Abstract
A number of medications do not have a licence, or label, for use in the paediatric age group nor for the specific indication for which they are being used in children. Over recent years, mycophenolate mofetil has increasingly been used off-label (i.e. off-licence) in adults for a number of indications, including autoimmune conditions; progressively, this wider use has been extended to children. This review summarizes current use of mycophenolate mofetil (MMF) in children, looking at how MMF works, the pharmacokinetics, the clinical conditions for which it is used, the advantages it has when compared with other immunosuppressants and the unresolved issues remaining with use in children. The review aims to focus on off-label use in children so as to identify areas that require further research and investigation. The overall commercial value of MMF is limited because it has now come off patent in adults. Given the increasing knowledge of the pharmacodynamics, pharmacokinetics and pharmacogenomics demonstrating the clinical benefits of MMF, new, formal, investigator-led studies, including trials focusing on the use of MMF in children, would be of immense value.
Keywords: child, mycophenolate, mycophenolic acid, off-label, pharmacogenomics, pharmacokinetics
Introduction
A number of medications do not have a licence for use in the paediatric age group or are used outside their licensed indication in children, i.e. off-label. Mycophenolate mofetil falls into this category. It has increasingly been used in adults for a number of indications, including autoimmune conditions; progressively, this wider use has been extended to children. This review summarizes the current use of mycophenolate mofetil (MMF) in children in order to identify areas that require further research and investigation.
Search methodology and selection criteria
A search of MEDLINE from 1996, the year in which MMF was first licensed in adults, to October 2011 was carried out using the terms ‘mycophenolate mofetil’ and ‘mycophenolic acid’ in combination with ‘humans’, ‘English’, ‘neonate’, ‘infant’ and ‘child’. Publications were then selected which were of relevance, and some citations made in these were also searched.
What is mycophenolate mofetil?
Mycophenolate mofetil is the 2-morpholinoethyl ester of mycophenolic acid (MPA). The development of mycophenolate mofetil as an active medicinal compound has its roots in a discovery made in 1893 by the Italian physician Bartolomeo Gosio. In the 1890s, he was studying fungal contamination of corn, working on the then widely held belief that there was a link between deterioration of corn by mould and pellagra. In his attempts to find this link, Gosio isolated a crystalline substance from mould found on corn, which he later discovered inhibited anthrax bacilli [1]. Gosio presented these findings at Reale Accademia Medicina di Torino in May 1893 [1]. Unfortunately, as Gosio had isolated only a small amount of this crystalline material he was unable to take his research further. Consequently, Gosio did not come to realize what a growing impact his unnamed crystals would have in the future field of medicine.
Likewise, Alsberg and Black, working on corn moulds from an agricultural perspective at the US Department of Agriculture, isolated a substance which they named mycophenolic acid in a 1913 bulletin by the Bureau of Plant Industry [2]. Mycophenolic acid is now generally accepted as being one and the same substance as Gosio's crystals [1]. Gosio/Alsberg and Black's discovery continued to go unnoticed until 1969, when the potential of MPA was to be rediscovered by a research team in England. The team at the Pharmaceuticals Division of Imperial Chemical Industries Limited (ICI) in Cheshire was routinely screening mould compounds for activity against mouse fibroblasts, when they found that MPA, obtained from a culture of Penicillium stoloniferum, suppressed mitosis and hence, cell proliferation. Although the mechanism at this time was not fully understood, it was proposed as a potential anticancer agent [3].
Over the subsequent years, the antibacterial, antifungal and antiviral properties of MPA have been demonstrated in vitro but not so successfully in vivo[1], with the consequence that the mechanism of these particular actions of MPA remain, as yet, not fully explained. Unfortunately, the trials as an anticancer agent, initiated by the team at ICI, were not very promising. Likewise, earlier trials in the 1970s for psoriasis showed limited success; the rationale for use in this condition being that other agents that slowed cell turnover were effective in psoriasis [1]. Solubility problems and fears that medications which suppress the immune system may have the potential to cause cancer meant that development as a therapeutic agent was hampered at this time. Consequently, interest in MPA waned. It was not reignited until the 1980s, when there was an increasing focus on immunosuppressant drugs, and the long road to develop a derivative with improved uptake and, thus, potential activity within the body began. This renewed interest led directly to the development of MMF by a research group at Syntex Research (now part of F. Hoffmann-La Roche) [4]. Mycophenolate mofetil is much more soluble than MPA. As a consequence, when taken orally, it has better absorption, after which it is metabolized to MPA, i.e. MMF is a prodrug of MPA.
How does mycophenolate work?
Replicating cells in the body need to synthesize purine (adenine and guanine) nucleotides to produce DNA and RNA during cell division and replication. There are two ways in which cells can produce the purine nucleotides: de novo synthesis and the salvage pathway. T and B lymphocytes are dependent on de novo synthesis of guanine nucleotides, mediated by inosine 5′-monophosphate dehydrogenase (IMPDH), in order to proliferate and participate in immune responses, while in general, other cells in the body can form guanine nucleotides by using alternative, salvage pathways. The de novo synthesis of guanosine nucleotide is shown diagrammatically in Figure 1.
Figure 1.
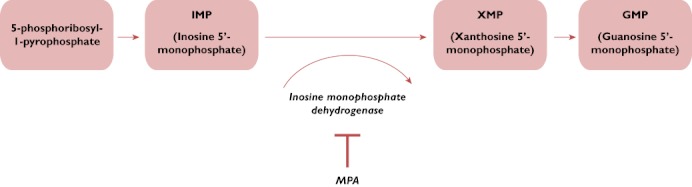
Mycophenolic acid (MPA) inhibition of de novo synthesis of guanine nucleotide
Mycophenolic acid is a potent, selective, uncompetitive and reversible inhibitor of IMPDH, a crucial enzyme in lymphocyte proliferation. The prodrug, MMF, is rapidly metabolized in the body to pharmacologically active MPA (see Figure 2), which inhibits IMPDH.
Figure 2.
