Controller General of India) and ethics committee approval was obtained from an independent ethics committee for all the centers participating in the study. Ethical committee notifications as per Good Clinical Practice guidelines issued by Central Drugs Standard Control Organization (CDSCO) of India and ethical guidelines for biomedical research on human subjects, issued by Indian Council of Medical Research (ICMR) and ICH were followed.
Study population
A total of 182 patients of either sex, above 50 years of age, diagnosed with mild to moderately severe AD consistent with NINCDS-ADRDA (National Institute of Neurologic and Communicative Disorder and Stroke and the AD and Related Disorders Associated Work Group) and DSM-IV (American Psychiatric Association's Diagnostic and Statistical Manual-IV) criteria for AD and having a Mini Mental State Examination (MMSE) score of 10 to 26 at screening were enrolled from the study sites after obtaining on informed written consent. All patients needed to have a computerized tomography (CT) or magnetic resonance imaging (MRI) scan in the past 18 months to be consistent with the diagnosis of AD by excluding any other causes of dementia; also essential was the availability of a caregiver, able to provide information on the patient's status and ensure patient compliance with treatment and clinic visits for follow-up. The patients needed vision and hearing to be sufficient for compliance with the testing procedures with eye glasses and hearing aids allowed for inclusion.
Patients with known hypersensitivity to donepezil or any other ChE inhibitors and those with clinical evidence of any medical, neurological, and psychiatric disorders which in the opinion of investigators were likely to interfere with the study were excluded from the study. Also excluded were patients with dementia complicated by delirium or who had a known or suspected history within past one year of alcohol dependence or substance abuse and all those who received an investigational new drug in past one month.
Procedures
Enrolled patients and caregivers were explained about the nature of the study. A general and systemic examination followed by neuropsychological assessment evaluating the baseline Hachinski ischemia scale score, MMSE score,[11] and Activities of Daily Living (ADL) index[12] were recorded. Patients were dispensed with 5 mg donepezil hydrochloride (tablet Aricept® Mfg. Eisai India Pvt. Ltd.) and were instructed about the dosage schedule. Dose of donepezil was titrated from 5 mg/day to 10 mg/day after first 4 weeks and continued with the same dose for the remaining 8 weeks of study period.
Investigators were recommended to exercise caution in prescribing ketoconazole, itraconazole, erythromycin, fluoxetine, rifampicin, carbamazepine, phenytoin, and beta-blockers.
After the baseline screening and enrollment visit (day 0), patients were followed up 4 times weekly, for 12 weeks. Thus, each patient made four visits.
Assessment parameters
The primary efficacy assessment included cognition evaluation on a scale of 0-30 using MMSE scale conducted by trained clinicians. MMSE is a 30 item instrument which evaluates orientation, registration of information, attention and calculation, recall, language, and constructions. It has three main factors: verbal functions, memory abilities, and construction.
Secondary efficacy assessments were the ADL score for ADL index weeks and efficacy assessed by clinicians and caregivers on a 5-point likert scale of very poor to very good. ADL index evaluates the activities of daily living using 17 questions, which evaluates instrumental and basic activities of daily living. It is a reported on a 3 point scale for every question wherein ‘1’ designates activity performed without assistance, ‘2’ designates activity performed greater part without assistance with some verbal or physical assistance required, and ‘3’ designates complete inability to perform, even with assistance or refusal to perform even if deemed able. The minimum possible score on the scale is ‘17’ indicating no apparent handicap and the score is ‘51’, if totally unable to carry out any activity even with help.
Safety was assessed based on the side effects reported by the patients/caregivers during the course of treatment. Safety was also assessed by the clinicians and caregivers on a 5-point likert scale from very poor to very good.
Compliance to therapy was assessed based on the tablets count and non-consumption of study medication for three successive days was termed as ‘non-complaint’.
Statistical analysis
Measurement data and data for MMSE score, ADL index is expressed as mean and standard deviation (SD). Change in the scores from baseline over the study period is analyzed using paired t-test and one way analysis of variance (ANOVA) (repeat measures) with Dunnett's post-hoc analysis using baseline value as a reference.
