Abstract
Objective:
Sumatriptan succinate is a selective 5-hydroxytryptamine-1 receptor agonist effective in the acute treatment of migraine headaches, having low bioavailability of about 15% orally due to first-pass metabolism. The purpose of this research was to mask the intensely bitter taste of Sumatriptan succinate and to formulate fast-acting, taste-masked sublingual tablet formulation.
Materials and Methods:
Taste masking was performed by solid dispersion method with mannitol and ion exchange with Kyron T 114 because it releases the drug in salivary pH. The resultant batches were evaluated for in-vivo taste masking as well compatability study (Fourier transform infrared (FTIR) and differential scanning calorimetry (DSC)). For a better feel in the mouth, menthol and sweetener Na saccharine were added to the tablet formulation. The tablets were prepared by direct compression and evaluated for weight variation, thickness, friability, drug content, hardness, disintegration time, wetting time, in vitro drug release, and in vitro permeation study.
Results and Discussion:
Optimized batches disintegrated in vitro within 28-34 s. Maximum drug release could be achieved with in 10 min for the solid dispersion batches and 14-15 min for the ion-exchange batches with Kyron T 114. The optimized tablet formulation showed better taste and the formulated sublingual tablets may act as a potential alternate for the Sumatriptan succinate oral tablet.
Conclusion:
Sumatriptan succinate can be successfully taste-masked by both the solid dispersion method using mannitol by the melting method and Ion exchange resin with Kyron T114. It was also concluded that prepared formulation improve bioavailability by prevention of first pass metabolism.
Keywords: Ion-exchange resin Kyron T 114, mannitol, solid dispersion, taste masking
INTRODUCTION
Migraine is a common disorder characterized by a unilateral headache, which is often associated with nausea, vomiting, gastrointestinal disturbance, and extreme sensitivity to light and sound.[1] Sumatriptan succinate is the first member of a new class of anti-migraine compounds that act as a specific and selective 5-hydroxytryptamine-1 receptor agonist. Sumatriptan has low bioavailability after oral administration (about 15%), with a large inter-individual variation, although not affected by concomitant food intake. The dose is 50-100 mg orally. Tmax is reached at approximately 2 h and is slightly delayed by the presence of food and during an acute migraine attack.
The pharmacokinetics of Sumatriptan is linear over the dose range 25-200 mg, with the exception of rate of absorption. Sumatriptan is extensively metabolized in the liver predominantly by monoamine oxidase type A and is excreted mainly in the urine as the inactive indole acetic acid derivative and its glucuronide. Total plasma clearance is 1160 ml/min, of which 20% is renal. The elimination half-life is about 2 h.[2]
Through the subcutaneous route, injected Sumatriptan works the fastest of all the dosage forms available and is the most effective, but it is inconvenient due to pain at the injection site and it also requires a trained person to administer the dose. Nasal spray bypasses the stomach, gets absorbed more quickly than the oral form, and relieves the pain within 15 min after administration. However, it is less effective when the patient has nasal congestion from cold or allergy, and it also leaves a bad after-taste. Oral administration in the form of a conventional tablet is a convenient method, but in some instances, such as during travel where patients have little or no access to water, administration of the drug is not feasible and carries a risk of choking. Moreover, a substantial proportion of patients suffer from severe nausea or vomiting during their migraine attack. In such conditions, even if the patient has access to water, ingestion of the conventional tablet could lead to vomiting and expulsion of a portion or the entire dose administered, which leads to treatment failure. All these factors limit the utility of conventional tablets.[3]
Thus, the oral sublingual tablet delivery system for Sumatriptan succinate may be a viable alternative for self-administration, whereby these limitations could be overcome. Our aim is to overcome the limitation observed in the oral, subcutaneous, and nasal route; to provide fast dissolution or disintegration in the oral cavity, without the need for water or chewing; to avoid hepatic first-pass metabolism and improve the oral bioavailability of administered drug; and to give rapid onset of action.
For better patient compliance and mouth feel, in this research work taste masking was the first approach. Systemic drug delivery through the sublingual route had emerged from the desire to provide immediate onset of pharmacological effect. Dysphagia (difficulty in swallowing) is a common problem of all age groups; elderly; children; and patients who are mentally retarted, uncooperative, nauseated, or on reduced liquid intake/ diets especially have difficulties in swallowing these dosage forms.
