Abstract
Background:
The purpose of this study was to develop microemulsion-based hydrogel formulation for topical delivery of bifonazole with an objective to increase the solubility and skin permeability of the drug.
Materials and Methods:
Oleic acid was screened as the oil phase of microemulsions, due to a good solubilizing capacity of the microemulison systems. The pseudo-ternary phase diagrams for microemulsion regions were constructed using oleic acid as the oil, Tween 80 as the surfactant and isopropyl alcohol (IPA) as the cosurfactant. Various microemulsion formulations were prepared and optimized by 32 factorial design on the basis of percentage (%) transmittance, globule size, zeta potential, drug release, and skin permeability. The abilities of various microemulsions to deliver bifonazole through the skin were evaluated ex vivo using Franz diffusion cells fitted with rat skins. The Hydroxy Propyl Methyl Cellulose (HPMC) K100 M as a gel matrix was used to construct the microemulsion-based hydrogel for improving the viscosity of microemulsion for topical administration. The optimized microemulsion-based hydrogel was evaluated for viscosity, spreadability, skin irritancy, skin permeability, stability, and antifungal activity by comparing it with marketed bifonazole cream.
Results:
The mechanism of drug release from microemulsion-based hydrogel was observed to follow zero order kinetics. The studied optimized microemulsion-based hydrogel showed a good stability over the period of 3 months. Average globule size of optimized microemulsion (F5) was found to be 18.98 nm, zeta potential was found to be -5.56 mv, and permeability of drug from microemulsion within 8 h was observed 84%. The antifungal activity of microemulsion-based hydrogel was found to be comparable with marketed cream.
Conclusion:
The results indicate that the studied microemulsion-based hydrogel (F5) has a potential for sustained action of drug release and it may act as promising vehicle for topical delivery of ibuprofen.
Keywords: Antifungal activity, bifonazole, oleic acid, permeability, phase diagram, zeta potential
INTRODUCTION
Fungal skin infections are infections on the skin caused by a fungus. Fungal skin infections usually affect the skin as they live off keratin, a protein that makes up skin, hair, and nails. The symptoms of a fungal skin infection include rashes with a variety of different appearances like red, scaly, itchy, and dry skin.[1]
Topical preparations are used for the localized effects at the site of their application by virtue of drug penetration into the underlying layers of skin or mucous membranes. The main advantage of topical delivery system is that it has ability to deliver drugs more selectively to a specific site (local action). It provides utilization of drugs with short biological half-life, narrow therapeutic window to increase the duration of action.[2]
Approximately 40% of new chemical entities exhibit poor aqueous solubility and presents major challenge to modern drug delivery systems which leads to poor absorption, poor bioavailability, and lack of dose proportionality. However, in many instances, oral administration is unsuitable when the drug undergoes significant degradation in the gastrointestinal tract or is metabolized to a high degree via the first pass effect in the liver. These disadvantages intensified the search for an alternative drug delivery in the form of microemulsion-based hydrogel for topical delivery.[3] The selected drug for the study was bifonazole, which is an antifungal agent having low solubility.
The natural barrier for topical delivery is skin, which makes the drug delivery difficult. Taking this factor into consideration, microemulsions are prepared, which have low skin irritation, high drug loading capacity, and may reduce the diffusion barrier of Stratum corneum by dissolving the lipids in the Stratum corneum and enhancing the permeation of drug.[4,5]
Microemulsions are thermodynamically stable isotropically clear dispersion of two immiscible liquids, such as oil and water, stabilized by an interfacial film of surfactant molecules, with a size range of 5-200 nm and have very low interfacial tension. Because of their unique solubilization properties, microemulsion have attracted increasing attention as potential drug delivery systems, either as vehicles for topical applications or as bioavailability enhancers for poorly water soluble active pharmaceutical ingredients (API).[6] The existence of microdomains of different polarity within the same single-phase solution enables both hydrophilic and lipophilic materials to be solubilized. Advantages associated with microemulsions include their thermodynamic stability, optical clarity, ease of preparation, and high diffusion and absorption rates when compared with solvent without the surfactant system.[7]
Microemulsions offer advantages over traditional creams, gels, and solutions as topical drug delivery. The main disadvantage of the current formulations, that is, topical gels and solutions for treating fungal infections is low solubilizing capacity with lipophilic drugs. The main advantage of microemulsions over gels is that they are used to solubilize drugs and to improve topical drug availability.[3,8] The advantage of microemulsions over the creams is that it improves hydration of stratum corneum, which will increase the drug dermal permeation and the skin flux.[9] Even though microemulsions offer several advantages for topical delivery, it is difficult to stabilize the system because of low viscosity.[7] This problem can be overcome by formulating microemulsion-based hydrogel using polymers such as hydroxy propyl methyl cellulose (HPMC), Carbopol, and xanthan gum.[10] In this study, microemulsion-based hydrogel formulation containing bifonazole was prepared using HPMC K100M as gelling agent.
Bifonazole is a broad spectrum azole antifungal agent, which is effective against yeasts, moulds, dermatophytes, and other fungi and it is used to treat skin infections such as tinea, Athlete's foot (tinea pedis), and ringworm of the body. Bifonazole is topically administered once a day (100 mg/day) for 2-3 weeks to treat Athlete's foot, but it shows very low absorption following topical administration (0.6% of an applied dose) through bifonazole gel. Hence, to increase the topical skin permeability and solubility of bifonazole, microemulsion formulation was developed. Additionally, the half life of bifonazole is only 1-2 h. Thus, to sustain the release of bifonazole and to increase the duration of action, microemulsion-based hydrogel was formulated.[11]
MATERIALS AND METHODS
Materials
Bifonazole was obtained as a gift sample from Vital Healthcare Pvt Ltd., Vapi. Oleic acid, Tween 80, isopropyl alcohol (IPA), and HPMC K100M were obtained from Chemdyes Corporation, Vadodara. All other chemicals used were of laboratory reagent grade.