Discrete data for efficacy and safety assessments by clinicians and caregivers are expressed as numbers and percentages and analyzed using Chi-square test. All analyses were done at α=0.05 and 95% confidence level.
RESULTS
Of the 182 enrolled patients, 8 patients were lost to follow-up and 2 patients had violation of inclusion criteria. Thus, 10 patients were excluded from the analysis and the data of 172 patients were evaluated by Intent-to-Treat (ITT) analysis method. For missing data on MMSE and ADL, last observation carried forward (LOCF) was applied, whereas for missing data on other parameters, observed cases (OC) analysis was done.
The demographic data and baseline patient characteristics are given in [Table 1]. Abnormality in respiratory system was observed in 5 (2.9%) patients, cardiovascular system (CVS) in 9 (5.2%) patients, and central nervous system (CNS) in 10 (5.8%) patients.
Table 1.
Demographic data and baseline patient characteristics
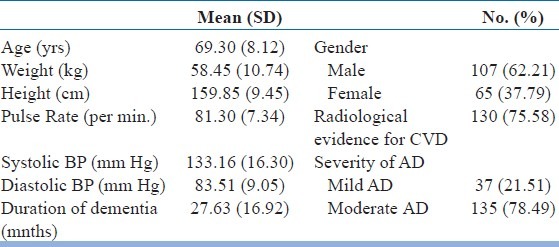
There was no significant change in vital parameters like pulse rate and systolic and diastolic blood pressure throughout the study period. Patient compliance was good throughout the study period. More than 80% of the patients received some form of concomitant medication. Anti-hypertensives (calcium antagonists, ACE-inhibitors, angiotensin receptor antagonists, and beta receptor antagonist) and aspirin were the most frequently used medications, followed by statins and multivitamins. Other concomitant drugs included oral hypoglycemics, antidepressants and antipsychotics, benzodiazepines, anti-epileptics, and prokinetics.
[Table 2] shows the mean scores and improvement in scores for MMSE and ADL index at baseline and during the study period. There was a significant improvement in the cognition status (MMSE score) at 4, 8, and 12 weeks (P<0.0001), whereas improvement in ADL index score was significant (P, 0.003) at 12 weeks but not significant (P>0.05) at 4 and 8 weeks. However, at 8 and 12 weeks, the improvement was significant for MMSE scores (P<0.0001). Analysis of MMS subsets showed that improvement was greater for the domains of orientation, attention and calculation, recall, and language; whereas lesser improvement was seen in registration domain of MMSE [Figure 1].
Table 2.
Scores for MMS* and ADL index#
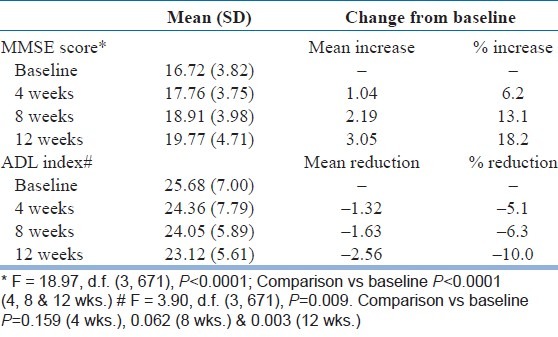
Figure 1.
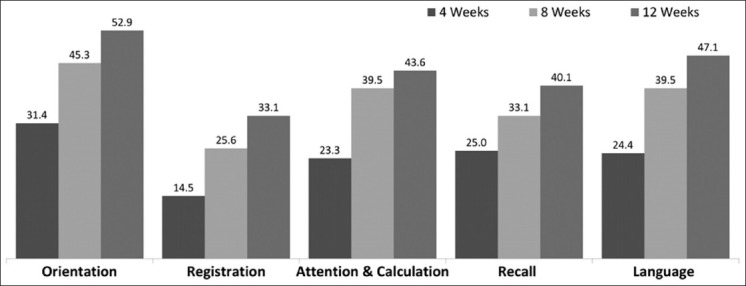
Improvement in MMSE subsets (% pts.)
Individual component analysis of ADL index showed a significant improvement in 13 of 17 domains of ADL index scale. There was a significant improvement in both instrumental and basic activities of daily living like transfer from floor to chair, walking indoors and outdoors, ascending and descending a flight of stairs, dressing, washing, bathing, using lavatory, grooming, and using taps.
