Abstract
Aim:
To report our experience with the fibrin sealant as a suture substitute for securing the human scleral patch graft during implantation of Ahmed glaucoma valve (AGV).
Materials and Methods:
A retrospective, non-comparative study of 12 eyes of 12 patients who underwent an AGV implantation with fibrin sealant for part of the procedure during June 2009 to September 2010.
Results:
The mean patient age was 21.5 ± 20.6 years. Male: Female ratio was 2 : 1. Seven (58.3%) patients were monocular. The indications for AGV were varied. The mean number of intra-ocular surgeries prior to an implantation of AGV was 1.8. The mean follow-up duration was 24.5 ± 17.9 weeks. There was a statistically significant reduction in the mean IOP and in the mean number of anti-glaucoma medications at the final visit compared to the pre-operative values (P < 0.01, paired t test). Conjunctival retraction was seen in 1 (8.3%) case. The scleral patch graft was retracted posteriorly in another (8.3%) case. There was no case of AGV tube exposure, tube-cornea touch, or conjunctival erosion. Vision threatening complication viz. late post-operative rhegmatogenous retinal detachment, unlikely to be related to the use of the fibrin sealant, occurred in 2 (16.6%) eyes.
Conclusion:
The fibrin sealant offers the advantages of safety and convenience to the placement of a scleral patch graft during an AGV implantation.
Keywords: Ahmed glaucoma valve, fibrin glue, fibrin sealant
The Ahmed glaucoma valve (AGV) is rapidly gaining popularity in the management of refractory glaucoma cases. It is a common practice to use both absorbable and non-absorbable sutures during an implantation of a glaucoma drainage device (GDD). Suture material is typically used for securing the GDD plate to the sclera, securing the silicone tube to the sclera, suturing a patch graft or scleral flap over the silicone tube and for conjunctival closure.
Fibrin sealant has been used successfully in ophthalmic surgery as a suture substitute.[1,2] A couple of small scale studies have favorably reported on the safety and efficacy of the fibrin sealant as a suture substitute for placement of the patch graft and/or closure of the conjunctiva during GDD implantation surgery.[3,4] There is concern about the fibrin sealant not providing enough tensile strength to keep a patch graft or conjunctiva in place. High price of the fibrin sealant is of additional concern. Here, we report our experience with the fibrin sealant as a suture substitute for placement of the human scleral patch graft during implantation of AGV. We also suggest measures to reduce the per-patient-price of the fibrin sealant.
Materials and Methods
Study design
This was a retrospective, non-comparative study. Patients who underwent an AGV implantation with fibrin sealant for part of the procedure during June 2009 to September 2010 were identified by means of operative records. Post-surgical follow-up of <6 weeks was the exclusion criterion. All patients signed a written, informed consent.
Surgical procedure
All surgeries were performed by 1 of the 2 authors (NSC, AN) using an identical technique. The surgical procedure was performed under general anesthesia or a peribulbar block. The conjunctival incision was made 4 - 5 mm behind and parallel to the corneal limbus for approximately 100° in the supero-temporal quadrant. A careful dissection was done antero-posteriorly in the sub-conjunctival plane. The AGV (model FP7 or FP8, New World Medical, Rancho Cucamonga, LA) was primed by injecting 1 - 2 ml balanced salt solution. The plate of an AGV was placed at 8 mm behind the corneal limbus and secured to the sclera with 8-0 nylon suture material (M/S GN Corporation Ltd., Yamanashi, Japan). This was followed by placement of the silicone tube into the anterior chamber or pars plana region through a 23-gauge needle track. The silicone tube was shortened to the desired length prior to insertion. The anterior part of the tube was covered with previously prepared human donor scleral patch graft. The fibrin sealant (Tisseel kit, Baxter AG, Vienna, Austria) was used for gluing the patch graft. An overlying conjunctiva was sutured with 8-0 polyglactin suture material (Ethicon inc., Aurangabad, India). The eye was inspected for any leaks as the anterior chamber was inflated to a proper pressure using balanced salt solution. Postoperatively, all cases were prescribed Ciprofloxacin eye drops (Cipla Ltd, Mumbai, India) 4 times a day for a week and 6 weeks tapering regimen of Prednisolone acetate eye drops (Allergan India Private Limited, Bangalore, India).