The main mechanism for absorption of the drug into the oral mucosa is via passive diffusion into the lipoidal membrane. Absorption of the drug through the sublingual route is 3-10 times greater than the oral route and is only surpassed by hypodermic injection. For these formulations, a small volume of saliva is usually sufficient to result in tablet disintegration in the oral cavity. Sublingual absorption is mostly rapid in action, but also short-acting in duration.[4]
In terms of permeability, the sublingual area of the oral cavity is more permeable than the buccal (cheek) area, which in turn is more permeable than the palatal (roof of the mouth) area. The differences in permeability are generally based on the relative thickness, the blood supply, and the degree of keratinization of these membranes. In addition to the differences in the permeability of the various mucous membranes, the extent of drug delivery is also affected by the physicochemical properties of the drug to be delivered.[5] So, through this research our aim was to formulate taste-masked sublingual tablets.
MATERIALS AND METHODS
Sumatriptan succinate was gifted by Aurobindo Pharma Ltd. (Indrad, India), Kyron T 114 and Kyron T 314 were gifted by Corel Pharm Chem. (Ahmedabad, India), Mannitol was gifted by Sigma Aldrich (Mumbai, India), and other materials were purchased as follows: Cross PVP from Yarrow Chem. (Mumbai, India), sodium starch glycolate from Loba Chem. (Mumbai, India), and microcrystalline cellulose (MCC) from Chemdyes Corporation (Ahmedabad, India).
Taste masking of Sumatriptan succinate
The intensely bitter taste of the drug was tried to be masked by several methods as follows:[6,7]
Physical mixture with mannitol
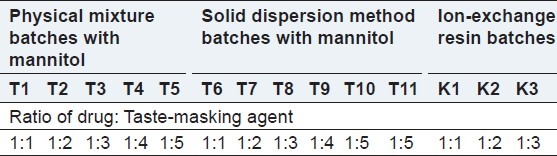
Sumatriptan succinate (14 mg)[8] was mixed with mannitol at a ratio of 1:1-1:5 for masking the bitter taste of the drug. Following are the different batches prepared as the preliminary trial for taste masking. The compositions of the batches are shown in Table 1.
Table 1.
Taste masking batches
Solid dispersion with mannitol
First mannitol was melted in a porcelain dish at 120°C on a hot plate. The drug Sumatriptan succinate was added and mixed properly, then immediately cooled in an ice bath. The solid dispersion was stored for 24 h then passed through 80#.[9] The compositions of the batches are shown in Table 1.
Taste masking of drug by Kyron T 114 (ion-exchange resins)
Kyron T 114 was added to 25 ml of water in a beaker. The mixture was stirred for half an hour, then Sumatriptan succinate was added to it and the mixture was stirred for 2 h at 1000 r.p.m. The complex was filtered using Whatman filter paper and dried. Different ratios of drug to Kyron T114 were taken as preliminary trials of taste masking as follows.[10,11] The compositions of the batches are shown in Table 1.
To check the effect of stirring time on drug loading, six different batches were stirred at 200 r.p.m on a magnetic stirrer for 1-6 h with an optimized ratio of 1:3 (drug:polymer). Out of the six batches, the batch stirred 2 h showed maximum drug loading, so further trial were carried out for 2 h. To check the effect of r.p.m. on drug loading, three batches at three different r.p.m (500, 1000, and 1500) were put for 2 h. The drug–resin complex was evaluated for the de-comlpexation process in phosphate buffer (pH 6.8) and estimated at 227 nm spectrophotometrically. It was found that there were no interaction was found between Kyron T114 and drug as shown in Figure 1.
Figure 1.
