Abstract
Aims:
To derive a reliable estimate of the frequency of pupillary involvement and to study the patterns and course of anisocoria in conjunction with ophthalmoplegia in diabetes-associated oculomotor nerve palsy.
Materials and Methods:
In this prospective analytical study, standardized enrolment criteria were employed to identify 35 consecutive patients with diabetes-associated oculomotor nerve palsy who were subjected to a comprehensive ocular examination. Standardized methods were used to evaluate pupil size, shape, and reflexes. The degree of anisocoria, if present and the degree of ophthalmoplegia was recorded at each visit.
Results:
Pupillary involvement was found to be present in 25.7% of the total number of subjects with diabetic oculomotor nerve palsy. The measure of anisocoria was < 2 mm, and pupil was variably reactive at least to some extent in all cases with pupillary involvement. Majority of patients in both the pupil-involved and pupil-spared group showed a regressive pattern of ophthalmoplegia. Ophthalmoplegia reversed much earlier and more significantly when compared to anisocoria.
Conclusions:
Pupillary involvement in diabetes-associated oculomotor nerve palsy occurs in about 1/4th of all cases. Certain characteristics of the pupil help us to differentiate an ischemic insult from an aneurysmal injury to the 3rd nerve. Ophthalmoplegia resolves much earlier than anisocoria in diabetic oculomotor nerve palsies.
Keywords: Anisocoria, diabetic oculomotor nerve palsy, ophthalmoplegia
Diabetics are predisposed to certain acute mononeuropathies, including cranial neuropathies, which could involve oculomotor nerves. The oculomotor nerve is quite commonly involved in diabetes.[1,2]
The size and reactivity of the ipsilateral pupil is generally considered a useful guide to help clinicians distinguish oculomotor nerve injury, caused by aneurysmal compression (dilated and poorly reacting pupil) from peripheral nerve infarction in which the pupil is usually spared.[3–5]
While pupil involvement is a sensitive predictor of aneurysmal compression, the specificity of this sign remains less clear in regard to diabetes-associated infarction. In the recent past, it has been noted that more patients present with pupillary involvement in diabetes-associated oculomotor nerve palsy. Similarly, there have been incidences of oculomotor nerve palsy associated with aneurysms but presenting with sparing of the pupil.
As oculomotor nerve palsy caused by posterior communicating artery aneurysm can lead to devastating outcomes, a dilemma arises in patients with pupil involving oculomotor nerve palsies with diabetes.
The reported frequency of pupil involvement based on retrospective analysis ranges from 14% to 32%.[1,3,6–8]
The purpose of this study was to derive a more reliable estimate of the frequency of pupil involvement and to study the course of anisocoria and ophthalmoplegia in patients with diabetes-associated oculomotor nerve palsy.
Finding the correct incidence could help in deciding the need for extensive investigative procedures like an MRI (magnetic resonance imaging) and MRA (magnetic resonance angiography) of brain.
Materials and Methods
The study population consisted of 35 consecutive patients with diabetes-associated oculomotor nerve palsy from July 2007 to May 2008 who were referred to the neuro-ophthalmology outpatient department.
All patients with oculomotor nerve palsy due to diabetes as diagnosed clinically and documented appropriately with Hess charting and diplopia charting were recruited for the study.
All patients with 3rd nerve palsies due to other conditions such as head trauma, compressive lesions such as intracranial aneurysm and space occupying lesions, carotid-cavernous fistula, vasculitic infarction such as giant cell arteritis, meningeal inflammation, herpes zoster, cavernous sinus thrombosis, ophthalmoplegic migraine and post-viral demyelination were excluded from the study. Similarly, patients with oculomotor nerve palsy due to diabetes along with one or more of the above-mentioned conditions were also excluded.
A detailed medical history and past history of the subjects was taken. All patients were subjected to a comprehensive ocular examination, which included visual acuity and slit lamp biomicroscopy. Particular attention was paid towards lid examination, pupillary reflexes, and extraocular movements.
Ptosis, if present, was graded. Pupils were checked for size, shape, and light reflexes. Pupillary involvement was scrutinized by measuring the pupil size and its reactivity to light.
Standardized methods were used to measure pupil size. Patients were instructed to look at a target kept 6 meters away under stable room light conditions.
A pupil gauge accurate to within 0.5 mm was used to measure the pupil diameters. The patients were engaged in conversation to ensure that they were alert. The degree of anisocoria, if present, was recorded.
Anisocoria, if present, was again measured under dim light conditions to rule out simple (physiological) anisocoria. An anisocoria was termed as simple if it remained similar in room light as well as in dim light. The quality of direct pupillary light reaction was also recorded.