Preparation of donor scleral graft
The donor scleral tissue preserved in absolute alcohol was used in every case. The tissue was cleaned of all the uveal tissue attachments, washed thoroughly with balanced salt solution and cut into the desired size (4 - 5 × 4 - 5 mm).
Fibrin sealant
Tissel kit (Baxter AG, Vienna, Austria), a biodegradable, 2 component fibrin sealant, was used in every case. Before use, bottles containing the 2 components were thawed to room temperature.[5] The fibrinolysis inhibitor, Aprotinin, was added to the sealer protein concentrate vial followed by warming in a patented fibrotherm device.[5] The second component was prepared by injecting calcium chloride solution into the thrombin 4 vial, which was then warmed.[5] We preferred thrombin 4 over thrombin 500 as it allows sufficient time (60 seconds versus 10 seconds, respectively) for approximation of the patch graft to the underlying sclera. The required dose of the fibrin sealant was 0.1 to 0.2 ml of each of thrombin and fibrinogen solutions. After application, the donor tissue was pressed gently over the sealant for 3 minutes for firm adhesion.
Data collection and analysis
The data collection included information on patient demography, diagnosis of glaucoma, prior ocular surgeries, measurements of visual acuity; intraocular pressure (IOP); number of anti-glaucoma medications at the pre-operative and every post-operative follow-up visit and complications, if any. Visual acuity was measured using Snellen visual acuity chart. We measured IOP either by applanation tonometer viz. Goldmann tonometer (Haag-Streit, Switzerland), a hand-held Perkin's tonometer (Haag-Streit, Essex, UK) or by Tonopen XL (Reichert ophthalmic instruments, Walden ave. Depew, NY, USA). Finger tension of the globe was assessed whenever measurement of IOP was not possible. The cause(s) for low vision and post-operative reduction in visual acuity, if any, were also recorded. Surgical success was defined as a final IOP between 5 and 22 mm Hg without (complete success) or with topical anti-glaucoma medication(s) (qualified success) and without any vision threatening complication. Descriptive statistics were calculated. Paired t test was used to compare the measurements of IOP and the number of anti-glaucoma medications at the pre-operative and the final visits. Data analysis was done using SPSS for Windows (SPSS Inc., Chicago, IL).
Results
13 patients underwent implantation of AGV with fibrin sealant for part of the procedure during the study period. One patient was excluded because of an insufficient follow-up. The data from 12 eyes of 12 patients was analyzed. Table 1 shows the patient data. Table 2 shows the demographic and pre-operative data. 7 (58.3%) patients were monocular. The mean number of intra-ocular surgeries prior to an implantation of AGV was 1.8. One eye (case 9) received FP8 model of AGV. All other eyes received FP7 model of AGV. Table 3 shows the post-operative data. The mean pre-operative IOP in 9 eyes, in which it could be measured, was 34.4 mm Hg. Post-operatively, IOP could be measured in 6 eyes. Its mean value in these eyes was 9.5 mm Hg at the final post-operative visit. The mean number of anti-glaucoma medications decreased from 2.5 at the pre-operative visit to 0.6 at the final post-operative visit. The postoperative reductions in IOP and in number of anti-glaucoma medications were statistically significant (P < 0.01, paired t test).
Table 1.