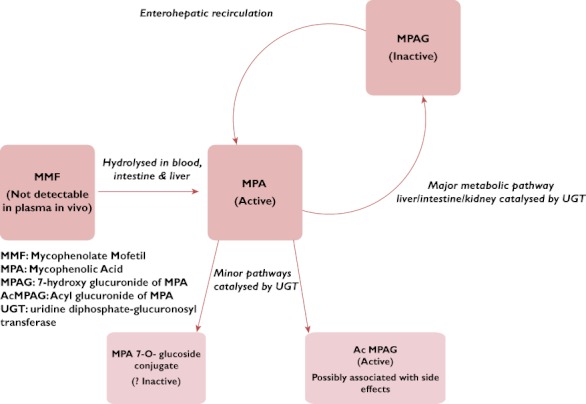
Known metabolism of mycophenolate mofetil (MMF) in humans
Inosine 5′-monophosphate dehydrogenase catalyses the oxidation of inosine 5′-monophosphate (IMP) to xanthosine 5′-monophosphate (XMP), which is then converted to guanosine 5′-monophosphate (GMP), a precursor of guanine nucleotides. Inosine 5′-monophosphate dehydrogenase exists in two isoforms: IMPDH1 and IMPDH2. Inosine 5′-monophosphate dehydrogenase 2 is the isoform expressed in activated T and B lymphocytes, and MPA has a more potent effect on this isoform [4]. Thus, inhibition of IMPD2 selectively depletes the pool of guanine nucleotides available for proliferation of T and B lymphocytes.
Guanine nucleotides are also required for the synthesis of glycoproteins, such as selectins and integrins, which are important in the leucocyte inflammatory immune response [5]. Depletion of tetrahydrobiopterin, a limiting cofactor in nitric oxide production, follows a decrease in the pool of guanine nucleotides. Nitric oxide has been implicated in nephropathy and allograft rejection, and it is thought that the indirect effects on nitric oxide production may contribute to the clinical effect of MPA [4]. Thus, although the primary effect of MPA is to decrease production of lymphocytes, it has secondary effects on cell signalling and inflammatory responses. By targeting IMPDH, MPA has a selective, but potent, cytostatic effect on lymphocytes. More recently, however, in experimental studies MPA has been shown to have additional effects other than its primary one of IMPDH inhibition, as follows.
Angiogenesis – MPA can block tumour-induced angiogenesis in vivo[6], indicating that it may have a wider role in therapy rather than only as an immunosuppressant.
Maturation of dendritic cells – MPA can have a direct effect on antigen presenting cells by disrupting the maturation of dendritic cells, which present antigens to T lymphocytes [7].
Endothelin 1 – MPA can reduce endothelin 1 concentrations, which has been implicated in ischaemic and drug-induced renal failure [8].
The latter two of these effects may partly explain the apparent advantages of MMF over other commonly used oral immunosuppressant agents. For example, MMF is considered more potent than azathioprine, possibly due to its effects on maturation of dendritic cells, yet it has less adverse effects on kidney function than ciclosporin A (CsA), which may be as a result of reduction of endothelin 1.
Pharmacokinetics in children
When taken orally by adults, MMF is rapidly metabolized in the liver to MPA (Figure 2), achieving a bioavailability of 94% relative to intravenously administered MMF in adults, based on MPA area under the curve (AUC) measurements. The AUC is directly proportional to the total amount of MPA in the plasma. The manufacturer's pharmacokinetic data [9] confirm similar findings in paediatric renal transplant patients given 600 mg m−2. Mycophenolic acid is 97% bound to plasma proteins and shows two peak levels of MPA as a result of enterohepatic recirculation of glucuronidated MPA, mycophenolic phenolic glucuronide (MPAG) [10]. Apart from MPAG, there are two other minor metabolites of MPA which have been identified, the 7-O-glucoside and acyl glucuronide (AcMPAG) [11]. Initial investigations revealed that the 7-O-glucoside had no activity [11]. Additionally, in further studies it has been established that MPAG has no immunosuppressant activity. Notwithstanding, AcMPAG is pharmacologically active; it may thus contribute to the immunosuppressive action and to the adverse effects of MMF treatment [12, 13].
Table 1 summarizes published studies of MMF used for an unlicensed indication or off-label in children under the age of 13 years. Some of these studies investigated the implications of pharmacokinetics and plasma concentrations of MPA on clinical outcome [14–16]. For example, the low AUC for MPA in the paediatric study by Dorresteijn et al. was cited as the possible reason for relapse in a patient with nephrotic syndrome [15].
Table 1.