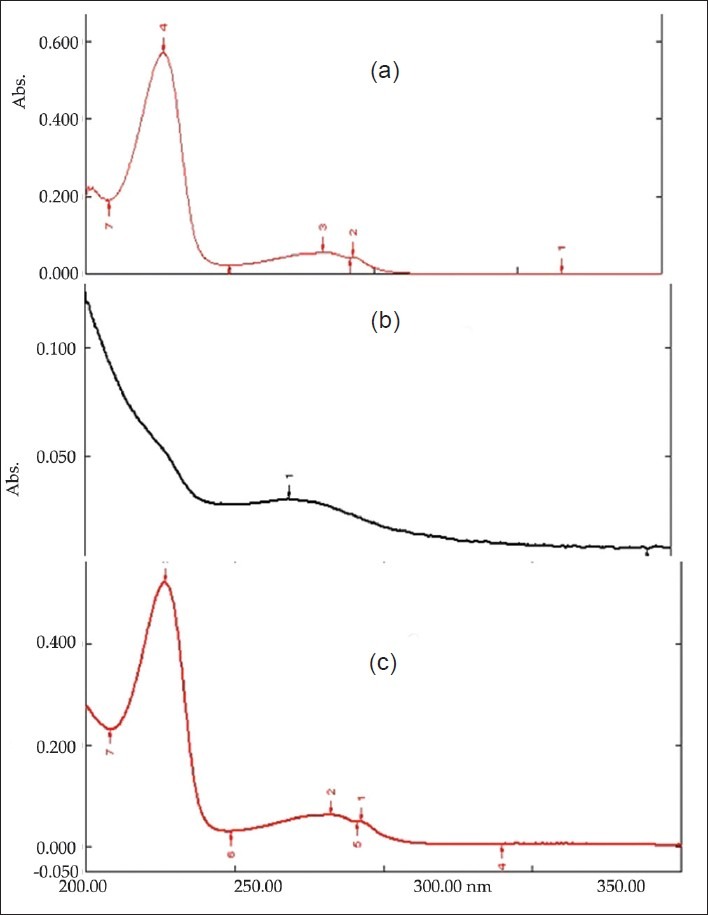
UV λmax of: 1. Pure drug, 2. Kyron T 114, 3. Drug and Kyron T 114 complex
Evaluation of taste by volunteers
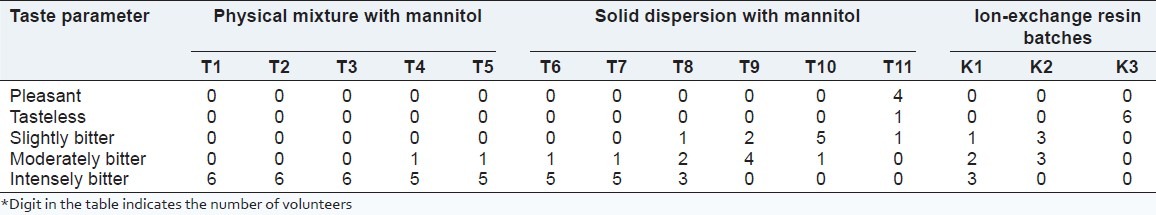
A protocol for conducting taste assessment by taste panel studies was accepted by the Institutional review board SSPC with protocol SSPCIRB/4/2012/19.[12] Healthy volunteers who met the inclusion and exclusion criteria and who provided informed consent would be recruited into this study. Healthy volunteers not knowing about the taste of the formulation would be selected. A single-blind study was designed for the taste masking test. Six healthy volunteers participated in the test. They were asked to rate the bitter taste of the 11 formulations (formulation T11-T11) for physical mixture and solid dispersion, and batches K1-K3 for ion- exchange batches. For mouth feel, the same human volunteers who participated in the taste evaluation test were asked to give their opinion about the feel of the prepared formulation. The evaluation of taste parameters is shown in Table 2.
Table 2.