Patient data
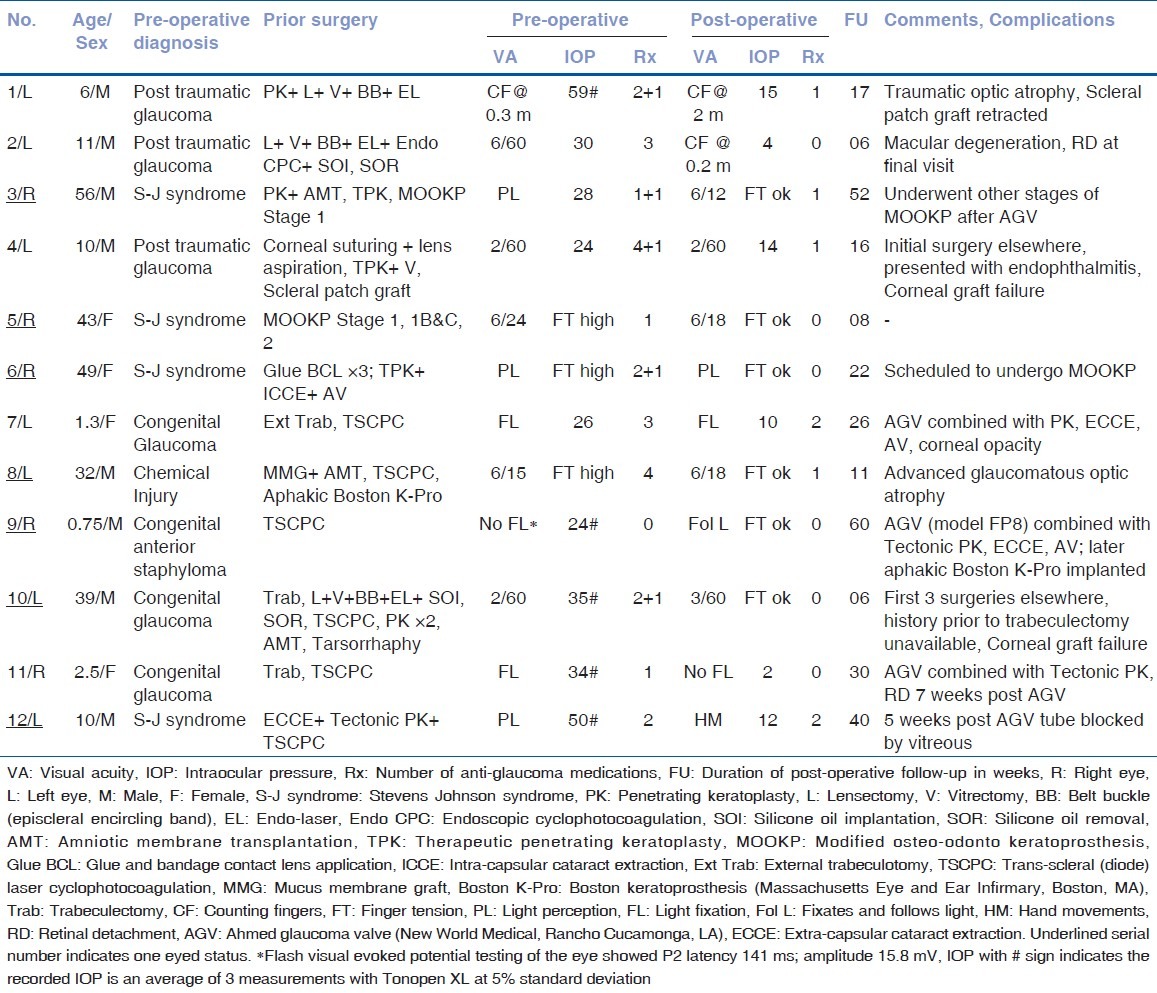
Table 2.