Studies with more than five subjects that have used mycophenolate mofetil (MMF) in children <13 years old for an off-label indication or in an off-label manner
| Indication | Study | Study question and relationship to MMF | Type of study | Age range (years) | Total number of people in study | Number of children (age) |
|---|---|---|---|---|---|---|
| Studies that demonstrated a positive clinical benefit | ||||||
| Transplantation | ||||||
| Renal | Birkeland et al. [28] | To monitor height, growth patterns and other factors, patient and graft survival, graft function, and incidence of acute rejection in CsA monotheray and CsA with MMF regimens | Retrospective case series | 2–14 | 14 | 10 (<13 years) |
| 4 (13–14 years) | ||||||
| Cransberg et al. [36] | Safety and efficacy of MMF as maintenance immunosuppressive drug in paediatric kidney transplantation compared with CsA, both in combination with corticosteroids | Open-label randomized controlled trial | Paediatric mean age: 11.9 years for MMF group and 10.9 years for CsA group | 36 (18 in each group) | 42 | |
| Nematalla et al. [30] | To elucidate the safety and efficacy of a steroid-free immunosuppressive regimen in live-donor renal transplant recipients. All patients were given tacrolimus and MMF and then assigned either to stop steroids after 3 days or to continue steroids | Open, one-to-one, prospective, randomized, parallel group, controlled, double-arm, comparative study | 5–60 years | 100 | Not stated | |
| Krischock et al. [35] | To determine whether calcineurin inhibitors can be withdrawn safely in paediatric patients with declining renal allograft function receiving MMF and steroids for long-term immunosuppression following renal transplantation | Retrospective review | Paediatric mean 7.3 ± 3.7 years | 38 | 38 | |
| Hocker et al. [32] | Late steroid withdrawal (≥1 year post-transplant) in paediatric renal allograft recipients with stable allograft function and low or regular immunological risk, undergoing CsA- and MMF-based immunosuppression (2 year follow-up) | Multicentre, randomized, controlled trial | <18 years | 42 | 42 (<18 years) | |
| Hocker et al. [39] | Comparison of MMF efficacy and safety in paediatric vs. adult renal transplantation: subgroup analysis of the randomized, multicentre trial comparing fixed dose with concentration-controlled MMF dosing | Subgroup analysis of a multicentre, prospective, open-label randomized study | Children and adults >2 years | 901 | 62 (<16 years) | |
| Heart | Dipchand et al. [45] | Experience with MMF in paediatric heart transplant recipients, particularly with reference to treatment of rejection, facilitation of a reduced dosage or steroid-free regimen, and the side-effect profile in the paediatric population | Retrospective chart review | 11 months to 16 years | 21 | 21 (<18 years) |
| Heart valves | Shaddy et al. [77] | To determine whether MMF can blunt the HLA antibody response to valved allografts in children | Pilot study | 3–12 years | 8 | 8 |
| Haematopoietic stem cells | Alousi et al. [37] | Phase 2 trial to identify the most promising agent(s) for initial therapy for acute graft vs. host disease. Efficacy and toxicity data suggest the use of MMF plus corticosteroids is the most promising regimen | Randomized, controlled trial | 7–70 years | 180 (45 in MMF, 47 in denileukin, 42 in pentostatin and 46 in etanercept group) | Not stated |
| Ostronoff et al. [78] | MMF–CsA combination for graft vs. host disease prophylaxis after allogeneic bone marrow transplantation | Prospective descriptive trial | 6–48 years | 47 | 9 (<18 years) | |
| Liver | Chardot et al. [44] | Use of MMF rescue therapy after paediatric liver transplant | Pilot trial | 7 months to 1 year | 19 | 19 |
| Tannuri et al. [79] | Efficacy and security of long-term use of an MMF protocol with reduced doses of calcineurin inhibitors in stable liver-transplanted children with renal dysfunction secondary to prolonged use of CsA or tacrolimus | Prospective within-subject comparison study | 3–15 years | 11 | 8 (<13 years) | |
| 3 (>13 years) | ||||||
| Intestine | Tzakis et al. [80] | Adjuvant immunosuppression with MMF to improve patient and graft survival after intestinal transplant with tacrolimus (FK506) immunosuppression | Pilot study | 5 months to 47 years | 34 | 16 (<13 years) |
| 1 (>13 <18 years) | ||||||
| Rheumatology/autoimmune | ||||||
| Systemic lupus erythematosus | Buratti et al. [81] | MMF treatment of renal disease in paediatric onset SLE | Prospective case series of first clinical experience of MMF | 9–15 years | 11 | 8 (<13 years) |
| 3 (>13 years) | ||||||
| Falcini et al. [82] | To evaluate the efficacy of MMF either in decreasing disease activity or in preventing the development of renal involvement in patients with juvenile SLE and to evaluate its safety profile | Multicentre, retrospective chart analysis | 5–16 years | 26 | 26 (<16 years) | |
| Rivera et al. [83] | To evaluate responses to MMF and intravenous cyclophosphamide in lupus nephritis in a multiethnic population | Single-centre, retrospective chart review | 5–78 years | 237 | Not stated | |
| Wong et al. [84] | Review of clinical presentation, treatment and medium-term outcome in paediatric patients with lupus nephritis in Chinese children. Three patients received MMF in combination therapy | Retrospective case series | 3–22 years | 13 (3 patients received MMF combination maintenance therapy: 1 had positive therapy outcome and 2 had no clinical benefit) | Not stated | |
| Posalski et al. [85] | Does MMF prevent extrarenal flares in systemic lupus erythematosus? | Single-centre, retrospective, centre cross-sectional analysis | 11–68 years | 75 | Not stated | |
| Aragon et al. [86] | Examined the outcomes of children with proliferative lupus nephritis using MMF- and CsA-based induction protocol | Single-centre, prospective study | 3–14 years | 16 | 16 | |
| Kazyra et al. [87] | Effectiveness and safety of MMF in children and adolescents with SLE | Retrospective analysis | 5–18 years | 26 | 26 (5–18 years) | |
| Scleroderma | Martini et al. [88] | To evaluate the efficacy of MMF in the treatment of severe refractory juvenile localized scleroderma | Multicentre, retrospective chart review | 2–16 years | 10 | 8 (<13 years) |
| 2 (>13 years) | ||||||