Taste evaluation
Preparation of sublingual tablets by direct compression method
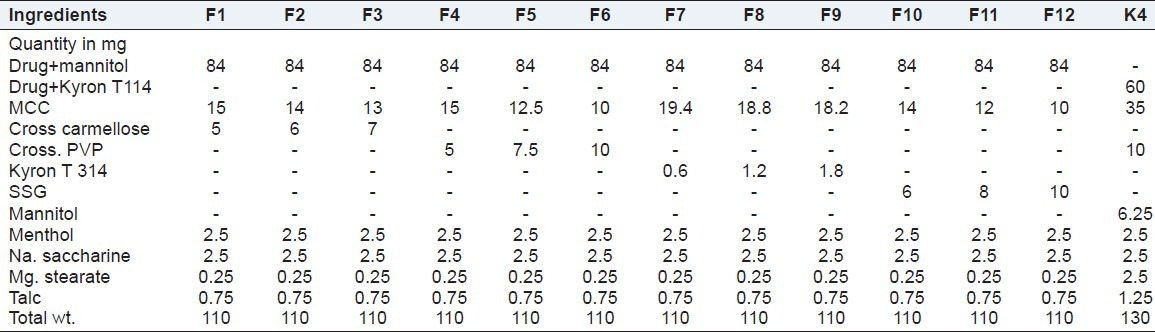
Sublingual tablets of Sumatriptan succinate were prepared by direct compression. All ingredients were passed through a #80 mesh separately. Then the ingredients were weighed and mixed in geometrical order and compressed into tablets of the 110 mg by direct compression method using 6-mm bi-concave punches on a Double Rotary Tablet Compression Machine (Rimek 10 station minipress). Batches F1-F12 were prepared by using four disintegrants for optimization of the disintegrant. Batches F1-F12 prepared were taste-masked by solid dispersion techniques. Powder blends of batches F1-F12 were evaluated for powder characteristics like bulk density, tapped density, angle repose. Batch K4 was prepared using a drug–Kyron T114 complex with the optimized disintegrant from batches F1-F12. The compositions of batches F1-F12 and batch K4 are shown in Table 3.
Table 3.
List of materials for batches F1-F12 and batch K4
Compatibility study
Physicochemical compatibility of drugs and excipients (FTIR)
Drug–excipients interactions play a vital role in the release of drug from formulation. Fourier transform infrared spectroscopy (FTIR) has been used to study the physical and chemical interactions between drug and the excipients used. FTIR spectra of Sumatriptan succinate, solid dispersion of Sumatriptan succinate with mannitol, and Sumatriptan succinate and Kyron T114 were recorded using the KBr mixing method on an FTIR instrument of the institute (FTIR-1700; Shimadzu, Kyoto, Japan).
DSC study for compatibility
Differential scanning calorimetry (DSC) is used to detect melting point and quantify transitions and interaction displayed as endothermic and crystallization (exothermic) events, which results in a baseline shift as the specific heat capacity of the sample changes. Specific interactions can be identified by DSC. For DSC spectra, the instrument used was DSC-6O (Shimadzu).
Evaluation of sublingual tablets
Weight variation
Ten tablets were weighed individually and then collectively, and the average weight of the tablets was calculated.
Drug content
Tablets (n = 5) were weighed individually, and the drug was extracted in phosphate buffer (pH 6.8), and the solution was filtered by Whatman filter paper. Absorbance was measured at 227 nm after suitable dilution using a Shimadzu UV-1601 UV/Vis double-beam spectrophotometer.
Friability
The tablets were tested for friability testing using a Roche Friabilator. For this test, six tablets were weighed and subjected to a combined effect of abrasion and shock in the plastic chamber of the Friabilator revolving at 25 r.p.m. for 4 min, and the tablets were then dusted and re-weighed.
Thickness
The thicknesses of buccal tablets were determined by using a micrometer screw gauge. Ten individual tablets from each batch were used and the average thickness was calculated.
Hardness
The hardness of the tablets was determined by a Pfizer hardness tester. A tablet hardness of about 3-4 kg is considered adequate for mechanical stability. Determinations were made in triplicate.
In vitro disintegration test
In the USP (United States Pharmacopoeia) disintegration test for sublingual tablets, the disintegration apparatus for oral tablets is used without the covering plastic disks, and 2 min is specified as the acceptable time limit for tablet disintegration fulfilling the official requirements (<2 min) for the sublingual dosage form.[13] The test was carried out using a tablet disintegration apparatus (Model ED-2L; Electrolab, Mumbai, India). Distilled water was used as the disintegrating medium at 24 ± 0.2°C. The time required to obtain complete disintegration of all the tablets was noted.