Demographic/Pre-operative data
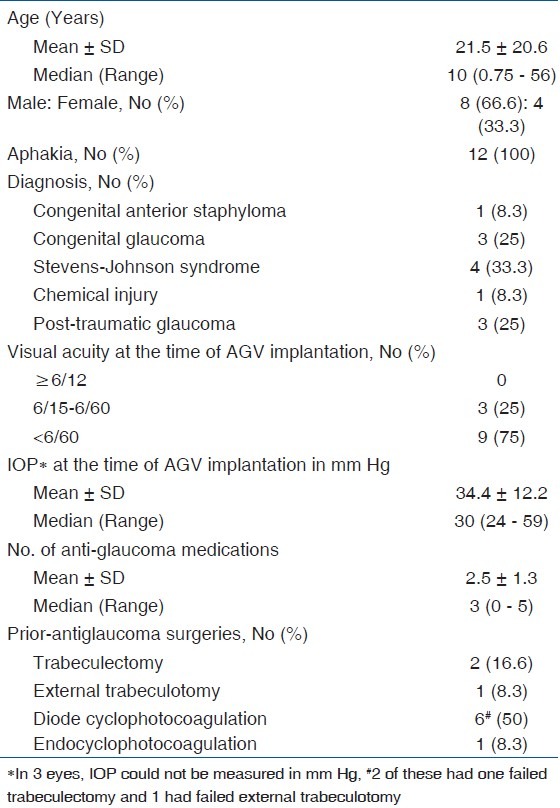
Table 3.
Post-operative data
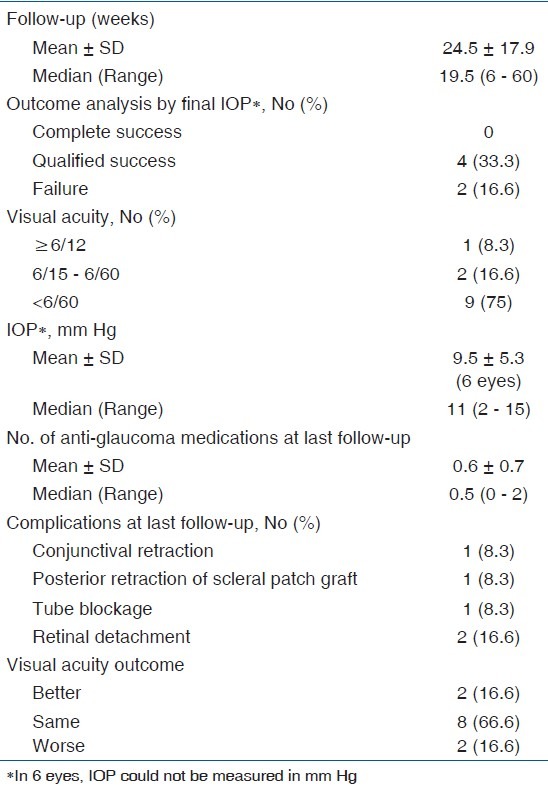
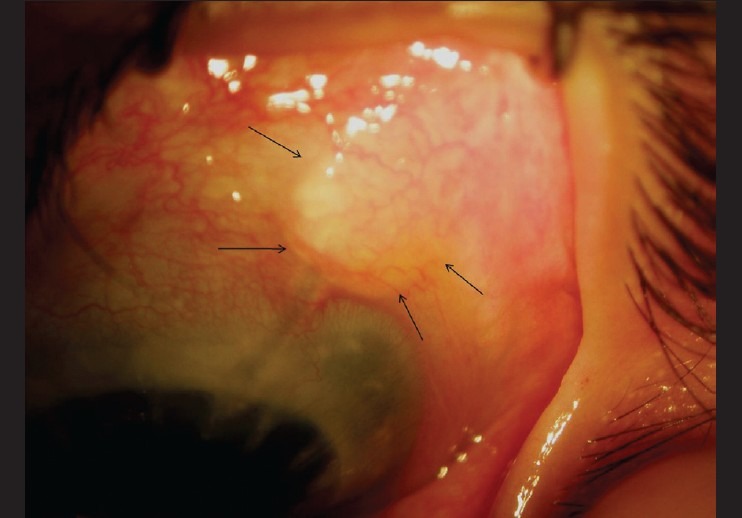
Fig. 1 shows a well-stuck scleral patch graft. The scleral patch graft was posteriorly retracted in case 1 at the last follow-up [Fig. 2]. Conjunctival retraction occurred in case 10 in the second post-operative week, the scleral patch graft was partly exposed, and it epithelized over the next 3 weeks. There was no case of AGV tube exposure, tube-cornea touch, or conjunctival erosion. The IOP was 38 mm Hg, and the bleb was shallow in case 12 at 5 weeks post-surgery. The AGV tube was suspected to be blocked by vitreous strands, requiring anterior vitrectomy and intra-cameral flushing of the tube with balanced salt solution. The vision-threatening complications were few [Table 3]. Two patients (cases 2 and 11) experienced a decrease in visual acuity due to late post-operative rhegmatogenous retinal detachment.