| Myasthenia gravis | Meriggioli et al. [89] | Use of MMF patients with autoimmune myasthenia gravis | Two-centre, retrospective analysis | 12–86 years | 85 | Not stated |
| Autoimmune liver disease | Aw et al. [90] | To evaluate the outcome of MMF therapy in children with autoimmune liver disease resistant to or intolerant of standard immunosuppression | Single-centre, retrospective chart review | 1–14 years | 26 (MMF was effective for children with autoimmune hepatitis but not for those with autoimmune sclerosing cholangitis) | 18 (<13 years) |
| 8 (>13 years) | ||||||
| Autoimmune lymphoproliferative syndrome | Rao et al. [91] | Use of MMF for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome | Prospective within-subject comparison study | 9 months to 17 years | 13 | 10 (<13 years) |
| 3 (>13 years) | ||||||
| Inflammatory eye disease | Thorne et al. [92] | To evaluate treatment outcomes with MMF in patients with inflammatory eye disease | Retrospective case series | 8–79 years | 84 | Not stated |
| Doycheva et al. [93] | To assess the efficacy of MMF in uveitis in children and to analyse the possible side-effects | Single-centre, retrospective analysis | 2–13 years | 17 | 17 | |
| Sobrin et al. [94] | To evaluate the outcomes of treatment with MMF in patients with scleritis and uveitis refractory to or intolerant of methotrexate | Retrospective, noncomparative case series | 3–62 years | 85 | 36 (<18 years) | |
| Chang et al. [95] | To evaluate effectiveness and safety of MMF monotherapy in paediatric autoimmune uveitis | Retrospective review | 3–18 years | 52 | 52 (<18 years) | |
| Congenital uropathy | Trachtman et al. [96] | To determine the feasibility of treatment with MMF to prevent a decrease in kidney function in paediatric patients with congenital uropathies | Open-label pilot study | 3–16 years | 12 | 8 (<13 years) |
| 4 (>13 years) | ||||||
| Juvenile dermatomyositis | Rouster-Stevens et al. [97] | To determine whether MMF diminishes skin and muscle disease activity in children with juvenile dermatomyositis, thereby permitting a decrease in corticosteroid dose | Retrospective review | 4–23 years | 50 | Not stated |
| Renal | ||||||
| Nephrotic syndrome | Barletta et al. [98] | Use of MMF as third-line treatment in steroid-dependent and – resistant nephrotic syndrome | Retrospective review | 3–15 years | 14 | 12 (<13 years) |
| 2 (>13 years) | ||||||
| Al-Akash et al. [42] | Safety and efficacy of MMF in steroid-dependent and/or frequently relapsing nephrotic syndrome | Retrospective chart review | 2–10 years | 11 | 11 | |
| Hogg et al. [99] | To evaluate the safety and the efficacy of MMF for children who had frequently relapsing nephrotic syndrome who had not been exposed to other imunosuppressive drugs | Multicentre, prospective, open-label study | 2–15 years | 32 | 31 (<13 years) | |
| 1 (>13 years) | ||||||
| Afzal et al. [100] | Effectiveness of MMF and alternate-day prednisolone in patients with steroid-dependent nephrotic syndrome | Prospective and retrospective analysis | 2–15 years | 42 (19 prospective, 23 retrospective) | 42 (<16 years) | |
| Okada et al. [101] | To evaluate MMF treatment in paediatric patients with CsA-resistant intractable nephrotic syndrome | Prospective case series | 10–22 years | 11 | Not stated | |
| Wong et al. [102] | Epidemiology and treatment of children with nephrotic syndrome, histological findings of minimal change nephrotic syndrome and mesangial C1q deposition with calcineurin inhibitors and/or MMF as maintenance therapy | Retrospective cohort study | 1–15 years | 18 (9 with minimal change nephrotic syndrome and mesangial C1q deposition, | 17 (<13 years) | |
| 9 with minimal change nephrotic syndrome and without C1q deposition) | 1 (>13 years) | |||||
| Dorresteijn et al. [15] | The efficacy and side-effects of MMF compared with CsA in children with frequently relapsing nephrotic syndrome | Randomized controlled trial | 3–17 years | 24 (12 in each group) | 24 (<18 years) | |
| de Mello et al. [43] | To assess the results of therapy with MMF in children with idiopathic nephrotic syndrome who were both steroid and cyclophosphamide resistant | Prospective, within-subject comparison study | 2–17 years | 52 | 52 (<18 years) | |
| Li et al. [103] | Efficacy and side-effects of MMF in children <2 years of age with steroid-resistant nephrotic syndrome | Prospective, single-centre trial | 0–2 years | 24 | 24 (8 months to 2 years) | |
| Gargah & Lakhoua [104] | To evaluate the efficacy and tolerance of MMF therapy in children with steroid-resistant nephrotic syndrome | Single-centre, prospective study | 9–13 years | 6 | 5 (<13 years) | |
| 1 (>13 years) | ||||||
| Dermatology | ||||||
| Atopic dermatitis | Heller et al. [105] | To evaluate the safety and efficacy of MMF in the treatment of severe childhood atopic dermatitis | Retrospective analysis | 2–16 years | 14 | 8 (<13 years) |
| 6 (>13 years) | ||||||
| Studies with no clinical benefit demonstrated | ||||||
| Opsoclonus–myoclonus syndrome | Pranzatelli et al. [14] | Efficacy of tandem combination of rituximab and MMF compared with MMF alone | Pilot study | Paediatric: mean age 4.7 ± 0.7 years | 19 | 19 |
| Aplastic anaemia | Scheinberg et al. [106] | Outcome of patients with aplastic anaemia treated with horse anti-thymocyte globulin, CsA and MMF compared with standard horse anti-thymocyte globulin and CsA therapy | Phase 2 comparative study | 3–76 years | 103 | Not stated (26 patients <20 years) |
| Renal transplant | Benfield et al. [40] | To compare the efficacy of AZA vs. MMF in paediatric renal transplantation maintenance therapy after induction with muromonab-CD3 | Induction was a randomized controlled trial; maintenance was a consecutive group comparison | Paediatric: 9.4 ± 5.1 years for AZA group and 10.7 ± 5.3 years for MMF group | 67 (31 in AZA group and | 67 |
| 36 in MMF group) | ||||||
| Type 1 new-onset diabetes | Gottlieb et al. [107] | Whether MMF alone or with daclizumab could arrest the loss of insulin-producing β-cells in subjects with new-onset type 1 diabetes | Multicentre, randomized, placebo-controlled, double-blind trial | 8 to adult | 142 | Not stated |
Abbreviations: AZA, azathioprine; CsA, ciclosporin A; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus.