Hess charting and diplopia charting were done in all cases to confirm oculomotor nerve palsy. Standardized method to quantify the degree of ophthalmoplegia by recording the relative limitation of ocular ductions of the superior, inferior, and medial recti muscles and inferior oblique using a 0 to 4 scale was used.[9] 0 represented full duction; 4 complete absence of function; and 1, 2 and 3, 25%, 50% and 75 % impairment of duction, respectively. A single ophthalmoplegia grade was determined by calculating the arithmetic mean of the relative limitation of ocular ductions of the involved 4 muscles.
All patients were subjected to a fundus examination with a 90 D lens to document any signs of diabetic retinopathy. MRI and MRA of brain were done in all cases to rule out surgical lesions. Blood pressure measurement, random blood sugar, erythrocyte sedimentation rate and serum cholesterol were recorded in all cases.
All patients were treated with oral methylcobalamin.[10] They were advised to control diabetes and other associated systemic disorders and to undergo ocular physiotherapy. All patients were reviewed again after 2 weeks and 8 weeks from the baseline visit. Lid position, extra-ocular movements, pupil size, and reaction to light were recorded at every visit.
The data collected from the patients were coded and tabulated. Appropriate inferential, descriptive statistics, analysis of variance (ANOVA), and correlation were compiled using Statistical Package for the Social Sciences (SPSS) version 17. The results of the analysis were presented in the form of tables and graphs. The statistical significance is tested at 5% level (P ≤ 0.05).
Results
Of the total 35 subjects screened, none had bilateral oculomotor nerve involvement, and MRI with MRA of brain was normal in all the cases. None of the patients had isolated weakness of extraocular muscles innervated by only the superior or inferior division of the oculomotor nerve.
Table 1 shows demographic and clinical characteristics of all the 35 patients recruited in the study.
Table 1.
Demographic and clinical characteristics
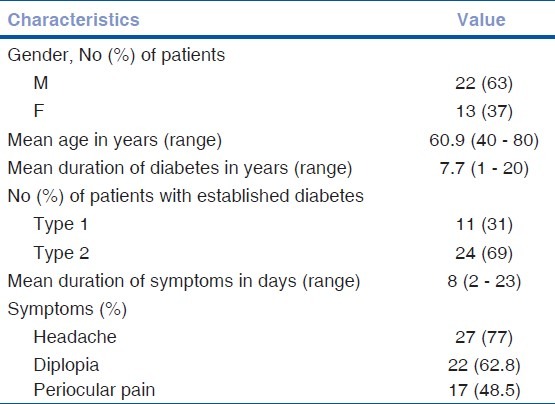
Among the other risk factors associated with the development of vasculopathic oculomotor nerve palsy, hypertension was seen most frequently (42.8%), followed by hypercholesterolemia (40%), smoking (28.57%), coronary artery disease (14.2%) and alcoholism (11.3%).
9 patients (25.7%) were found to have an internal ophthalmoplegia along with external ophthalmoplegia. The mean age in this group was 57.56 ± 11.98 years (ranging from 40 to 78 years), and mean duration of diabetes was 7.27 ± 5.7 years. Patients presented to us on an average 9.1 ± 6.5 days after the onset of symptoms. Some degree of anisocoria (pathological and simple anisocoria) was measured in 31.1% of the patients at presentation. Based on pupillary findings at the final visit, 4 kinds of patterns could be identified in all subjects [Table 2].
Table 2.
Pattern and degree of anisocoria
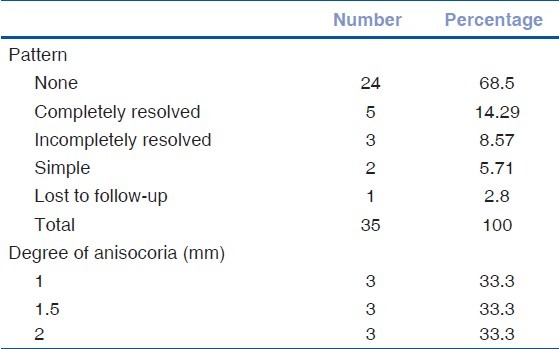
In all 9 patients, the measure of anisocoria ranged from 1 mm to 2 mm (median size 1.5 mm) with the frequency distributed equally between 1, 1.5, and 2 mm of anisocoria (33.3%) [Table 2]. None of these patients had a fully-dilated, non-reactive pupil.
Fig. 1 represents the course of anisocoria at each visit for each of the 9 patients (A - I) who had pupillary involvement. In patients with incomplete resolution, residual anisocoria was ≤ 1 mm. The direct pupillary reaction was impaired variably in all subjects during the 1st 2 visits, but the reaction normalized or near normalized as ophthalmoplegia resolved.