Wetting time
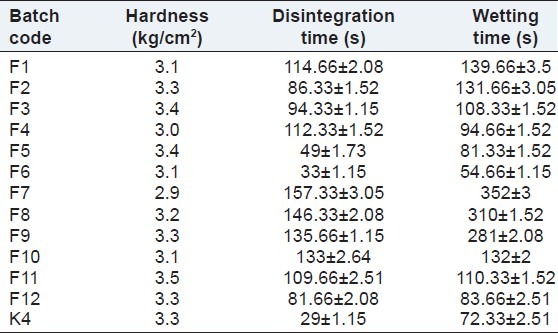
The tablet was placed at the center of two layers of absorbent paper fitted into a petridish.[13] After the paper was thoroughly wetted with distilled water, excess water was completely drained out of the dish. The time required for the water to diffuse from the wetted absorbent paper throughout the entire tablet was then recorded using a stopwatch. The evaluation parameters of batches F1-F12 are shown in Table 4.
Table 4.
Evaluation parameters of batches F1-F12 and batch K4
In vitro drug release study
Dissolution study was conducted for all formulations using USP dissolution rate test apparatus type-II (TDT-08L; Electrolab). Five hundred milliliters of phosphate buffer (pH 6.8) was taken in a dissolution apparatus, which was maintained at 37°C ±0.5°C at 50 r.p.m. Ten-milliliters aliquots were periodically withdrawn and the sample volume was replaced with an equal volume of fresh dissolution medium. Samples were collected at 2-min intervals and filtered by Whatman filter paper. The samples were diluted 10 times and analyzed spectrophotometrically at 227 nm.
Ex vivo permeation study of sublingual tablets
The buccal mucosa is very similar to the sublingual mucosa, so in this study, goat buccal mucosa was used to check the permeation of drug through the mucosa using a Franz diffusion cell at 37 ± 0.5°C. Fresh goat buccal mucosa was mounted between the donor and receptor compartments. The sublingual tablet was placed with the core facing the mucosa, and the compartments were clamped together. The donor compartment was filled with 1 ml of phosphate buffer (pH 6.8). The receptor compartment (45 ml capacity) was filled with phosphate buffer (pH 6.8) and the hydrodynamics in the compartment was maintained by stirring with a magnetic bead at uniform slow speed. Five- milliliter samples were withdrawn at pre-determined time intervals and analyzed for drug content using an ultraviolet (UV) spectrophotometer at 227 nm.
Stability studies of the optimized formulation
Stability testing of drug products begins as a part of drug discovery and ends with the demise of the compound or commercial product. To assess drug and formulation stability, stability studies were done according to International Conference on Harmonization (ICH) guidelines Q1C. The stability studies were carried out on the most satisfactory formulations (batches F6 and K4) as per ICH guidelines Q1C. The most satisfactory formulation was sealed in aluminum packaging and kept in a humidified chamber maintained at 40 ± 2°C/75 ± 5% relative humidity (RH) for 1 month. The optimized formulation sealed in aluminum foil was also kept at room temperature and humidity. At the end of the studies, the samples were analyzed for % drug release and drug content.[14]
RESULTS AND DISCUSSION
Taste masking of Sumatriptan succinate
Taste masking was carried out by the physical mixture and solid dispersion method using mannitol, and by ion-exchange resins using Kyron T 114. No taste masking was achieved in physical mixture formulation. Batch T6 of solid dispersion was found to be pleasant, so batch T6 with a drug: Mannitol ratio of 1:5 was optimized. Whereas in the case of ion-exchange complex with Kyron T 114, batch T3 with a drug: Polymer ratio of 1:3 was found tasteless, hence it was optimized. The results of the taste masking evaluations of all the batches are shown in Table 2.
Compatibility study
FTIR study
Sumatriptan succinate exhibits a peak due to N–H stretching at 3371.34 cm–1, S=O stretching at 1083.92 cm–1, and C–S stretching at 636.47 cm–1. Sumatriptan succinate with mannitol (solid dispersion) exhibits respective peaks at 3379.05 cm–1, 1083.92 cm–1, and 632.61 cm–1, whereas complexation of Sumatriptan succinate with Kyron T 114 gave respective peaks at 3336.05 cm–1, 1083.92 cm–1, and 632.61 cm–1 [Figure 2]. It was observed that there were no changes in these main peaks in the FTIR spectra of a mixture of drug, polymers, and excipients. Hence, it was concluded that no physical or chemical interactions of Sumatriptan succinate with mannitol and Kyron T 114 were found.