Solubility study of bifonazole
Screening of various oils, surfactants and cosurfactants were done for Bifonazole solubility. Solubility studies were conducted by placing an excess amount of Bifonazole (approximately 200 mg) in a 2 ml microtube containing 1 ml of each vehicle. Then, the mixture was vortexed and kept for 3 days at 37°C in a shaking water bath to facilitate the solubilization. The samples were centrifuged at 10,000 rpm for 10 min to remove the undissolved bifonazole. The supernatant was taken, diluted with methanol up to 10 times and filtered through Whatman filter paper for quantification of bifonazole by ultraviolet–visible (UV) spectroscopy at 254 nm.[12]
Selection of oil, surfactant, and cosurfactant
Based on the solubility data,[13] oleic acid, Tween 80 and IPA were selected as oil, surfactant, and cosurfactant, respectively.
Construction of pseudo-ternary phase diagrams
Pseudo-ternary phase diagrams were constructed using CHEMIX school 3.51software (. Arne Standnes). The surfactant and cosurfactant used were Tween 80 and IPA, having hydrophilic nature.[14] The pseudo-ternary phase diagrams of oil, surfactant, cosurfactant, and water were constructed using water titration method to obtain the components and their concentration ranges that can results in large existence area of microemulsion. Surfactant was blended with cosurfactant in fixed weight ratios (1:1, 2:1, 3:1, and 4:1). Aliquots of each surfactant and cosurfactant mixture (Smix) were then mixed with oil at room temperature. For each phase diagram, the ratios of oil to Smix were varied as 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9 (w/w). Water was added drop wise to each mixture under vigorous stirring by using magnetic stirrer. No heating was done during the preparation. Then, each mixture was visually observed for transparency. The samples were marked as points in the phase diagram. The area covered by these points was considered as the microemulsion region of existence. Quantities of all three phases were taken in %w/w.[15,16]
Preparation of microemulsion
After the identification of microemulsion region in the phase diagram, the microemulsion formulations were selected at desired component ratios. The preparation of selected microemulsion was simply performed by adding the weighed components together and stirring to form a clear microemulsion.[17,18]
Optimisation of microemulsion by 32 full factorial design
After determination of the concentration range, such as 2.5- 10%w/w for oil and 50-60%w/w for Smix as per preliminary trial batches, the 32 factorial design was applied for the optimization of final formulation.[19]
The developed microemulsion formulation was evaluated for the responses and the experimental values obtained were compared with those predicted by the mathematical models. On the basis of globule size, zeta potential, % drug release, and % permeability, formulation 5 (F5) was selected for the further study.
Method of preparation of microemulsion-based hydrogel
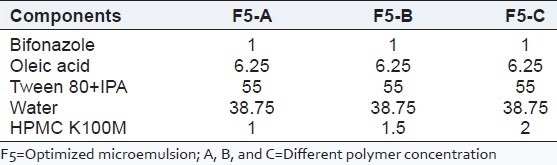
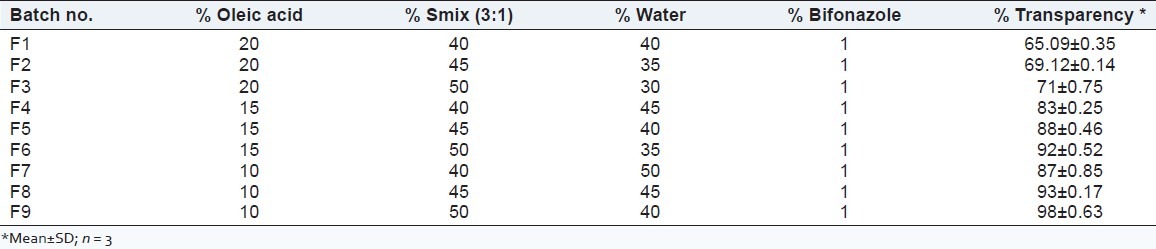
The HPMC K100M as a gel matrix was used to construct the microemulsion-based hydrogel for improving the viscosity of microemulsion for topical administration. The hydrogel was slowly mixed with microemulsion under stirring. As the microemulsion was added to the hydrogel, the viscosity of system microemulsion-based hydrogel was decreased. Hence, to obtain sufficient viscosity of microemulsion-based hydrogel, the hydrogels were prepared at various concentrations (1, 1.5, and 2%w/w), and the final concentration was selected on the basis of viscosity and transparency[20,21] [Table 1].
Table 1.