Figure 1.
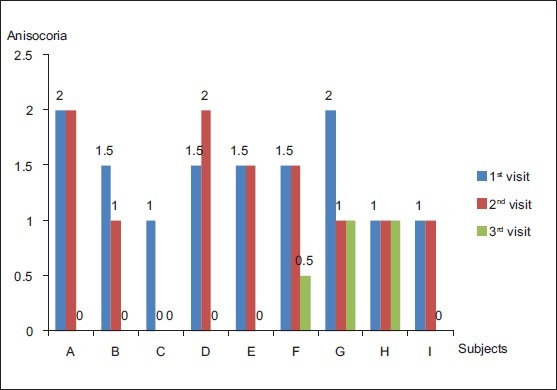
Course of anisocoria [The graph represents the degree of anisocoria for each of the nine patients (A-I) with pupillary involvement at each visit. Patient ‘C’ and ‘E’ were lost to follow-up at 2nd and 3rd visit, respectively]
Comparison of anisocoria at different visits (Post Hoc tests) revealed that there was a significant difference in the degree of anisocoria between the 1st and 3rd visits (P = 0.02) and the 2nd and 3rd visits (P = 0.02). In most of the patients with pupillary involvement, the maximum anisocoria developed within the 1st 2 weeks after the onset of symptoms [Fig. 2]. Mean time between the onset of symptoms and maximum anisocoria was 9 days.
Figure 2.
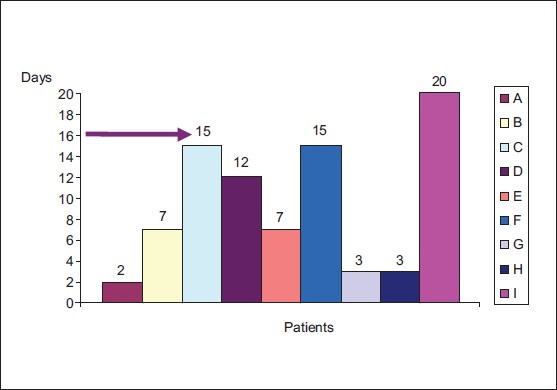
Time taken to develop maximum anisocoria (The last data point plotted for each patient (A-I) represents the maximum anisocoria recorded)
In 62.5% of the patients with pupillary involvement [Fig. 3], a complete resolution of ophthalmoplegia was seen (A, B, C, D, and F). Comparison of grades of ophthalmoplegia between different visits by the Post Hoc test revealed that there was a significant difference in the ophthalmoplegia grades between the 1st and 2nd visit (P = 0.033), the 2nd and 3rd visit (P = 0.002) and the 1st and 3rd visit (P = 0.00). Correlation analysis (Pearson correlation) didn’t reveal any significant association between the course of anisocoria and ophthalmoplegia (P = 0.086).
Figure 3.
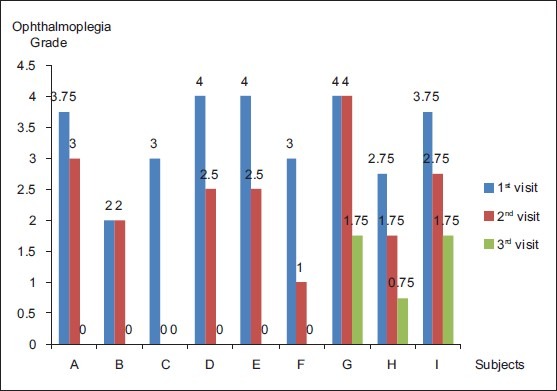
Course of ophthalmoplegia [The graph represents the grades of ophthalmoplegia of each of the 9 patients (A-I) with pupillary involvement at each visit. Patient ‘C’ and ‘E’ were missed for follow-up at 2nd and 3rd visit, respectively]
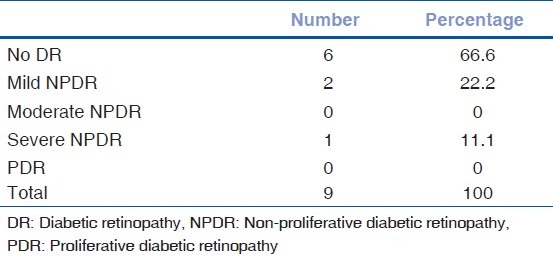
Majority of patients with pupil involvement didn’t have diabetic retinopathy changes [Table 3].
Table 3.