Global efficacy assessment as reported by the clinician and caregiver is shown in [Figure 2]. At the end of study period, the mean response on efficacy ranged near between good to very good by clinician as well as caregiver. At the end of 12 weeks, 84.7% of clinicians reported the efficacy to be good to very good, 14.0% of clinicians reported the efficacy to be average, and 1.3% reported it to be poor. Similarly, 86.8% of caregivers reported the efficacy to be good to very good, 12.0% reported average, and 1.3% caregivers reported efficacy to be poor.
Figure 2.
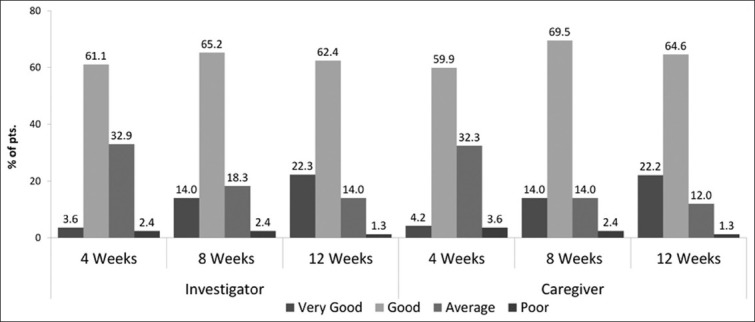
Global efficacy assessment by clinician and caregiver (% of pts.)
Global efficacy assessment as reported by the clinician and caregiver is shown in [Figure 3]. At the end of 12 weeks for 96.2% patients, clinician reported the safety to be good to very good, for 2.5% patients, it was average, and only for 1.3% patients, it was poor. Similarly, 96.2% of caregivers reported the safety to be good to very good, 2.5% of caregivers reported the safety to be average, and 1.3% of caregivers reported safety to be poor.
Figure 3.
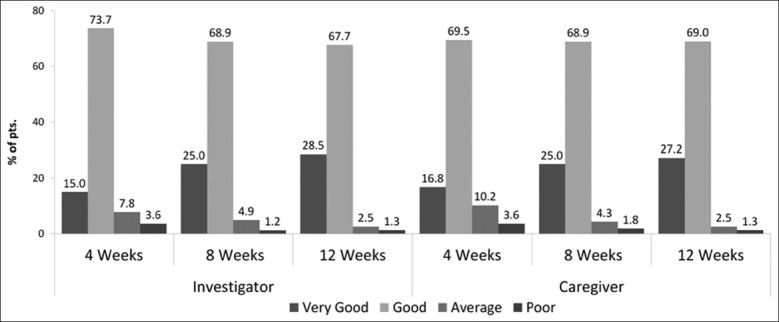
Global tolerability assessement by clinician and caregiver (% of pts.)
Adverse events
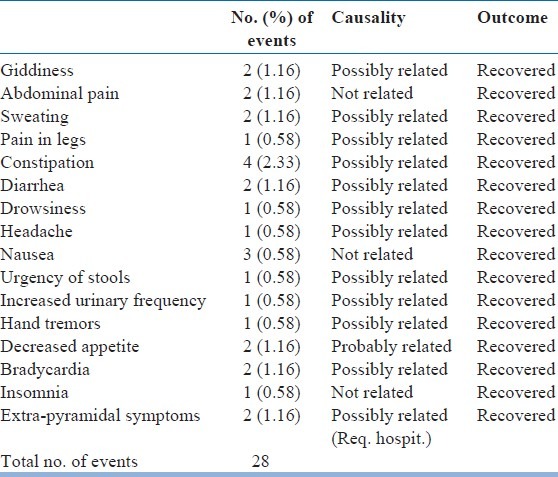
A total 28 adverse events were reported in 19 (11.05%) patients, of which, 22 were considered to be related to study medication, and 6 were considered as not related to study medication. A total of 19 (11.05%) patients reported adverse events during the study period (18 at 4 weeks, 8 at 8 weeks, and 2 at 12 weeks). [Table 3] shows the adverse events reported during the study period. Serious adverse events were reported in the two patients reporting with extra-pyramidal symptoms, as they had to be hospitalized. However, both the patients recovered without any sequel. The study medication was stopped in both these patients and they were withdrawn from the study. In all other cases, patients were continued on study medication for rest of the study period. All adverse events recovered without any residual effect.