Figure 2.
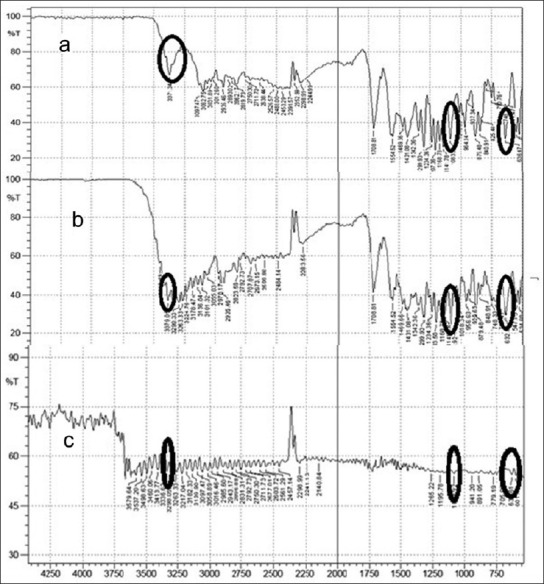
FTIR spectra of: a. Pure drug, b. Solid dispersion, c. Ion- exchange resin
DSC study
The DSC spectra of Sumatriptan succinate showed one endothermic peak at 173.15°C, which is associated with melting point. The DSC spectra of Sumatriptan succinate with mannitol showed an endothermic peak at 169.83°C, which represented the compatibility of the drug with mannitol [Figure 3]. In the case of Sumatriptan succinate with Kyron T 114, endothermic peak changes represented complex formation. DSC was taken by Shimadzu DSC-60.
Figure 3.
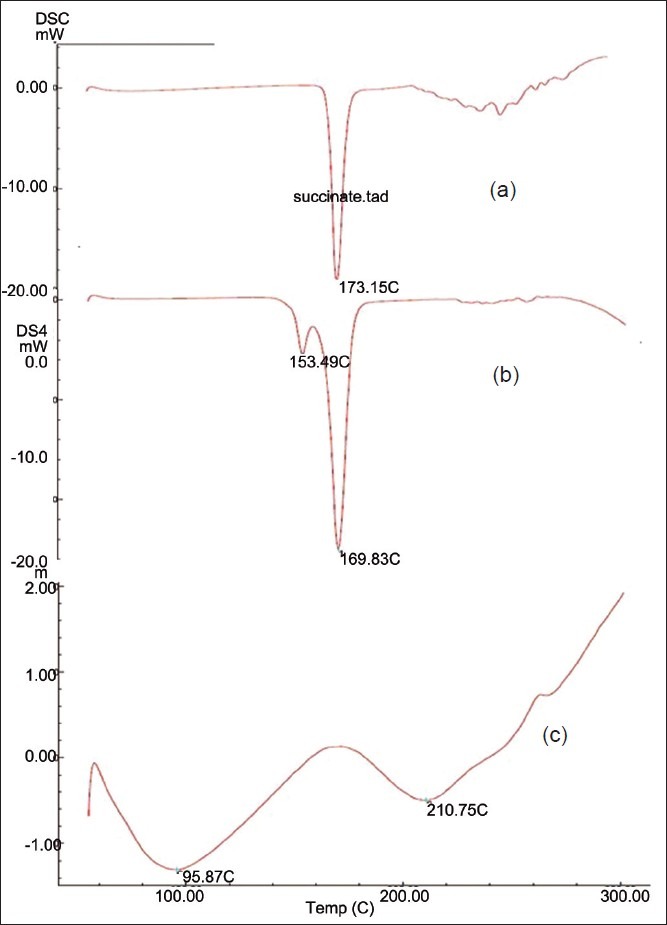
DSC spectra of: (a) Pure drug, (b) Solid dispersion, (c) Ion-exchange resin
Evaluation of sublingual tablets
The results of powder characteristics of blends of all batches showed good flow properties and compressibility.