More specific paediatric pharmacokinetic studies have been carried out in different patient groups. For stable liver transplant patients, large interpatient variations in AUC levels for MPA have been found [17]. In children following renal transplant, no relationship between MMF dose and drug exposure was found, the conclusion being that children could be overexposed to MPA when given doses based on weight or surface area rather than doses based on MPA blood levels [18]. In another study, children receiving stem cell transplantation under the age of 12 years appeared to have a significantly different MPA pharmacokinetic profile compared with older children and adolescents; this could indicate that younger children require more frequent dosing [19]. In paediatric patients taking MMF for systemic lupus erythematosus, only a moderate correlation was shown between weight-adjusted dosing and improvement of the disease, with maximal clinical response seen at doses giving area under the curve, from 0–12 hours, of 30 mg h l−1 MPA [20].
A study by Tredger et al. showed that children with low albumin required higher doses of MMF, as do adults [21]. Mycophenolic acid is highly bound to plasma proteins, and as a result, low serum albumin decreases MPA protein binding. Consequently, more free MPA is available, which is then quickly cleared by the body, so lowering MPA levels in the plasma and requiring higher or more frequent dosing. However, the other observation in the adult population from Tredger's study, i.e. that patients with elevated creatinine levels had higher MPA predose concentrations, was not seen in the paediatric patients. When looking specifically at patient populations with abnormal creatinine concentrations, there appears to be little information on the correlation between MPA levels and creatinine concentrations for paediatric patients. A small paediatric pharmacokinetic study by Aigrain et al. [22] on only eight patients listed a wide range of MPA AUC levels, but the patients had only a narrow range of creatinine concentrations, hence a true relationship to renal function was impossible to determine.
In an attempt to improve consistency in studies for children, population pharmacokinetic models are being proposed which may help to address the issue of optimal paediatric dosing levels [23–26]. Furthermore, findings from these studies support earlier observations that serum albumin, bodyweight and coadministration of CsA all have a significant impact on clearance of MPA. Future pharmacokinetic studies in children may be easier to interpret and correlate using such models.
In which clinical conditions is MMF used in children?
In the UK, the only licensed paediatric indication for MMF is kidney transplantation. This UK licence, granted in 2001, is for combination therapy (with corticosteroids and ciclosporin) for children aged 2 years and over. In the USA, there is a much younger age limit of 3 months listed on the ‘label’ or licence. However, following licensing in adults in 1996, MMF had been used off-label in paediatric kidney transplantation well before 2001. Initial studies, such as that of Antoniadis et al. [25] in 1998, showed that MMF was well tolerated in children aged 4–12 years for the treatment of acute rejection, when used in combination with CsA and steroids. Mycophenolate mofetil is further licensed as a combination therapy in adults for heart and liver transplantation, leading to the off-label use for these same conditions in children.
Mycophenolate mofetil and steroid-free/steroid-reduced renal transplant regimens
The adverse effects of corticosteroids on growth in children are significant [26, 27] A restriction in growth cannot always be reversed on ceasing prolonged oral treatment, and this in turn can restrict final height. Other recognized side-effects of steroids, including hypertension, adrenal suppression and effects on behaviour/mood can have long-term, significant sequelae in children. These facts have prompted studies of MMF to try to reduce or avoid steroids altogether following kidney transplantation.
An early paediatric clinical study by Birkeland et al. [28] of maintenance therapy using steroid-free immunosuppressive protocols of CsA alone and in combination with MMF was promising. In this case series study, following the introduction of a local combination protocol that included MMF, there was an observed reduction in the number of acute kidney transplant rejection episodes at the study hospital. A further, retrospective paediatric study supported the finding that steroid-free immunosuppression is safe and efficacious in renal transplant patients [29].
Other studies have looked at attempts to restrict steroid use. These have often been conducted on adult patients, with only a few paediatric patients included. A trial by Nematalla et al. in 2007 with an unspecified number of children from the age of 5 years demonstrated that steroid avoidance after 3 days was feasible in renal transplant patients [30]. A well-conducted, although small, paediatric randomized controlled trial (RCT) by Hocker et al. investigated late withdrawal of steroids using MMF. The conclusion of this 2 year study was that in combination with CsA, withdrawal of steroids is possible, which in turn improves growth and the cardiovascular risk profile [31, 32]. Chavers et al. demonstrated that in paediatric patients over 5 years of age prednisolone could be discontinued after only 6 days by using MMF with CsA as maintenance after induction with thymoglobulin [33]. Likewise, the TWIST study, a large randomized paediatric trial, which assessed the impact on growth of early steroid reduction, showed that following induction with daclizumab, steroids could be withdrawn after 4 days with a maintenance regimen of tacrolimus and MMF [34].
In light of these studies, MMF appears to have a steroid-sparing effect that allows reduction or removal of steroids from immunosuppressant protocols. Admittedly, these studies are few in number, and future trials are needed to confirm this potential benefit, particularly in different clinical conditions.
Mycophenolate mofetil and calcineurin inhibitor-free renal transplant regimes
Ciclosporin A (a calcineurin inhibitor) is markedly toxic to kidney function. To date, two trials including paediatric subjects have investigated using MMF as an alternative to calcineurin inhibitors, such as CsA and tacrolimus. Krischock et al. demonstrated that for kidney transplant patients, complete withdrawal of CsA or tacrolimus could be successful in preserving renal function in a population treated with a maintenance MMF and corticosteroid regimen [35]. Cransberg et al. compared a MMF and prednisolone regimen with an established CsA and prednisolone regimen to prevent renal graft rejection in the long term [36]. This relatively small RCT involving a total of 44 patients demonstrated that MMF with prednisolone temporarily improved kidney function and dyslipidaemia. It also found that MMF may improve graft and patient survival in the long term. For five patients in this study, steroids were withdrawn, with MMF being continued as monotherapy for a mean of 6.71 years post-transplant. This demonstrates that in clinical practice, in a subgroup of patients, MMF can become the only immunosuppressant agent used.
Off-label conditions
The British National Formulary for Children 2010 (BNFc) gives off-label dosing guidance for hepatic transplantation and for renal transplantation with tacrolimus, as well as stating that MMF is used in severe refractory eczema. There are no dosing recommendations for any other off-label conditions, such as autoimmune disease. However, in clinical practice MMF is frequently being used in these other off-label conditions. Table 1 summarizes studies reporting off-label use of MMF in children following renal transplantation (without CsA/steroids), following transplantation of other organs, in paediatric rheumatological conditions and in other autoimmune disorders.