Composition of microemulsion-based hydrogel formulations (%W/W)
Evaluation of microemulsion, hydrogel, and microemulsion-based hydrogel
Microemulsion, hydrogel, and microemulsion-based hydrogel were evaluated for % transmittance, globule size, zeta potential, dilutability, dispersion stability studies, viscosity, pH, spreadability, drug content, skin irritation study, and in vitro drug permeation % Transmittance, Globule size, and Zeta Potential Determin % Transmittance was observed by UV spectrophotometer at 630 nm. The globule size and zeta potential of the microemulsion was measured by zeta sizer ZS nano series (Malvern Instruments U. K.).[22]
Viscosity and pH
The viscosity was determined by using Brookfield viscometer. The spindle number 2 was dipped in microemulsion and rotated at 5, 10, 20, and 50 rpm at room temperature.[23] For the viscosity of hydrogel, the spindle number 6 was dipped in the preparation and rotated at 5, 10, 20, and 50 rpm at room temperature. The viscosity of microemulsion-based hydrogel was determined by using Brookfield viscometer. For viscosity determination of microemulsion-based hydrogel, 175 g of microemulsion-based hydrogel was filled in a 250 ml beaker and the viscosity was measured by using Spindle number LV4.[24]
The pH was determined using digital pH meter. Microemulsion-based hydrogel (2.5 g) was weighed accurately and dispersed in 25 ml of purified water. The pH meter was calibrated before each use with buffer solution of pH 4.0, 7.0, and 9.0. The measurement of pH of formulation was done in triplicate and mean values were calculated.[25]
Dilutability
The microemulsions formed were diluted in 1:10, and 1:100, ratios with double distilled water to check if the system shows any signs of separation.[26]
Dispersion stability studies
Selected formulations were centrifuged at 3000 rpm for 30 min. The formulations having no phase separations were taken for the heating and cooling cycle (freeze thaw cycle). Six cycles between the temperatures 4°C (refrigerator) and 45°C in a hot air oven with storage at each temperature for not less than 48 h were done. The formulations which were stable at these temperatures were selected for further studies.[27]
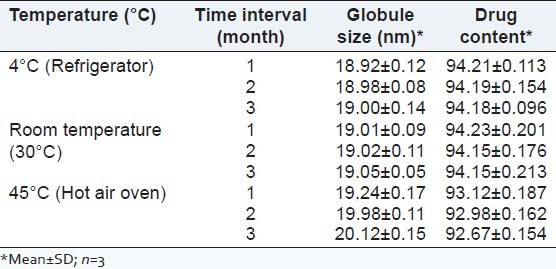
The optimized microemulsion formulation was stored at 4°C, room temperature and 45°C for 3 months and samples were evaluated for physicochemical parameters like globule size and drug content at 1 month interval.
In vitro drug release studies
The in vitro drug release studies were performed by using Franz diffusion cell with cellophane paper. The water jacketed recipient compartment had total capacity of 25 ml and it had 2 arms, one for sampling and another for thermometer. The donor compartment had internal diameter of 2 cm.[28] The donor compartment was placed in such a way that it just touches the diffusion medium in receptor compartment. The receptor compartment contained phosphate buffer saline (PBS) that was maintained at 37°C ± 1°C. The membrane was equilibrated before application of the microemulsion equivalent to 10 mg of drug onto the donor side. Samples were periodically withdrawn from the receptor compartment, replacing with the same amount of fresh PBS solution, and assayed by using a spectrophotometer at 254 nm.
Ex vivo skin permeation studies
Ex vivo skin permeation study was performed by using Franz diffusion cells with an effective diffusion area of 2 cm2. The excised skin samples (dorsal side) of rat were clamped between the donor and the receptor compartment of Franz diffusion cells with the Stratum corneum facing the donor compartment. Then, 1 g of microemulsion containing 1% (w/w) bifonazole was applied on the donor compartment. The receptor compartment was filled with PBS pH 7.4 and maintained at 37°C with stirring at 100 rpm. At predetermined time intervals (30 min), 1 ml receptor medium was withdrawn and the same volume of pure medium was immediately added into the receptor compartment. The procedure was repeated up to 8 h.[29] All samples were filtered through Whatman filter paper and analyzed by UV spectrophotometer at 254 nm.
Spreadability study
An apparatus suggested by Mutimer et al.[30] modified suitably in the laboratory and was used for spreadability study. The apparatus was made of wooden block with scale and two glass slides having a pan mounted on a pulley. Excess formulation was placed between two glass slides and 100 g weight was placed on the upper glass slide for 5 min to compress the formulation to uniform thickness. Weight (100 g) was added to the pan. The time in seconds required to separate the two slides was taken as a measure of spreadability.
The spreadability was calculated by using the following formula:
S = (m × l)/t
where S is spreadability; m is weight tied to the upper slides; l is length of glass slide and; t is time taken in seconds.
Drug content studies
Microemulsion-based hydrogel equivalent to 10 mg of bifonazole was taken in 10 ml volumetric flask containing 5 ml methanol and stirred for 30 min. Volume was made up to 10 ml with methanol. From the above solution, 0.1 ml was further diluted with 10 ml methanol to get 10 μg/ml. The resultant solution was filtered through Whatman filter paper and absorbance of the solution was measured at 254 nm using UV spectrophotometer.
Skin irritancy studies
As the formulation was intended for dermal application, skin irritancy should be tested. Skin irritation tests were conducted in rabbits to determine irritancy after single application of microemulsion-based hydrogel. The back of rabbits after depilation was used in this experiment. About 0.5 g of commercial bifonazole cream and microemulsion-based hydrogel was applied on two different rabbits and then the applied area was covered with gauze and adhesive bandage. The formulation was removed after 24 h and the exposed skin was graded for formation of edema and erythema. Scoring was repeated 72 h later. Based on the scoring, the formulation was graded as ‘nonirritant’, ‘irritant,’ and ‘highly irritant.’[31,32]
The total scores for irritation test were calculated using the following equation:
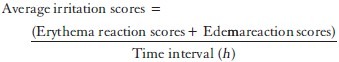
In vitro antifungal activity using cup-plate method
In vitro antifungal activity was carried out by cup plate (or cylinder plate) method. The cup plate method depends upon diffusion of antifungal from a vertical cylinder through a solidified agar layer in a petri dish or plate to an extent such that growth of added micro-organism is prevented entirely in a zone around the cylinder containing antifungal agent.[33]
The overnight grown culture of Candida albicans was inoculated into the sterilized agar media plates. After solidification, wells were cut into the media and fixed with 100 mg of the specimens to be tested using marketed bifonazole cream and microemulsion-based hydrogel. The plates were incubated at room temperature and the widths of zone of inhibitions resulting after drug diffusion into media were measured.