Table 3.
Adverse events reported by the patients
DISCUSSION
AD is a progressive, debilitating disorder estimated to affect some 5-10% of people over 65 years old and as many as 50% of those over 85 years of age.[13] The cognitive deficits are responsible for progressive impairment in activities of daily living such as driving, buying groceries, preparing meals, doing laundry and basic functions such as walking safety and maintaining personnel hygiene[14] AD is characterized by deficits in memory and cognition that are associated with significant losses of presynaptic cholinergic function in the brain, particularly the nucleus basalis of Meynert.[15] Bartus et al proposed the cholinergic hypothesis of AD suggesting that the deficiency of the neurotransmitter, acetylcholine, in the brain could be amenable to replacement therapy.[16]
Donepezil hydrochloride is a potent, reversible, and highly selective inhibitor of AChE, and as a piperidine-based agent, chemically distinct from the other ChE inhibitors.[1,17–18] The efficacy and safety of donepezil has been demonstrated in patients with AD through various global, double-blind, and open-labeled studies ranging from 12 weeks to 5 years duration.[19–23] Though donepezil is available in India since 2002, to the best of our knowledge, there are no studies evaluating the safety and efficacy in the India population.
The present study evaluated the safety and efficacy of donepezil hydrochloride in Indian patients suffering from mild to moderate AD. Progressive cognitive impairment is the hallmark of AD. In the present study, MMSE was used to evaluate cognition, as it is the most common instrument used by clinicians in their day to day practice in evaluating and managing patients with AD. Patients with AD are likely to have a relative preservation of long term memory, especially in the early stage of the dementia and greater deficits in frontal executive functioning like planning, organization, abstraction, category fluency initiation, reasoning, mental flexibility, sequencing, fine motor performance, and the allocation of attentional resources than patients with AD.[24] Significant improvement in MMSE was observed from as early as 4 weeks of study period (P<0.01).
The findings of the present study are consistent with the findings reported by Klinger T et al (2005)[25] and Relkin N et al (2003).[26] In the study conducted by Klinger T et al, which was a post-marketing surveillance study conducted on 913 patients with mild to moderate AD in Germany, patients who were not satisfied with existing anti-dementia medications and those who were treatment naïve were treated with donepezil 5 mg for 4 weeks. In the present study, patients showed a significant improvement in cognition as evaluated on MMSE by 2.21 (± 3.47) points at the end of 12 weeks. In the study conducted by Relkin N et al (2003), cognition was evaluated in patients with mild to moderate AD over 12 weeks period on standardized version of MMSE (sMMSE). At the end of 12 weeks, there was an improvement in cognition by 1.54 (± 3.05) points on sMMSE.
Studies of 6 months duration in patients with mild to moderate AD treated with donepezil have demonstrated the improvement on cognition to persist even at end of six months[27–28] and long term studies have demonstrated the improvement in cognition on Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) to be above baseline values for as long as 38 weeks and at any point-of-time retained better than the placebo in long term studies over 5 years of duration.[29]
The early symptoms of AD involve difficulty with the episodic memory, the ability to encode information and later recall.[1] There is visuospatial impairment evident on the inability to make drawings and other constructions or to orient themselves to their surroundings. Also, affected in AD is language, with initial word finding difficulty progressing to anomia and impaired comprehension. Early in the disease course, there may be an inability to retrieve words with circumlocution and poor wordlist generation, particularly for words in the given semantic category. As disease progresses, difficulty naming becomes apparent and spontaneous speech becomes increasingly empty.[30] Hence, improvement in cognition noted in the present study on MMSE and individual cognitive domains reflects a significant benefit in the real world setting from the patient's perspective when we relate to a progressive disorder like AD. When individual components of MMSE were analyzed, it demonstrated a trend for improvement in all the components of MMSE with significant improvement in orientation, attention, and recall in this group of patients.