Diabetic retinopathy and internal ophthalmoplegia
Discussion
The present study was aimed at deriving a reliable estimate of incidence of pupillary involvement in diabetes-associated oculomotor nerve palsy. It was unique in that it was a prospective study unlike many other previous studies and that pupillary measurements were done after minimizing variables, which could influence the size of the pupil. The level of scrutiny was increased by measuring pupil size to the nearest of 0.5 mm in all patients. Additionally, the course of anisocoria was compared with that of ophthalmoplegia.
Analysis of data shows that the incidence of pupillary involvement was 25.7%. In a similar study by Jacobson, the incidence was found to be 22.7%.[11] But, the difference in the incidences, found on comparing our study with that of Jacobson, was statistically not significant on doing chi square test (P = 0.288). Most previous studies quote much lower incidences than that derived from this study and the study by Jacobson.[1,3,6,7] The reason for this could be two fold. Most previous studies chose pupillary reaction to light as the primary end point for defining pupil involvement. This could have led to underestimation of the pupillary involvement as reaction of pupil to light could be influenced by various factors, like brightness of light, emotional stimuli and accommodation. In this study, the primary end point was anisocoria rather than pupillary reaction to light. As all other external factors which could influence the pupillary size were controlled, the difference in size of the involved pupil and the fellow pupil was more accurate in predicting pupillary involvement than pupillary reaction alone. Secondly, in our study, the level of accuracy of pupillary measurement was increased by using a pupil gauge with a unit measurement of 0.5 mm.
Most patients developed maximum anisocoria within the 1st 2 weeks after onset of symptoms, which is also similar to the observation in Jacobson's study.[11] However, this time interval could have been artifactually prolonged as patients were not followed up on a daily basis. Had all patients been seen on a daily basis, the maximum anisocoria might have been detected at a much earlier stage.
A fully-dilated, non-reactive pupil is found in 51% to 71% of patients with aneurysmal compression of oculomotor nerve.[5,12] In aneurysms compressing the 3rd nerve, anisocoria progresses and the pupil becomes maximally dilated within the 1st 2 weeks of the presentation. A certain number of patients in this study too showed progression of anisocoria during the 1st 2 weeks of presentation. But, the pupil remained incompletely involved and variably reactive in all patients in this study. Additionally, none of the patients had an anisocoria of > 2 mm. These characteristics of the pupil help to distinguish diabetic from aneurysmal injury of the oculomotor nerve.
From the statistical analysis performed on the course of anisocoria, it can be inferred that the degree of anisocoria normalized maximally by the 3rd visit (after 8 weeks) as compared to the 2nd visit (after 2 weeks). While 1 patient was lost to follow-up, pupil normalized in a majority of patients (55.5%). The direct pupillary reaction, which was impaired during the initial 2 visits, normalized or near normalized as the ophthalmoplegia reversed and the pupil came back to its original size.
Majority of subjects in both pupil-involved and pupil-spared groups showed a recovery of ophthalmoplegia over the initial 2 visits. This is in contrast to a study by Jacobson and Broste where an early progression of ophthalmoplegia was seen in 69% of the individuals and mentions that early progression may not be recognized as a common characteristic if the patient is first seen after 1 week of onset of double vision.[9] The reason for discrepancy between the findings of this study and that of the above-stated study might be due to the fact that majority of subjects in our study presented to us after 1 week of onset of their symptoms and also that the subjects were followed up 2 weeks after their initial visit. Ophthalmoplegia would have already-progressed and then recovered by the time we identified a change from the previous visit to a subsequent evaluation. Had all patients been seen on a daily basis, the progression would have been probably more evident.
It was also inferred that ophthalmoplegia recovers relatively more significantly and much earlier than anisocoria.
No statistically significant difference was found between the course of anisocoria and ophthalmoplegia although clinically both anisocoria and ophthalmoplegia showed an improvement and resolution at the 3rd visit in majority of the subjects. Although the P value was not significant (P value 0.086), it was close to 0.05, which denotes that a statistically significant association would have been seen had the sample size been more in the present series.
Majority of the patients with pupillary involvement showed no diabetic retinopathy changes or had less severe grades of diabetic retinopathy. A Similar result was reported in a study by Acaroglu et al., in which presence and level of diabetic retinopathy was found to be significantly lower in diabetics with cranial nerve palsy than in the age, sex, and disease-duration-matched controls.[13] The relatively milder form of diabetic retinopathy could be accounted for by the shorter duration of diabetes in the majority of subjects (Mean 7.27 years).