Is mycophenolate, used off-label, effective in children?
Many of the studies listed in Table 1 are case reports or small, retrospective, single-centre, uncontrolled studies. Those that are RCTs are typically adult trials that include a small, and sometimes unspecified, number of children of various ages [37]. Others, such as the study by Sinclair et al. [38], have a minimum age of 12 years and, here again, it is difficult to determine the exact numbers of young people who took part and thus the efficacy of MMF in children.
Randomized controlled trials of MMF which are exclusively paediatric are few and have small numbers of participants. One such trial compared the efficacy of MMF with that of CsA for maintaining remission in relapsing nephrotic syndrome [15]. This study suggested that CsA was more effective, but the adverse effect profile was much more acceptable in the MMF group. Although the study was small, there was no significant difference in the trough MPA blood levels between those who relapsed and those who did not. A limitation, however, was that trough-level data were not available for all patients who relapsed, with one child who had the lowest AUC relapsing several times. In a recent RCT, which analysed paediatric data separately, it was demonstrated that ‘overall efficacy and tolerability of MMF in paediatrics appear to be comparable with that in adults’[39].
As many of the studies contain only a few children amongst a majority of adult patients, it is possible that the efficacy results of MMF in children may be distorted by the adult data. This possibility appears to be supported in an early 1999 trial by Benfield et al. [40]. This RCT of paediatric transplant patients did not demonstrate a significant advantage in graft rejection through the addition of MMF to the immunosuppressive regimen. A number of theories were put forward by the authors as to why the result of this RCT failed to support evidence of MMF efficacy in this particular study, including a difference in how rejection was identified, the higher mass of renal graft to body mass of children compared with that of adults and the small number of patients studied. Although this trial did not support the efficacy of MMF in children, commercial trials did show MMF to be effective in paediatric renal transplantation, and its benefits outweighed it risks; therefore, MMF was granted a paediatric licence. There are few data to establish how effective MMF is in children, particularly for those conditions in which it has been tried off-licence. Further paediatric RCTs to compare MMF against a recognized, ‘gold standard’ or licensed treatment are needed to discover how effective MMF is in children.
What are the advantages of MMF compared with other immunosuppressants?
All commonly used immunosuppressants have adverse effects, which can limit their use, as summarized in Table 2. Azathioprine (AZA) was originally used in renal transplantation but is increasingly being replaced by MMF in paediatric renal transplant patients because it is associated with a reduced rejection rate and improved long-term graft function [35]. Azathioprine, like MMF, is another antiproliferative immunosuppressive agent, which affects DNA synthesis and thus production of lymphocytes. Compared with AZA, MMF is more potent and has a better clinical outcome in paediatric renal transplant patients [35]. Besides renal transplant, AZA has traditionally been used with clinical benefit in a number of autoimmune conditions [41]. This has led in turn to MMF being tried in these same autoimmune conditions, as observed in the off-label use studies listed in Table 1.
Table 2.
Advantages and disadvantages of mycophenolate mofetil (MMF) in comparison to some commonly used immunosuppressants
| MMF | Azathioprine | Cyclophosphamide | Ciclosporin A | Tacrolimus | |
|---|---|---|---|---|---|
| Primary mechanism of action | Inhibition of inosine 5′-monophosphate dehydrogenase, resulting in inhibition of T and B cell proliferation | Inhibition of purine synthesis, resulting in inhibition of T and B cell proliferation | Alkylating agent that leads to inhibition of DNA synthesis | Calcineurin inhibitor, resulting in inhibition of T cell proliferation | Calcineurin inhibitor, resulting in inhibition of T cell proliferation |
| Advantages | More selective for lymphocytes than Azathioprine | Extensive clinical experience as immunosuppressant agent | Can be effective as a second-line immunosuppressant after failure of first-line agent | Virtually nonmyelotoxic | Useful second-line immunosuppressant, also licensed as a topical agent for eczema |
| Disadvantages | Gastrointestinal disturbances | Risk of myelosupression in those with low activity of enzyme thiopurine methyltransferease | Gastrointestinal disturbances | Gastrointestinal disturbances | Gastrointestinal disturbances |
| Myelotoxic | Can cause haemorrhagic cystitis | Nephrotoxic | Increased risk of neurotoxicity compared with ciclosporin A | ||
| Myelotoxic | Hypertension | Can cause cardiomyopathy | |||
| Altered glucose metabolism |
Immunosuppressive agents, such as CsA and cyclophosphamide, adversely affect renal function. Mycophenolate mofetil does not have adverse effects on creatinine concentrations [42]. These advantages in renal function of MMF over CsA mean that MMF is also being evaluated for use in conditions where CsA is traditionally used therapeutically. Nephrotic syndrome, which primarily affects children, is one of these conditions; MMF has been shown to be a safe and effective alternative treatment option to CsA for nephrotic syndrome [42, 43].
As it becomes more commonplace to replace the use of AZA and CsA with MMF, this brings to the forefront a range of conditions for which MMF could be further trialled in the paediatric population, i.e. conditions in which these two drugs are currently being used as recognized, but not necessarily licensed, therapeutic agents.
Unresolved issues with the use of MMF in children
Further trials to investigate efficacy and use in clinical conditions outside of MMF's paediatric licence are important. Additionally, there are several unresolved issues which also require investigation to clarify the role of MMF in paediatric clinical practice.
Dosing
Dosage regimes for children are often extrapolated from adult data to give an initial dosage of around 40 mg kg−1 day−1[44], although doses of up to 50 mg kg−1 day−1 have been used [45]. However, medications for children are more usually quoted for surface area (in milligrams per square metre). This is because children have a larger surface area to body mass than adults. The rate of distribution or metabolism of a drug correlates with heat loss which, in turn, is generally considered as being proportional to surface area. Thus, surface area is widely accepted as being the best criterion when calculating drug doses in children.