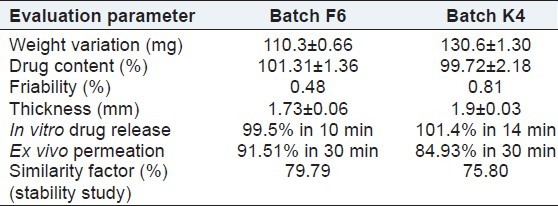
The prepared tablets were evaluated for weight variation, hardness, thickness, friability, drug content, wetting time, in vitro disintegration time, and in vitro dissolution. It was observed that all the tablets formulation passed the test for weight variation, as the percentage of weight variation was within the pharmacopeial limits. The prepared tablets in all formulations possessed good mechanical strength with sufficient hardness in the range of 2.9 to 3.5 kg/cm2. The tablets mean thickness was almost uniform, between 1.73 and 1.9 mm in all formulations. Friability varied between 0.48% and 0.81%. Friability values less than 1% were an indication of good mechanical resistance of tablets. The drug content in all formulations was highly uniform and in the range of 99.72%-101.31%. Wetting time was found to be in the range of 54.66-139.66 s. It was observed that with increase in the concentration of crospovidone, disintegration time did not decrease significantly, but formulation F4, containing 8% crospovidone as super-disintegrant showed faster disintegration rate as compared with other formulations. The results of the evaluation parameters of the sublingual tablets are depicted in Table 4.
The disintegration time of batch F5 and F6 was 49 ± 1.73 and 33 ± 1.15 s, respectively, and wetting time was 81.33 ± 1.52 and 54.66 ± 1.15 s, respectively. Batch F6 showed the lowest disintegration time and wetting time, hence it was considered an optimized batch. In vitro drug release of batch F6 was 99.5% in 10 min and ex vivo permeation was 91.51% in 30 min. For batch K4 with Kyron T 114, an in vitro drug release of 100.14% was achieved within 14 min and ex vivo permeation was 84.83% in 30 min. The in vitro release profile and ex vivo permeation of batch F6 and K4 are shown in Figures 4 and 5 respectively. The results of the evaluation parameters for batches F6 and K4 are depicted in Table 5.
Figure 4.
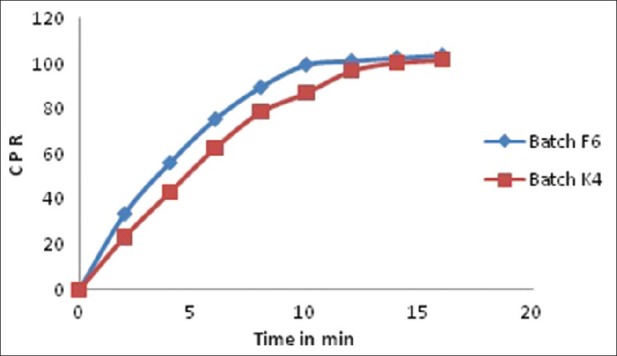
In vitro dissolution profile of batches F6 and K4
Figure 5.
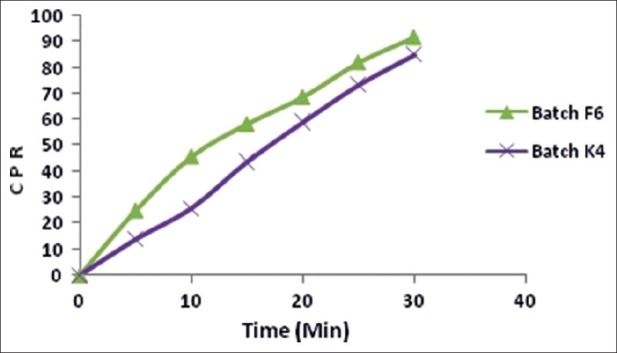
Ex vivo permeation study of batches F6 and K4
Table 5.