Figure 1.
Photograph of case 7 taken under surgical microscope at 26 weeks post-surgery. The scleral patch graft (outlined by arrows) was secured with the fibrin sealant
Figure 2.
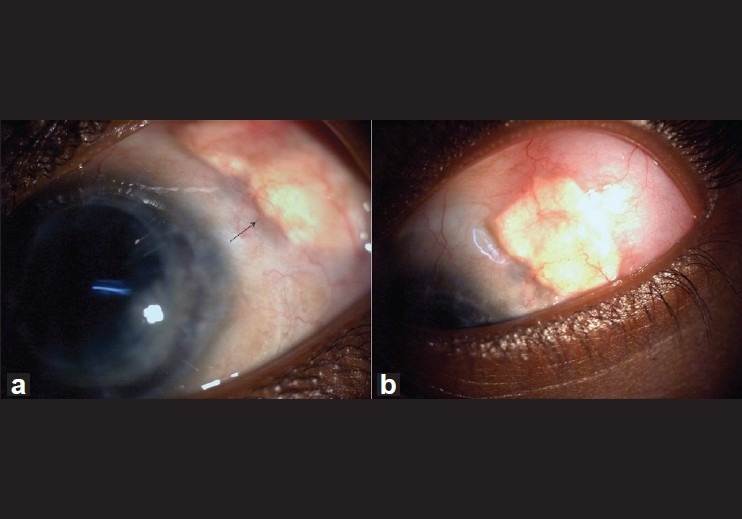
Slit lamp photograph of case 1. The arrow in (a) indicates posterior retraction of the scleral patch graft. Note a good bleb in (b)
Discussion
In this series, we used a fibrin sealant to secure the human scleral patch graft over the extra-ocular portion of the AGV tube. Although other fibrin adhesives are available, we preferred Tisseel since it is more extensively researched as a suture substitute in ophthalmic surgery. We did not encounter any case of AGV tube exposure, tube-cornea touch, or conjunctival erosion.
During an implantation of a GDD, a patch graft prevents the silicone tube from touching the corneal endothelium.[6] The graft also prevents erosion and extrusion of the silicone tube.[6] The graft doesn’t interfere with bleb formation either.[6] As soon as the silicone tube is inserted into the anterior chamber, aqueous drains and hypotony follows. Thus, the globe is hypotonic unless an anterior chamber maintainer is used while the scleral patch graft is being sutured in place. While superficial suture bites can make the graft unstable, deep scleral bites carry a considerable risk of globe perforation. Use of the fibrin sealant to stick the patch graft over the implant tube can avoid suturing the graft to the underlying sclera and thereby offers a safety advantage to the GDD implantation surgery.