Statistical analysis
The data obtained for different formulations was analyzed by one way analysis of variance (ANOVA).[34]
RESULTS AND DISCUSSION
Solubility study of bifonazole
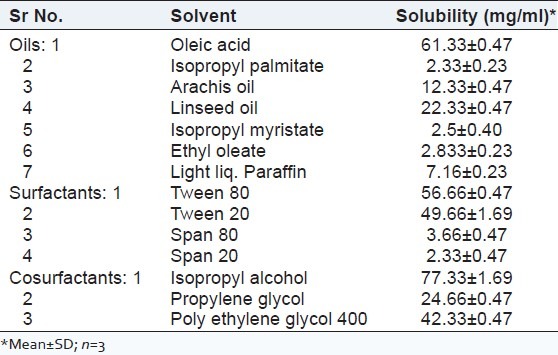
The solubility of Bifonazole in oleic acid, Tween 80, and IPA was highest among the other vehicles as shown in Table 2.
Table 2.
Solubility of bifonazole in various solvents saturated for 72 h at 37°C
Optimization of (s/co-s) ratio from pseudoternary phase diagram
From the pseudoternary phase diagrams for microemulsion s along with the ratios of surfactant and cosurfactant, as 1:1, 2:1, 3:1, and 4:1 shown in Figure 1, the phase diagram at 3:1 (s/co-s) weight ratio had obtained the highest area of emulsification and so it was selected for further optimization.
Figure 1.
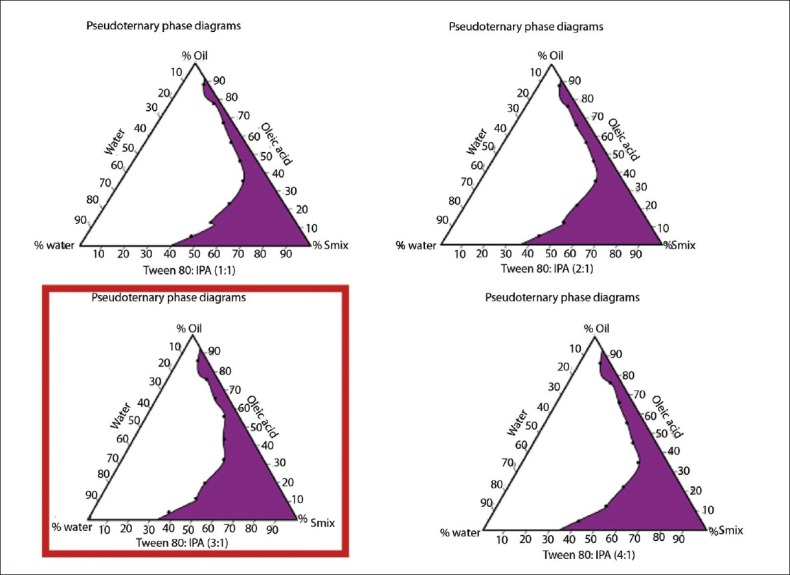
The pseudo ternary phase diagrams of the oil-surfactant-water system at 1:1, 2:1, 3:1, and 4:1 weight ratio of Tween 80 to IPA mixture at 25°C
Preliminary trial batches for optimization of oil and smix concentration range
By trial and error method, the concentration range of oil and Smix was determined based on water uptake in the formulation and the % transparency which was found to be 2.5-10%w/w for oil and 50-60%w/w for Smix. The %w/w of oil and Smix was optimized based on the considerations that high concentration of surfactant could cause toxicity and skin irritation. Sixty percent (60%) (w/w) S/Co-s was selected as maximum safe concentration. It has been reported that the highest skin flux and permeability coefficient was observed for the formulation that contains maximum amount of water. Hence, 47.5% (w/w) of water proportion was selected. Further, 37.5% (w/w) of surfactant and 12.5% (w/w) of cosurfactant were selected as minimum requirement for stable and successful microemulsion formulation, and 10% (w/w) of oil was selected as the highest quantity of oil that can be incorporated to form a stable microemulsion as shown in Table 3.
Table 3.
Trial batches for microemulsion made by water titration method
Optimization of microemulsion by 32 factorial design
After applying the 32 factorial design for the optimization of final batch of microemulsion, the 5th batch (F5) containing 6.25% oil and 55% Smix was selected as optimized microemulsion and the further characterization tests were done by using batch F5 [Table 4 and Figure 2].
Table 4.
Design matrix of experimental design and effect of formulation variables on globule size, zeta potential, % release and % permeability
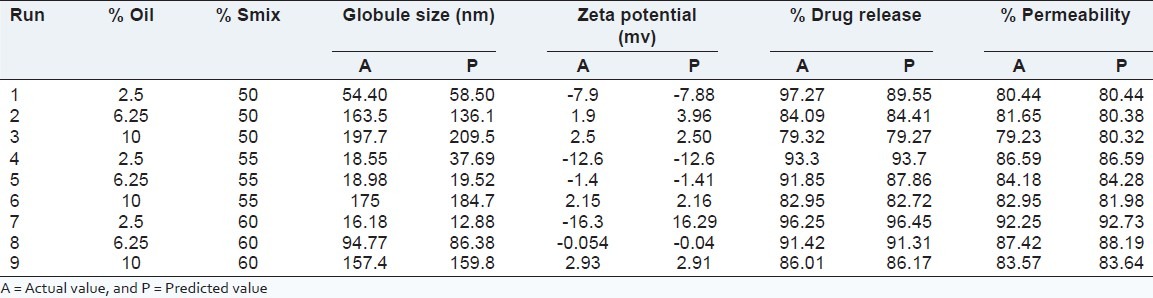
Figure 2.