The different evaluation parameters of batches F6 and K4
Results of stability studies
The optimized formulations (batches B6 and K4) stored at 40 ± 2°C/75 ± 5% RH were found to be stable. After storage at 40 ± 2°C/75 ± 5% RH, in vitro drug release, ex vivo permeation, % friability, and % drug content were found to be similar to the initial results. So, it was clear that drug and formulation were thermally stable as well as not affected by high humidity at 40 ± 2°C/75 ± 5% RH. The similarity factors of the batch after the stability study were found to be 79.79 and 75.80 for batch F6 and batch K4, respectively. The results of the stability tests are shown in Table 5.
CONCLUSION
From this study it was concluded that Sumatriptan succinate can be successfully taste-masked by both the solid dispersion method using mannitol by the melting method (1:5 ratio) and ion-exchange resin (1:3 ratio) with Kyron T 114, which helps to formulate sublingual tablets of Sumatriptan succinate, which is an alternative way to oral administration. It was also concluded that there was no physical and chemical interaction of the drug with mannitol and Kyron T 114. The drug permeates quickly because it was highly permeable. Good taste masking was achieved by ion-exchange resin complex with Kyron T 114.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Shojaie AH. Buccal mucosa as a route for systemic drug delivery: A review. J Pharm Pharm Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 2.Richman MD, Fox D, Shangraw RF. Preparation and stability of glyceryl trinitrate sublingual tablets prepared by direct compression. J Pharm Sci. 1965;54:447–51. doi: 10.1002/jps.2600540323. [DOI] [PubMed] [Google Scholar]
- 3.Milton KA, Scott NR, Allen MJ, Abel S, Jenkins VC, James GC, et al. Pharmacokinetics, pharmacodynamics, and safety of the 5-HT (1B/1D) agonist eletriptan following intravenous and oral administration. J Clin Pharmacol. 2002;42:528–39. doi: 10.1177/00912700222011580. [DOI] [PubMed] [Google Scholar]
- 4.Narang N, Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Pharma Sci. 2011;3:18–22. [Google Scholar]
- 5.Kurosaki Y, Takatori T, Nishimura H, Nakayama T, Kimura T. Regional variation in oral mucosal drug absorption permeability and degree of keratinization in hamster oral cavity. Pharm Res. 1991;8:1297–301. doi: 10.1023/a:1015812114843. [DOI] [PubMed] [Google Scholar]
- 6.Drug Bank, “Sumatriptan”. [Last accessed on 10 Nov 2011]. Available from: http://www.drugbank.ca/drugs/DB00669 .
- 7.Sheshala R, Khan N, Darwis Y. Formulation and optimization of orally disintegrating tablets of Sumatriptan succinate. Chem Pharm Bull. 2011;59:920–8. doi: 10.1248/cpb.59.920. [DOI] [PubMed] [Google Scholar]
- 8.Holl Richard J, Lentz Mathew. Enhanced transmucosal composition and dosage form. US Patent. 2011 Jan 26; 2011 / 0021583. [Google Scholar]
- 9.Wagh VD, Ghadlinge SV. Taste masking methods and techniques in oral pharmaceuticals. Curr Perspect J Pharm Res. 2009;2:1049–54. [Google Scholar]
- 10.Dhakana KG, Rajebahadur MC, Gorde PM, Gade SS. A novel approach for taste masking techniques and evaluation in pharmaceutical: An updated review. Asian J Bio Pharm Sci. 2011;1:18–25. [Google Scholar]
- 11.Devi NK, Rani AP, Madhvi BR. Studies on taste masking of levocetrizine dihydrochloride using ion-exchange resins. Res J Pharm Bio Chem Sci. 2010;1:245–53. [Google Scholar]
- 12.Gowthamarajan K, Kulkarni GT, Kumar MN. Pop the pills without bitterness. Taste-masking technologies for bitter drugs. J Sci Edu. 2004:25–32. [Google Scholar]
- 13.Bayrak Z, Tas C, Tasdemir U, Erol H, Ozkan CK, Savaser A, et al. Formulation of zolmitriptan sublingual tablets prepared by direct compression with different polymers: In vitro and in vivo evaluation. Eur J Pharm Biopharm. 2011;78:499–505. doi: 10.1016/j.ejpb.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Note for guidance on stability testing. Stability testing of new drug substances and products. [Last accessed on 10 Aug 2010]. Available from http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html .