The mean 3.05 point improvement from baseline in total MMSE scores for the evaluable population confirms that the cognitive benefits of donepezil observed in controlled studies can also be measured in routine clinical particle. These findings are consistent with the findings reported study conducted by Rockwood K et al.[31] In this multi-center, 6-month, open-label study of 101 primary care patients, changes in a 19-symptom checklist were assessed in relation to changes in standardized scales of cognition, activities of daily living, behavior, and caregiver burden and the clinicians reported significant improvement in cognitive symptoms like recall, attention, and spatial and temporal orientation over 12 and 24 weeks of study period.
Deterioration in functional abilities of daily living has a major impact on quality of life of an individual with AD.[30] Feelings of incompetency and loss of control are often expressed by the dementia victim in the earlier stages of the disease.[30] Functional deficits starts to occur at a stage when cognition deficits are mild and first become apparent in complex occupational tasks, such as work or hobbies and in social activities like forgetting appointments, difficulty in finding their way home, preparing meals, using telephone, etc. When cognition deficits become moderately severe, individuals start experiencing difficulties with basic activities of daily living like increasing ability to dress, bathe, and toilet. Deterioration in functional abilities has important effects on the life of individual with AD as well as on caregiver.[32]
In the present study, there was a significant improvement in ADL index and individual component analysis of ADL index in 13 of 17 domains of ADL index scale. More than 20% of the patients’ demonstrated improvement in either of the activities of daily living as measured on the ADL index scale. There was an improvement in dressing, washing, bathing, using lavatory and continence. Activities like grooming, brushing teeth, preparation for making tea, making tea, using taps and feeding also improved with donepezil. Similar findings were noted in the study conducted by Rockwood K et al in which ≥20% of patients reported improvement in domains of judgment, hygiene, dressing, and domestic activities.[33]
The findings of global assessment in the present study are similar to those reported Klinger T et al wherein the investigators assessment of safety was reported between very good to good in 93.5% of total patient populations.[34]
All the patients exhibited good patient compliance with >98% of patients being complaint throughout the study period.
The study drug was well tolerated in the present study with most of the adverse events being mild to moderate in intensity and not requiring discontinuation of study medication. Most of the adverse events were reported in the first 4 weeks of study period. In the study conducted by Relkin N et al (2003), the common adverse events reported were anorexia, diarrhea, nausea, abdominal disturbances, vomiting, generalized weakness, agitation, confusion, dizziness, and headache.[35]
The incidences of cholinomimetic properities of donepzil in the present study are highly consistent with the known tolerability profile of donepezil.[36,37] This low rate of adverse events may be partly attributed to the specificity of donepezil for AChE in the central nervous system. Donepezil significantly inhibits brain AChE, while having little effect on either the cardiac muscle or smooth muscle.[38]
While the present study extends the available data on tolerability of donepezil to the Indian population that is more community dwelling patients, it has its own limitations. Patients with unstable medical or psychiatric conditions were excluded from this study. However, these exclusions are consistent with safe medical practice and do not necessarily constitute limitations to this study. This was an open-labeled study, which introduces the possibility of rater bias in the assessment of efficacy. Nevertheless, the statistically significant improvements in cognitive function that were observed in patients treated with 5 and 10 mg/day of donepezil are consistent with those observed in previous double-blind, placebo-controlled studies and the post marketing surveillance studies conducted in Germany. This suggests that the efficacy of donepezil measured in pivotal trials worldwide can still be obtained in Indian patients, despite administration of a variety of concomitant medication.
Another limitation of the present study is its shorter duration. Three months was chosen as the minimum period of the study, since this enables the evaluators to establish first impressions of efficacy and safety data in the Indian patients without compromising the quality of the data due to attrition that can occur with longer trials in AD.
Based on the results reported, the present study offers support for the safety and efficacy of donepezil in the treatment of elderly, community-dwelling Indian patients with mild to moderately AD.