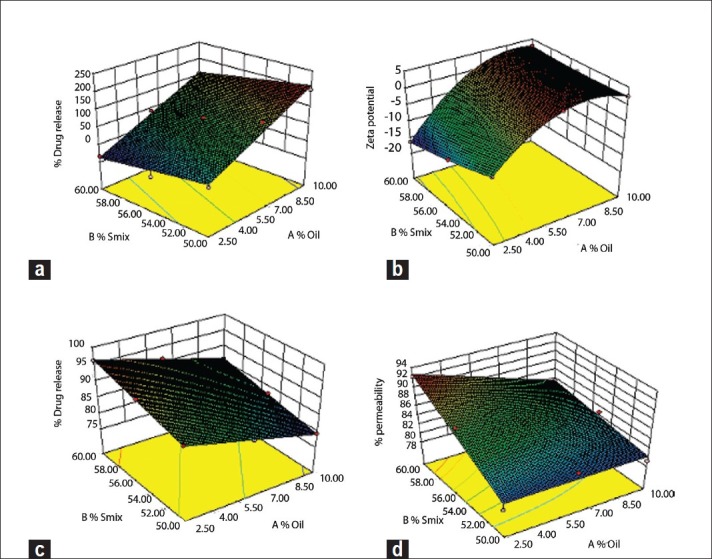
Response surface plot of (a) globule size, (b) zeta potential, (c) %release, and (d) % permeability
Fitting of data to the model
All the responses observed for nine runs were fitted to various models using response surface methodology (Design Expert software). It was observed that the best fitted model was linear.
Optimization of polymer concentration for preparation of microemulsion-based hydrogel
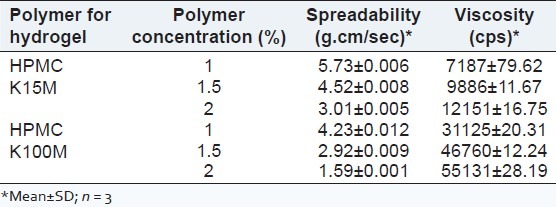
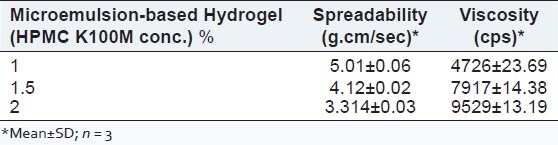
The polymer for preparation of microemulsion-based hydrogel was optimized on the basis of viscosity and 2% of HPMC K100M was selected as the suitable polymer concentration to provide sustained release of bifonazole from the microemulsion-based hydrogel. The viscosity of various hydrogels having different polymer concentration is shown in Table 5.
Table 5.
Viscosity and spreadability of various hydrogels
Evaluation of microemulsion, hydrogel and microemulsion-based hydrogel % transmittance, globule size, and zeta potential determination
The optimized microemulsion (F5) showed 94.1% transparency at 630 nm using UV spectrophotometer, 18.98 nm globule size [Figure 3] and-5.56 mv zeta potential using Malvern particle size analyzer.
Figure 3.
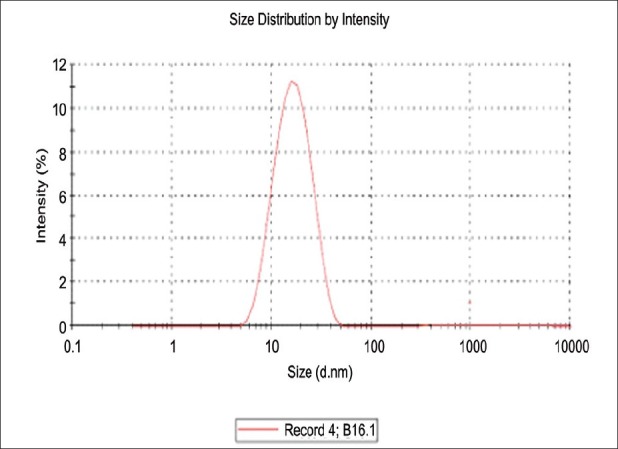
Globule size of F5
The negative zeta potential indicates that globules of microemulsion had no charge, that is, the system was stable. As there was no charge on globules, no flocculation of globules occurred and hence, microemulsion was found to be stable. The results are shown in Table 6 and Figure 4.
Table 6.
Various evaluation parameters of optimized microemulsion and microemulsionbased hydrogel
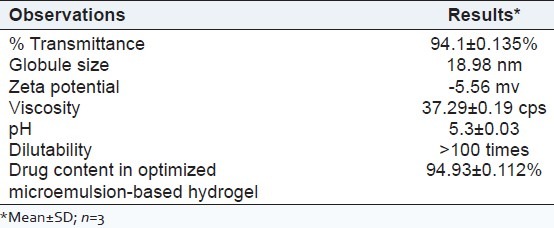
Figure 4.
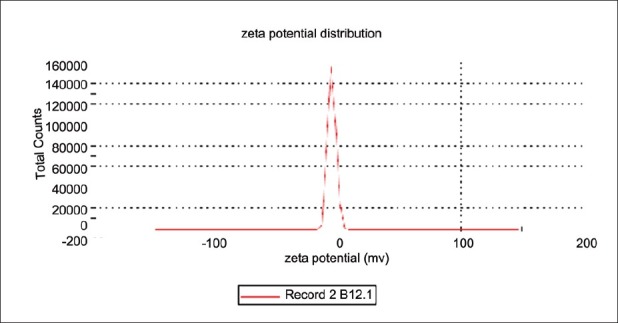
Zeta potential of F5
Dilutability
The microemulsions formed were diluted in 1:10, and 1:100, ratios with double distilled water to check the signs of separation. The optimized microemulsion did not show any signs of phase separation as shown in Table 6.
Viscosity and pH
The viscosity of optimized microemulsion (F5) was found to 37.29 ± 0.19 cps and is shown in Table 6. The viscosity of various hydrogels having different polymer concentration is shown in Table 5. As the viscosity of HPMC K100M (2%) was highest among other polymer concentrations, 2% concentration was selected as the suitable one. It was observed that the microemulsion-based hydrogel with 2% HPMC K100M had the highest viscosity among the other concentrations. Hence, it was selected as the suitable one for topical application. The viscosity of various microemulsion-based hydrogel is given in Table 7.
Table 7.