Kahook and Noecker[3] used a 6 × 6 mm2 pericardial patch graft of 0.4 mm thickness. Availability and cost are barriers to the routine use of pericardial patch graft to cover the GDD tube in our scenario. Potential for pericardial graft thinning and possible tube erosion are additional concerns. In a series, 5 (11.3%) out of 44 eyes developed thinning of the pericardial patch graft over a mean follow-up of 10.2 months.[7] We used human scleral patch graft to cover the AGV tube. The thickness of the sclera varies depending on its location on the globe. A human scleral patch graft can be thicker than the pericardial patch graft. The thickness of human sclera obtained from formalin-fixed eyes, varied from 0.39 ± 0.17 mm near the equator to 0.9 to 1.0 mm near the optic nerve.[8] There is a concern about fibrin sealant not providing enough tensile strength to keep the patch graft in place. Shigemitsu and Majima[9] compared tensile strength of the cataract surgery wounds sutured with various methods and glued with bio-tissue adhesives in rabbit eyes. The strength of the wound treated with fibrin sealant was much less (43 gf/mm2) compared to the wound sutured with a single 10-0 nylon suture (131 gf/mm2) at 4 days after surgery although the respective strengths were comparable at 28 days after surgery.[9] Zeppa et al.[4] prospectively studied 15 eyes for the safety and efficacy of a fibrin sealant to secure the human scleral patch graft during an AGV implantation. The scleral patch graft was found in place at each check during the follow-up period. There was no case of conjunctival erosion over the tube.[4] We did find scleral patch graft retraction in 1 eye [Fig. 2]. The eye had an episcleral encircling band and limited mobile conjunctiva. The complication can be attributed to additional conjunctival scarring and retraction following AGV implantation. He may develop tube erosion in future.
Use of fibrin sealant to stick the patch graft during GDD implantation can cut down the surgical time. Our retrospective and non-comparative study design did not allow commenting on this aspect. Earlier Kahook and Noecker[3] did report reduced mean surgical time by 10 minutes in the Tisseel-assisted group. They retrospectively compared 28 cases of GDD implantation using traditional suture material with 14 cases of GDD implantation using fibrin sealant for portions of the procedure. They used Baerveldt 250 mm2 GDD in all patients. Most of our cases were complicated. The time-saving benefit of fibrin sealant is desirable in such situations.
Kahook and Noecker[3] used the fibrin sealant to close the conjunctiva besides sticking the scleral patch graft. Most of our patients had undergone more than 1 ocular surgery with an inevitable conjunctival manipulation. The resultant conjunctival fibrosis can impact wound closure. Freely mobile conjunctiva is also necessary to limit postoperative conjunctival retraction, which can overcome the tensile strength of the glue. For these reasons, we did not use the fibrin sealant to close the conjunctival wound.
In our study, 2 patients experienced retinal detachment after implantation of AGV. Case 2 was a high myope, suffered from post-traumatic retinal detachment, and developed secondary glaucoma following retinal detachment repair. He presented with recurrent rhegmatogenous retinal detachment 6 weeks after the implantation of AGV. Case 11 had prior unsuccessful trabeculectomy and diode cyclophotocoagulation for intractable congenital glaucoma. She experienced rhegmatogenous retinal detachment 7 weeks after an implantation of AGV. Retinal detachment as a complication of GDD has been reported in as many as 16% cases.[10] Suggested causes of retinal detachment in the literature include previous intraocular operations other than GDD implantation, posterior vitreous detachment in patients with an underlying retinal pathologic condition, chorioretinal scar, trauma, uveitis, retinal apposition from suprachoroidal hemorrhage, vitreous incarceration, inadvertent scleral perforation, and retinal dialysis from the pars plana-positioned tube.[10,11] Nevertheless, the retinal detachment was unlikely to be related to the use of fibrin sealant to stick the scleral patch graft.
The duploject application system, a double-barrel syringe, is supplied with the Tisseel kit.[5] The required dose of the sealant in ophthalmic surgery is much less than the dose in general and cardiovascular surgery. Therefore, we prefer 2 separate 1 ml syringes over the double-barrel syringe to load the reconstituted components. This technique enables drop by drop application of the sealant and limits spillage. Moreover, the quantity of the sealant lost in the joining piece of the duploject system is utilized.