There are major ambiguities which may arise from the fact that the BNFc shows the MMF dose in milligrams per kilogram as well as in milligrams per square metre [46]. The licensed dose of MMF for renal transplant, used in combination with CsA and steroids, is 600 mg m−2 twice a day. A dose of 300 mg m−2 twice a day is shown for use with tacrolimus and steroids, an unlicensed combination. Such dosing takes account of the known interaction between CsA and MMF, necessitating an MMF dose reduction when tacrolimus rather than CsA combination is used. However, for hepatic transplantation, in combination with steroids and CsA or tacrolimus, a dose of 10–20 mg kg−1 twice daily is recommended in the BNFc. This same milligrams per kilogram dosing strategy was used for hepatic transplantation in a study by Tredger et al. [21], and it would appear that this is where the BNFc recommendations for the unlicensed indication of hepatic transplantation have stemmed. The BNFc hepatic transplantation dosing guidance does not take account of the anticipated dose reduction required when using MMF with tacrolimus rather than CsA, the dose being listed as the same for use in either combination. There is an absence of dose information for other conditions for which MMF is known to be used. Without an appreciation of the diversity of conditions being treated and the known interactions, dosing inconsistency can arise.
The study by Tredger et al. [21] was conducted on 147 adults and 63 children. Unlike many studies of both adults and children, the paediatric data in this particular study were analysed separately from those of the adults. Dosing aimed to achieve predose, trough levels of MPA of 0.3–5.2 mg mg l−1. However, it is not clear how the MMF starting dose of 5 mg kg−1 for the children in the study was determined. Doses were increased up to 20 mg kg−1 twice a day, if tolerated by the child, and were required to achieve the target predose level. No close correlation between MMF dose and MPA levels was observed in adults or in children. For adults, a therapeutic range of predose MPA of 1–3.5 mg l−1 was determined in this study; doses above 3.5 mg l−1 were associated with higher risk of leucopenia, infection and gastrointestinal disturbances. Conversely, levels below 1 mg l−1 were associated with acute rejection episodes. A therapeutic predose MPA level was not determined for the paediatric population, because there were insufficient adverse event data to make any calculations meaningful. However, it was noted that all three episodes of acute rejection occurred when MPA levels were less than 0.5 mg l−1. The adult therapeutic predose MPA range indicated by this study is similar to that advocated by Filler [47] of 1.6–3.5 mg l−1 for paediatric patients. These studies, therefore, show that MPA trough levels could be used to indicate whether a therapeutic level is being reached and maintained during MMF treatment. Nevertheless, a study by Pape et al. disputes the value of MPA trough-level monitoring in long-term follow-up of transplant patients. They indicate that MPA levels taken at 75 min after administration show a better correlation with MPA AUC and are of the opinion that such alternative monitoring of paediatric patients should be used [48]. There are substantial intra- and interpatient variations in MPA exposure in children; younger children require more frequent dosing than adolescents and adults [19, 49]. Such dosing discrepancy across age ranges is thought to be due to age-dependent variations in the transporter, P-glycoprotein [47], implicated in reduced absorption of medications.
APOMYGRE, a randomized multicentre trial of renal transplant patients, looked at MMF dosing based on MPA exposure, comparing fixed doses of MMF and concentration-controlled dosing. This adult trial found that dosing based on MPA blood concentrations significantly reduced treatment failure and acute rejection [50]. Although this is an adult trial, the data, like those of the Tredger study discussed above, do appear to support monitoring of MPA blood concentrations to maintain an optimal therapeutic concentration.
The value of therapeutic drug monitoring (TDM) during MMF treatment remains controversial [51]. The BNFc does not have a recommendation on monitoring concentrations of MPA. Therapeutic drug monitoring of MPA is not routine in the UK, although intermittent trough-level monitoring of MPA is known to be carried out in some UK centres, to guide dosing [35]. By contrast, in the USA, TDM is carried out more routinely, because there is a Food and Drug Administration-approved commercial assay for MPA [47].
To add to the controversy surrounding TDM, assay methods used to measure MPA have been questioned. It has been established that cross-reactivity of AcMPAG in assay techniques can result in an overestimation of MPA levels, making it difficult to establish a therapeutic level for MPA [13]. Measurement of IMPDH activity levels in paediatric patients, to guide initial MMF dosing, may provide an alternative to TDM [52]. There is a need for more research, in this connection, to establish the validity, timing of blood sampling and interpretation of relationship outcome. The recently proposed paediatric population pharmacokinetic models, discussed earlier, may assist in any further research.
Variable response as a result of genetic differences
It has been demonstrated that the way that the body metabolizes MMF is a ‘multigenic process’[53]. There are many steps involved in this processing of MMF. Potentially, each has its own, genetically different, components that contribute to the overall effect. Those so far identified as having potential for genetic variations are enzymes and transporter proteins.
The target enzyme of MPA is IMPDH, of which there are two forms, IMPDH1 and IMPDH2 [54]. Mycophenolate mofetil has a more potent effect on IMPDH 2, which is the form predominately present in activated leucocytes. Variations in the gene coding for IMPDH2 could have a dramatic effect on the response to MPA therapy. Healthy adult subjects with rs11706052 polymorphism of the IMPDH2 gene have been shown to have around a 50% reduction in the antiproliferative effect of MMF on lymphocytes [55]. Currently, there are few published data on the expression of IMPDH in children; early reports demonstrate that haplotypes of IMPDH2 are associated with neutropenia and haplotypes of IMPDH1 are associated with gastrointestinal intolerance [56, 57]. In addition, the main metabolizing enzyme of MMF is uridine diphosphate-glucuronosyl transferase (UGT), of which there are two isoforms, UGT1A8 and UGT1A9, which are significant to MMF metabolism [53]. Prausa et al. [58] have studied the association between the main two adverse effects of MMF in children, namely leucopenia and diarrhoea, with polymorphisms of UGT. The data obtained from this study implicated UGT polymorphisms as potential predictors of adverse events during MMF treatment in children. The strongest association was for those who were homozygous for the single-nucleotide polymorphism UGT1A9 −331T>C, all of whom experienced leucopenia. No association was found with diarrhoea and UGT polymorphism, suggesting that this side-effect could be due to genetic variations in other genes, including the transporter proteins involved in MMF absorption. This correlates well with the knowledge that MPA is a substrate and inhibitor of the transporter protein, P-glycoprotein (MDR1) [47]. In common with the isoforms of IMPDH, further investigation of UTG in children is desirable.