Spreadability and viscosity data for various microemulsion-based hydrogel having different polymer concentration
The pH of optimized formulation F5 was found to be 5.3, which was nearer to the skin pH and is shown in Table 6.
Stability study
Stability studies indicated that the preparation was stable at room temperature over the period of 3 months. The results are shown in Table 8.
Table 8.
Stability testing showing globule size and drug content of F5 after 1 month interval at various temperatures
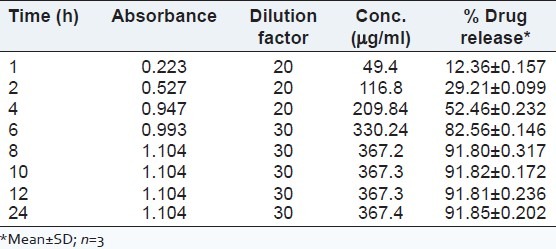
In vitro drug release
It was observed that maximum drug release from microemulsion was achieved within 8 h. The release profile of bifonazole from F5 formulation is shown in Figure 5 and Table 9.
Figure 5.
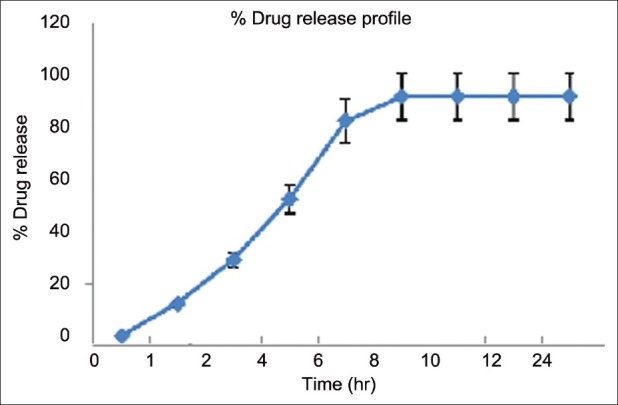
Drug release profile of microemulsions
Table 9.
Drug Release Data of Optimized Microemulsion
Ex vivo permeation study
From the ex vivo permeation studies, the drug permeability was found 84% within 10 h. The results are shown in Table 10 and Figure 6.
Table 10.
Ex vivo skin permeation study from microemulsion
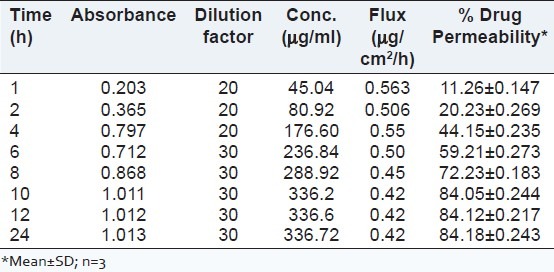
Figure 6.
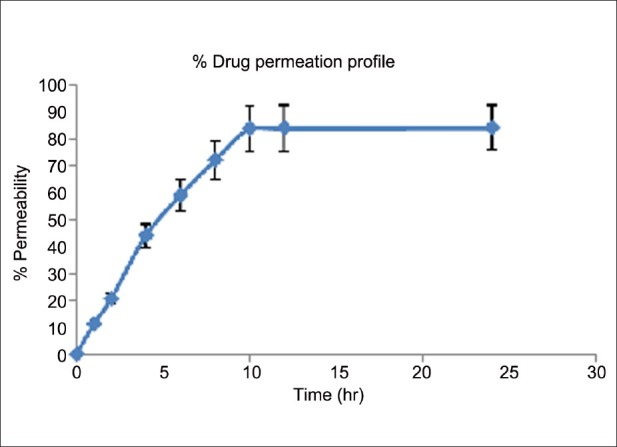
Ex vivo permeation profile of drug through rat skin from optimized microemulsion
Spreadability study
Spreadability of hydrogels was found to be 1.591 gcm/sec [shown in Table 5]. The spreadability of optimized microemulsion-based hydrogel was found to be 3.314 gcm/sec as shown in Table 7.
Drug content studies
The Bifonazole content in the optimized microemulsion-based hydrogel formulation was found to be 94.93% as shown in Table 5.
Evaluation of skin irritancy
The total scores for irritation test in each formulation were calculated and shown in Table 11.
Table 11.
Average response scores of skin irritation for single application

Skin flux, % permeability, and % drug release
As shown in Tables 12 and 13 as well as in Figure 7, the optimized microemulsion-based hydrogel (having 2% polymer concentration) showed the sustained release of Bifonazole from the formulation compared with other formulation.
Table 12.
Skin flux of bifonazole through hairless rat skin from microemulsion and microemulsion-based hydrogel formulations containing 1% of bifonazole

Table 13.
% Permeability and % cumulative amount of bifonazole released from F5 and microemulsion-based hydrogel
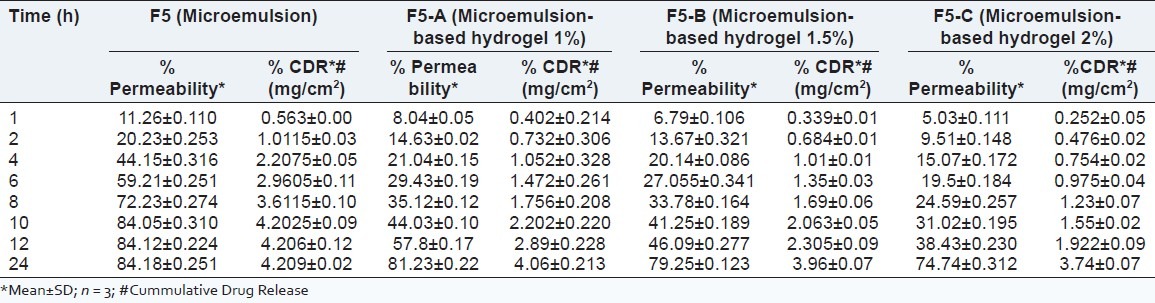
Figure 7.