The preparation of the components of the smallest available Tisseel kit gives 1.0 ml each of thrombin and fibrinogen solutions.[5] This amount is sufficient for the gluing of surfaces for an area of at least 10 cm2.[5] The reconstituted solutions must be used within 4 hours.[5] We elect cases requiring the fibrin sealant for various conditions besides GDD implantation e.g. pterygium surgery, forniceal reconstruction, amniotic membrane transplantation etc. and operate them simultaneously in adjacent operation theatres. The required amount of the reconstituted components of the fibrin sealant is taken into separate 1 ml syringes and used. We are able to use the components of 1 kit for an average of 4 patients. This approach allows significant reduction in per-patient-price of the fibrin sealant. However, this approach carries an inherent risk of kit contamination. We recommend strict aseptic precautions during usage of the components in multiple patients.
The retrospective and non-comparative nature of the study did not allow us to compare the safety and efficacy of the newer technique to the current practice of suturing the scleral patch graft during implantation of AGV. Also, a higher number of patients are necessary to understand the possible complications or difficulties with this technique. Our study at least presents an alternative to the suture-assisted scleral patch grafting during implantation of AGV.
Footnotes
Source of Support: Nil.
Conflict of Interest: Nil.
References
- 1.Chan SM, Boisjoly H. Advances in the use of adhesives in ophthalmology. Curr Opin Ophthalmol. 2004;15:305–10. doi: 10.1097/00055735-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Panda A, Kumar S, Kumar A, Bansal R, Bhartiya S. Fibrin glue in ophthalmology. Indian J Ophthalmol. 2009;57:371–9. doi: 10.4103/0301-4738.55079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahook MY, Noecker RJ. Fibrin glue-assisted glaucoma drainage device surgery. Br J Ophthalmol. 2006;90:1486–9. doi: 10.1136/bjo.2006.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeppa L, Romano MR, Capasso L, Tortori A, Majorana MA, Costagliola C. Sutureless human sclera donor patch graft for Ahmed glaucoma valve. Eur J Ophthalmol. 2010;20:546–51. doi: 10.1177/112067211002000302. [DOI] [PubMed] [Google Scholar]
- 5.Tisseel Kit(R) [package insert] Vienna, Austria: Baxter; 2010. [Google Scholar]
- 6.Freedman J. Scleral patch grafts with Molteno setons. Ophthalmic Surg. 1987;18:532–4. [PubMed] [Google Scholar]
- 7.Raviv T, Greenfield DS, Liebmann JM, Sidoti PA, Ishikawa H, Ritch R. Pericardial patch grafts in glaucoma implant surgery. J Glaucoma. 1998;7:27–32. [PubMed] [Google Scholar]
- 8.Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: Thickness and surface area. Am J Ophthalmol. 1998;125:237–41. doi: 10.1016/s0002-9394(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 9.Shigemitsu T, Majima Y. The utilization of a biological adhesive for wound treatment: Comparison of suture, self-sealing sutureless and cyanoacrylate closure in the tensile strength test. Int Ophthalmol. 1996-1997;20:323–8. doi: 10.1007/BF00176885. [DOI] [PubMed] [Google Scholar]
- 10.Hill RA, Heuer DK, Baerveldt G, Minckler DS, Martone JF. Molteno implantation for glaucoma in young patients. Ophthalmology. 1991;98:1042–6. doi: 10.1016/s0161-6420(91)32179-1. [DOI] [PubMed] [Google Scholar]
- 11.Waterhouse WJ, Lloyd MA, Dugel PU, Heuer DK, Baerveldt G, Minckler DS, et al. Rhegmatogenous retinal detachment after Molteno glaucoma implant surgery. Ophthalmology. 1994;101:665–71. doi: 10.1016/s0161-6420(94)31280-2. [DOI] [PubMed] [Google Scholar]