In conclusion, pupil involvement in patients with diabetes-associated oculomotor nerve palsy occurs in about 1/4th of all cases. Although pupil may be involved in both, certain pupil characteristics like an incomplete involvement and anisocoria < 2 mm may help to distinguish diabetic (ischemic) from aneurysmal (compressive) injury of the oculomotor nerve. Imaging may not be required in pupil-sparing oculomotor nerve palsies in patients over 50 years with known vasculopathic risk factors although this is associated with the rare risk of missing an aneurysm sparing the pupils. These patients could be just treated conservatively and followed up on a regular basis, if possible almost daily for 2 weeks for progression of anisocoria and ophthalmoplegia to diagnose an early aneurysm as mortality rate due to aneurysm rupture could reach up to 86% and 20% of untreated aneurysms will re-bleed within 2 weeks of the 1st bleed.[14,15]
Imaging should be considered in those cases of pupil-involved oculomotor nerve palsies if patient presents with additional cranial nerve palsy or neurological abnormalities and pupil shows characteristics of a compressive lesion even if history is suggestive of an ischemic lesion.
Majority of cases of ischemic oculomotor nerve palsy show spontaneous resolution with medical treatment alone in contrast to nerve palsy due to aneurysmal injury where earliest possible surgical intervention is required.
Limitations of this study include the following issues. The incidence could have been more accurate had the sample size been more than that in the current study. The course of ophthalmoplegia and anisocoria could have been more precisely-studied had the patients been followed up at closer intervals or even on a daily basis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rucker CW. Paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1958;46:787–94. doi: 10.1016/0002-9394(58)90989-9. [DOI] [PubMed] [Google Scholar]
- 2.Rucker CW. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1966;61:1293. doi: 10.1016/0002-9394(66)90258-3. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JE, Cogan DG. Diabetic third nerve palsy with special reference to the pupil. Arch Ophthalmol. 1960;64:592–600. doi: 10.1001/archopht.1960.01840010594018. [DOI] [PubMed] [Google Scholar]
- 4.Trobe JD. Third nerve palsy and the pupil. Arch Ophthalmol. 1988;106:601–2. doi: 10.1001/archopht.1988.01060130655019. [DOI] [PubMed] [Google Scholar]
- 5.Keane JR. Aneurysms and third nerve palsies. Ann Neurol. 1983;14:696–7. doi: 10.1002/ana.410140622. [DOI] [PubMed] [Google Scholar]
- 6.Green WR, Hackett ER, Schlezinger NS. Neuro-ophthalmic evaluation of oculomotor nerve paralysis. Arch Ophthalmol. 1964;72:154–67. doi: 10.1001/archopht.1964.00970020154005. [DOI] [PubMed] [Google Scholar]
- 7.Zorrilla E, Kozak GP. Ophthalmoplegia in diabetes mellitus. Ann Intern Med. 1967;67:968–76. doi: 10.7326/0003-4819-67-5-968. [DOI] [PubMed] [Google Scholar]
- 8.Teuscher AU, Meienberg O. Ischaemic oculomotor nerve palsy; Clinical features and vascular risk factors in 23 patients. J Neurol. 1985;232:144–9. doi: 10.1007/BF00313889. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson DM, Broste SK. Early progression of ophthalmoplegia in patients with ischemic oculomotor nerve palsies. Arch Ophthalmol. 1995;113:1535–7. doi: 10.1001/archopht.1995.01100120065011. [DOI] [PubMed] [Google Scholar]
- 10.Yaqub BA, Siddique A. Effects of methylcobalamin on diabetic neuropathy. Clin Neurol Neurosurg. 1992;94:105–11. doi: 10.1016/0303-8467(92)90066-c. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DM. Pupil involvement in patients with diabetes-associated oculomotor nerve palsy. Arch Ophthalmol. 1998;116:723–7. doi: 10.1001/archopht.116.6.723. [DOI] [PubMed] [Google Scholar]
- 12.Kissel JT, Burde RM, Klingele TG, Zeiger HE. Pupil sparing oculomotor palsies with internal carotid-posterior communicating aneurysms. Ann Neurol. 1983;13:149–54. doi: 10.1002/ana.410130207. [DOI] [PubMed] [Google Scholar]
- 13.Acaroglu G, Akinci A, Zilelioglu O. Retinopathy in patients with diabetic ophthalmoplegia. Ophthalmologica. 2008;222:225–8. doi: 10.1159/000130070. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsumi K, Ueki K, Morita A, Kirino T. Risk of rupture from incidental cerebral aneurysms. J Neurosurg. 2000;93:550–3. doi: 10.3171/jns.2000.93.4.0550. [DOI] [PubMed] [Google Scholar]
- 15.Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the Cooperative Study. J Neurosurg. 1966;25:321–68. doi: 10.3171/jns.1966.25.2.0219. [DOI] [PubMed] [Google Scholar]