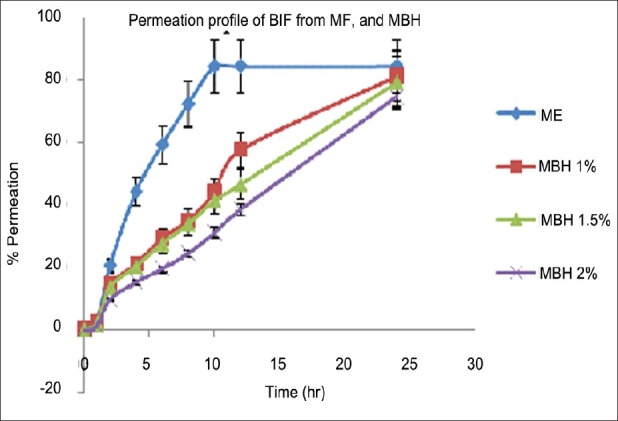
Permeation profiles of Bifonazole through hairless rat skin from microemulsion and microemulsion-based hydrogel formulations containing 1% of drug (n = 3)
F5 = Optimized microemulsion; F5-A = Microemulsion-based hydrogel (1%); F5-B = Microemulsion-based hydrogel (1.5%) and F5-C = Microemulsion-based hydrogel (2%).
Kinetic modeling and mechanism of drug release
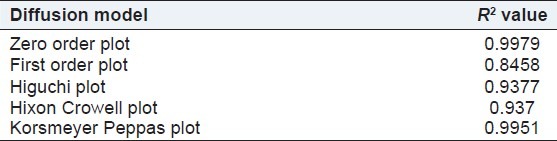
The correlation coefficient (R2) of the zero order model was found to be 0.9979, slightly higher when compared with the Peppas plot and Higuchi's plot for final selected optimized batch of microemulsion-based hydrogel. Hence the release of drug from the preparation followed zero order kinetics [Table 14].
Table 14.
Results of kinetic model fitted for microemulsion-based hydrogel
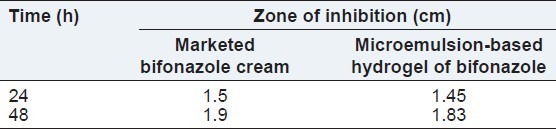
Comparision of microemulsion-based hydrogel with marketed bifonazole cream for in vitro antifungal activity
Results of in vitro antifungal activity are shown in Table 15. Microemulsion-based hydrogel exhibited maximum antifungal activity after 48 h comparable to marketed bifonazole cream.
Table 15.
Comparision of antifungal activity of microemulsion-based hydrogel with marketed cream formulation
Statistical analysis
The data obtained for different formulations was analysed by one way ANOVA. The values were considered to be statistically significant when the P value ≤ 0.05. It was observed that the P value of all responses for linear model was found to be < 0.05. Hence, the results obtained considered as significant.
CONCLUSION
The study demonstrated that the microemulsion formulation can be employed to improve the solubility and skin permeability of bifonazole. Microemulsion-based hydrogel was successfully prepared with HPMC K100M (2%) as a gelling agent to impart viscosity to the preparation as well as to sustain the action of the drug by increasing residence time. The contents of the developed microemulsion-based hydrogel were bifonazole (1%, 10 mg/g), oleic acid (6.25%), Tween 80, IPA (55%, 3:1), water (38.75%), and HPMC K100M (2%). The formulated microemulsion-based hydrogel was optimized for viscosity, spreadability, drug content, skin irritancy, and skin permeability. The permeability of bifonazole achieved from microemulsion within 8 h was observed 84%, which proves the permeability enhancement of drug with the use of microemulsion. There was no change in globule size and drug content of the microemulsion after 3 months of stability testing. Thus, the prepared microemulsion was found to be stable at room temperature for 3 months. The skin irritancy and antifungal activity of microemulsion-based hydrogel was comparable with the marketed bifonazole cream.
In conclusion, microemulsion-based hydrogel was successfully formulated to sustain the action of the drug and also to improve its poor solubility and permeability.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the assistance of Mr. Rajesh Vaidya, Sr. Manager P. and A., Vital Laboratories Pvt. Ltd., Vapi for the kind supply of Bifonazole as gift sample. Authors are also thankful to Dr. Archana Paranjape, Principal, Baroda College of Pharmacy and Dr. Devanshu Patel (Managing Trustee, Parul Trust) for providing the facilities to carry out the research work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Edinburgh, UK: Churchill Livingstone; 2005. p. 666. [Google Scholar]
- 2.Mitchel J. Transdermal drug delivery. Americal academy of dermatology–58th Annual Meeting, San Francisco, CA. 2000 [Google Scholar]
- 3.Patel V, Kukadiya H, Mashru R, Surti N, Mandal S. Development of microemulsion for solubility enhancement of clopidogrel. Iran J Pharm Res. 2010;9:327–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Yamane M, Williams A. Effect of terpenes and oleic acid as skin penetration enhancers. Int J Pharm. 1995;116:237–51. [Google Scholar]
- 5.Touitou E, Godin B, Karl Y, Bujanover S, Becker Y. Oleic acid, a skin penetration enhancer, affects Langerhans cells and corneocytes. J Control Release. 2002;80:1–7. doi: 10.1016/s0168-3659(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. J Control Release. 2002;81:75–82. doi: 10.1016/s0168-3659(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 7.Lawrencea M, Gareth D. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 8.Singh V, Bushettii SS, Raju AS, Ahmad R, Singh M, Bisht A. Microemulsions as promising delivery systems: A Review. Indian J Pharm Educ Res. 2011;45:392–401. [Google Scholar]
- 9.Chandra A, Sharma PK. Microemulsion: An Overview. Pharm Rev. 2008;6:2–4. [Google Scholar]
- 10.Chen H, Moub D. Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm. 2007;341:78–84. doi: 10.1016/j.ijpharm.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Lackner TE, Clissold SP. Bifonazole. A review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs. 1989;38:204–25. doi: 10.2165/00003495-198938020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar B, Hardenia S. Microemulsion drug delivery system: For oral bioavailability enhancement of glipizide. J Adv Pharm Educ Res. 2011;1:195–200. [Google Scholar]