CONCLUSIONS
This study confirms findings of global studies demonstrating improvement in cognition and function in Indian patients with mild to moderately AD; and donepezil is well tolerated with adverse events similar to those reported in global studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Medez MF, Cummings JL. Dementia Significance, Definition and Epidemiology. 3rd ed. Philadelphia: Elseveir; 2006. Dementia: A Clinical Approach; pp. 1–12. [Google Scholar]
- 2.Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer's disease: Characteristics and treatment. Neurol Clin. 2000;18:829–46. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter K, Hall G. Alzheimer's disease. In: McBride AB, Austin JK, editors. Psychiatric Mental Health Nursing. Philadelphia Pa: WB Saunders Co; 1996. pp. 348–2. [Google Scholar]
- 4.Cardozo MG, Iimura Y, Sugimoto H, Yamanishi Y, Hopfinger AJ. ‘QSAR anaylsis of the substituted indandone and benzylpiperidine rings of a series of indandone-benzylpiperidine inhibitors of acetylcholinesterase. J Med Chem. 1992;35:584–9. doi: 10.1021/jm00081a022. [DOI] [PubMed] [Google Scholar]
- 5.Cardozo MG, Kawai T, Iimura Y, Sugimoto H, Yamanishi Y, Hopfinger AJ. Conformational analysis and molecular shape comparisons of a series of indanone-benzylpiperidine inhibitors of acetylcholinesterase. J Med Chem. 1992;35:590–601. doi: 10.1021/jm00081a023. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi Y, Ogura H, Kosasa T, Araki S, Sawa Y, Yamatsu K. Inhibitory action of E2020, a novel acetylcholinesterase inhibitor, on cholinesterase. Comparison with other inhibitors. In: Nagatsu T, Fisher A, Yoshida M, editors. Basic, clinical, and therapeutic aspects of Alzheimer's and Parkinson's diseases. New York: Plenum Press; 1990. pp. 409–3. [Google Scholar]
- 7.Rogers SL, Yamanishi Y, Yamatsu K. E2020 – The pharmacology of a piperidine cholinesterase inhibitor. In: Becker RE, Giacobini E, editors. Cholinergic basis for Alzheimer therapy. Vol. 3. Boston: Birkhäuser; 1991. pp. 314–20. [Google Scholar]
- 8.Rogers SL, Friedhoff LT The Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer's disease: Results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 9.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–45. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SL, Doody RS, Mohs RC, Friedhoff LT Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–31. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 11.Folstein W, Folstein SE, McHigh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh K, Smith DS, Meade TW, Goldenberg E, Brennan PJ, Kinsella G. Repeatability and validity of a modified Activities of Daily Living (ADL) Index in studies of chronic disability. Int Rehabil Med. 1979;1:51–8. doi: 10.3109/03790797909164024. [DOI] [PubMed] [Google Scholar]
- 13.Evans DA, Scherr PA, Smith LA, Albert MS, Funkenstrin HH. The east Boston Alzheimer's Disease Registry. Aging (Milano) 1990;2:298–302. doi: 10.1007/BF03323937. [DOI] [PubMed] [Google Scholar]
- 14.Henderson AS. Epidemiology of Dementia Disorders. Adv Neurol. 1990;51:15–25. [PubMed] [Google Scholar]
- 15.Katzman R. Education and the prevalence of dementia and Alzheimer's Disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 16.Canadian Study of Health and Aging: Study Methods & Prevalence of Dementia. CMAJ. 1994;150:899–912. [PMC free article] [PubMed] [Google Scholar]
- 17.Bachman DL, Wolf PA, Linn RT, Knoefel JE, Cobb JL, Belanger AJ. Incidence of Dementia a probable Alzheimer's Disease in a general population: The Framingham study. Neurology. 1993;43:515–9. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- 18.Jorn AF. Cross-national comparisons of the occurrence of Alzheimer's & Vascular Dementias. Eur Arch Psychiatry Clin Neurosci. 1991;240:218–22. doi: 10.1007/BF02189530. [DOI] [PubMed] [Google Scholar]