- 13.Dhamankar A, Manwar J, Kumbhar D. The novel formulation design of O/W microemulsion of ketoprofen for improving transdermal absorption. Int J PharmTech Res. 2009;1:1449–57. [Google Scholar]
- 14.Naguib G. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug Dev Ind Pharm. 2012;38:64–72. doi: 10.3109/03639045.2011.590731. [DOI] [PubMed] [Google Scholar]
- 15.Chandra A, Sharma PK, Irchhiaya R. Microemulsion-based hydrogel formulation for transdermal delivery of dexamethasone. Asian J Pharm. 2009;3:30–6. [Google Scholar]
- 16.Rasal A, Mahajan HS, Shaikh HT, Surana SJ. Development and characterization of nasal mucoadhesive microemulsion of sumatriptan succinate. Indian J Novel Drug Deliv. 2010;2:103–8. [Google Scholar]
- 17.Gibaud S, Attivi D. Microemulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv. 2012;9:937–51. doi: 10.1517/17425247.2012.694865. [DOI] [PubMed] [Google Scholar]
- 18.Bagley DM, Gardner JR, Holland G, Lewis RW, Regnier JF, Stringer DA, et al. Skin irritation: Reference chemicals data bank. Toxicol In Vitro. 1996;10:1–6. doi: 10.1016/0887-2333(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 19.Shah P, Swarnkar D. Development and characterization of microemulsion containing antihypertensive agent using factorial design. J Pharm Bioallied Sci. 2012;4:69–70. doi: 10.4103/0975-7406.94143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EA, Balakrishnan P, Chng Kil Song CK, Choi JH, Noh GY, Park CG, Choi AJ, Chung SJ, Shim CK, Kim DD. Microemulsion-based hydrogel formulation of itraconazole for topical delivery. J Pharm Invest. 2010;40:305–11. [Google Scholar]
- 21.Gohel MC, Nagori SA. Fabrication and evaluation of hydrogel thickened microemulsion of ibuprofen for topical delivery. Indian J Pharm Educ Res. 2010;44:189–96. [Google Scholar]
- 22.Behera J, Keservani RK, Yadav A, Tripathi M, Chadoker A. Methoxsalen loaded, chitosan coated microemulsion for effective treatment of psoriasis. Int J Drug Deliv. 2010;2:159–67. [Google Scholar]
- 23.Chudasama A, Patel V, Nivsarkar M, Vasu K, Shishoo C. Investigation of microemulsion system for transdermal delivery of itraconazole. J Adv Pharm Technol Res. 2011;2:30–8. doi: 10.4103/2231-4040.79802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puranajoti P, Patil RT, Sheth PD, Bommareddy G, Dondeti P, Egbaria K. Design and development of topical microemulsion for poorly water-soluble antifungal agents. J Appl Res Clin Exp Ther. 2002;2:1. [Google Scholar]
- 25.Garala K, Patel J. Design and development of a self nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Invest. 2011;1:112–8. doi: 10.4103/2230-973X.82431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicentini FT, Vaz MM, Fonseca YM, Bentley MV, Fonseca MJ. Characterization and stability study of a water-in-oil microemulsion incorporating quercetin. Drug Dev Ind Pharm. 2011;37:47–55. doi: 10.3109/03639045.2010.491078. [DOI] [PubMed] [Google Scholar]
- 27.Kantarci G, Ozgüney I, Karasulu HY, Arzik S, Güneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: Preparation, characterization, release rate, skin irritation studies. AAPS Pharm Sci Tech. 2007;8:E91. doi: 10.1208/pt0804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG. Effect of formulation components on the in vitro permeation of microemulsion drug delivery system of fluconazole. AAPS PharmSci Tech. 2009;10:917–23. doi: 10.1208/s12249-009-9286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasool Abdul BK, Abu-Gharbieh EF, Sahar A, Fahmy SA, Saad HS, Khan SA. Development and evaluation of ibuprofen transdermal gel formulations. Trop J Pharm Res. 2010;9:355–63. [Google Scholar]
- 30.Mutimer MN, Riffkin C, Hill JA, Glickman ME, Cyr GN. Modern ointment base technology II. Comparative evaluation of bases. J Pharm Sci. 1956;45:212–8. doi: 10.1002/jps.3030450406. [DOI] [PubMed] [Google Scholar]
- 31.Dreher F, Walde P, Luisi PL, Elsner P. Human skin irritation studies of a lecithin microemulsion gel and of lecithin liposomes. Skin Pharmacol. 1996;9:124–9. doi: 10.1159/000211408. [DOI] [PubMed] [Google Scholar]
- 32.Zhua W, Guo C. Microemulsion-based hydrogel formulation of pencyclovir for topical delivery. Int J Pharm. 2009;378:152–8. doi: 10.1016/j.ijpharm.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Odds FC, Webster CE, Abbott AB. Antifungal relative inhibition factors: BAY 1-9139, bifonazole, butoconazole, isoconazole, itraconazole (R 51211), oxiconazole, sulconazole, terconazole and vibunazole compared in vitro with nine established antifungal agents. J Antimicrob Chemother. 1984;14:105–14. doi: 10.1093/jac/14.2.105. [DOI] [PubMed] [Google Scholar]
- 34.Suppaibulsuk B, Prasassarakich P, Rempel GL. Factorial design for nanosized polyisoprene synthesis via differential microemulsion polymerization. Polym Adv Technol. 2010;21:467–75. [Google Scholar]