- 19.Rogers SL, Friedhoff LT The Donepezil Study Group. The efficacy and safety of donepezil in patients withAlzheimer's disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 20.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–45. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 21.Rogers SL, Doody RS, Mohs RC, Friedhoff LT Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–3. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 22.Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: Final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000;10:195–203. doi: 10.1016/s0924-977x(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg SM, Tennis MK, Brown LB, Gomez-Isla T, Hayden DL, Schoenfeld DA, et al. Donepezil therapy in clinical practice: A randomized crossover study. Arch Neurol. 2000;57:94–9. doi: 10.1001/archneur.57.1.94. [DOI] [PubMed] [Google Scholar]
- 24.Stephens S, Kalaria R, Kenny RA, Ballard C. Progression of Cognitive Impairments associated with cerebrovascular disease. In: Paul RH, Cohen R, Ott BR, Salloway S, editors. Vascular Dementia – Cerebrovascular Mechanisms and Clinical Management. Totowa, NJ: Humana press; 2005. pp. 145–6. [Google Scholar]
- 25.Klinger T, Ibach B, Schoenknecht P, Kamleiter M, Silver G, Schroder J, et al. Effect of Donepezil in patients with Alzheimer's Disease previously untreated or treated with memantine or nootropic agents in Germany: An observational study. Curr Med Res Opin. 2005;21:723–32. doi: 10.1185/030079905x43668. [DOI] [PubMed] [Google Scholar]
- 26.Relkin NR, Reichman WE, Orazem J, McRae T. A large Community-Based, Open-label Trial of Donepezil in the Treatment of Alzheimer's disease. Dement Geiatr Cogn Disord. 2003;16:15–24. doi: 10.1159/000069988. [DOI] [PubMed] [Google Scholar]
- 27.Ibbotson T, Goa TL. Management of Alzheimer's Disease: Defining the Role of Donepezil. Dis Manage Health Outcomes. 2002;10:41–54. [Google Scholar]
- 28.Dooley M, Lamb HM. Donepezil: A review of its use in Alzheimer's disease. Drugs Aging. 2000;16:199–226. doi: 10.2165/00002512-200016030-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: Final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000;10:195–203. doi: 10.1016/s0924-977x(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 30.Mendez MF, Cummings JL. Alzheimer's Disease in ‘Dementia – A Clinical Approach’. 3rd Ed. Vol. 4. Philadelphia: Elsevier; 2006. pp. 68–121. [Google Scholar]
- 31.Rockwood K, Black S, Bedard MA, Tran T, Lussier I for TOPS study investigators. ‘Specific symptomatic changes following donepezil treatment of Alzheimer's disease: A multicentre, primary care, open-label study. Int J Geriatr Psychiatry. 2007;22:312–9. doi: 10.1002/gps.1675. [DOI] [PubMed] [Google Scholar]
- 32.Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–8. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 33.Relkin NR, Reichman WE, Orazem J, McRae T. A large Community-Based, Open-label Trial of Donepezil in the Treatment of Alzheimer's disease. Dement Geiatr Cogn Disord. 2003;16:15–24. doi: 10.1159/000069988. [DOI] [PubMed] [Google Scholar]
- 34.Klinger T, Ibach B, Schoenknecht P, Kamleiter M, Silver G, Schroder J, et al. Effect of Donepezil in patients with Alzheimer's Disease previously untreated or treated with memantine or nootropic agents in Germany:an observational study. Curr Med Res Opin. 2005;21:723–32. doi: 10.1185/030079905x43668. [DOI] [PubMed] [Google Scholar]
- 35.Relkin NR, Reichman WE, Orazem J, McRae T. A large Community-Based, Open-label Trial of Donepezil in the Treatment of Alzheimer's disease. Dement Geiatr Cogn Disord. 2003;16:15–24. doi: 10.1159/000069988. [DOI] [PubMed] [Google Scholar]
- 36.Ibbotson T, Goa TL. Management of Alzheimer's Disease: Defining the Role of Donepezil. Dis Manage Health Outcomes. 2002;10:41–54. [Google Scholar]
- 37.ARICEPT® (Donepezil hydrochloride tablets) Teaneck, NJ: Eisai Inc; 2000. [Google Scholar]
- 38.Klinger T, Ibach B, Schoenknecht P, Kamleiter M, Silver G, Schroder J, et al. Effect of Donepezil in patients with Alzheimer's Disease previously untreated or treated with memantine or nootropic agents in Germany: An observational study. Curr Med Res Opin. 2005;21:723–32. doi: 10.1185/030079905x43668. [DOI] [PubMed] [Google Scholar]