As well as MDR1, the organic anion transporter ABCC2 is known to be important in MMF pharmacokinetics, and certain polymorphisms of this transporter protein can change the proportions of each of the different metabolites of MMF [53]. In turn, this can change the effectiveness and toxicity of MMF. There are few data on expression of transporter proteins in children, although it has been shown that some transporter proteins show highly variable expression relating to age and distribution in intestines and liver [47]. In a small study of paediatric heart transplant patients, ABCC2 polymorphisms have been shown to be associated with gastrointestinal intolerance and bone marrow toxicity [56].
Adverse drug reactions
Information relating to adverse drug reactions in children is sparse, which has prompted the setting up of a formal study, adverse drug reactions in children, currently ongoing, to examine the extent of adverse drug reactions in children [59]. The data for adverse effects of MMF in children are limited. The adverse effect profile has been described as being similar to that of adults, principally being abdominal pain, diarrhoea, vomiting, sepsis, leucopenia, anaemia, hypertension and infection. This profile is more frequent and severe in children under 6 years of age [9, 10].
Postmarketing surveillance data, which includes the UK Yellow Card scheme, have identified isolated cases of progressive multifocal leukoencephalopathy (PML) in adults taking MMF in combination with other immunosuppressants. Progressive multifocal leukoencephalopathy is a rare and usually fatal demyelinating condition found only in severely immunosupressed patients, which is caused by a human polyomavirus, JC virus [60]. This observation of PML in extremely immunosupressed patients prompted a drug safety alert for MMF in 2008. The contributory role of MMF in development of PML has not been ruled out, and recently there has been a reported case of PML in a child. The child, an 11-year-old, post-renal transplant patient, developed PML, with clinical improvement seen when MMF was discontinued [61].
The gastrointestinal symptoms are often the cause of cessation of treatment in children. As a result, an enteric preparation of mycophenolate sodium has been developed, which was reported in a pilot study to reduce gastrointestinal complications, such as diarrhoea, nausea or abdominal discomfort, in children [62, 63], although this product is not currently licensed in children. As with any enteric coated preparation, the peak drug concentration can be delayed, and this effect was seen in a small trial of post-renal transplant paediatric patients [64].
Some less common adverse effects have been reported in children, as follows: an abnormal chromatin clumping syndrome in two children associated with leucocytosis [65]; pseudotumour cerebri syndrome in a 5-year-old child taking MMF for autoimmune lymphoproliferative disease [66]; and two cases of children with severe colitis [67]. Perhaps the most significant, albeit rare, adverse effects have been due to respiratory problems, including a single case of reversible chronic mineralizing pulmonary elastosis in a 7-year-old boy [68] and several instances of bronchiectasis [69, 70]. Postmarketing surveillance has also identified isolated reports of interstitial lung disease and pulmonary fibrosis in adult patients treated with MMF in combination with other immunosuppressants [9]. However, whether there is a true causal association between the use of MMF and the occurrence of these respiratory adverse effects needs further study, including the definition of mechanisms.
Reports of congenital malformations as a result of MMF exposure in utero are now emerging. An ‘MMF embryopathy’ is described as a combination of craniofacial abnormalities and complex cardiac defects, thought to be as a result of interference with neural crest cell migration [71, 72]. Many of the side-effects, such as leucopenia, susceptibility to infection and lymphoma, are understandable and associated with most immunosuppressive agents. However, there are adverse effects, such as the respiratory and gastrointestinal problems, which are not so readily explained. Until such time as the pharmacokinetics and associated pharmacogenomics of MMF are researched further, the reason for these will continue to be a challenge to investigators.
Interactions with other drugs
Drug–drug interaction studies have not been reported in children, but adult studies are listed in the product licence information for MMF [9, 10]. The requirement to carry out drug–drug interactions studies in children is not necessarily a prerequisite for paediatric licensing. Presently, studies are carried out on healthy adult volunteers, and extending them to healthy children would raise ethical questions. The adult drug–drug interactions listed in the licence literature for MMF are often with medications already well recognized as causing interactions with other drugs, such as rifampicin, ciprofloxacin and CsA. For example, drugs which affect absorption from the gut, such as iron preparations, antacids and cholestyramine, do reduce absorption of MMF and lead to lower MPA levels in adults [9]. Interactions of MMF with other drugs can also be predicted, if it is given with medications known to interfere with absorption and distribution around the body. Thus, in addition to the ethical argument surrounding the use of healthy children for drug–drug interactions studies, there is probably no real new information to be gained by exposing children to these known drug–drug interactions.
When MMF is used off-label without CsA, there needs to be a substantial dose reduction to compensate for the significant interaction engendered by CsA. Such a drug–drug interaction is due to the inhibitory effect of CsA on the transporter protein, multidrug-resistant protein 2 [47]. This again highlights the importance of pharmacokinetic considerations when using MMF in children. Furthermore, from the pharmacogenomic aspect, it can be proposed that individual differences in the UGT expression might provide another explanation for certain drug–drug interactions in children. This requires further research.
Mycophenolate in children: the future
The lack of controlled trials in children is an issue that was addressed several years ago by changes in legislation for licensing of medications in the USA [73]. In the USA, rules requiring mandatory testing in paediatric populations in all new drug label applications were introduced in 1998. With similar changes in the legislation in Europe [74] in 2007, it is anticipated that the number and quality of trials orientated towards children will improve [75]. Public fears over testing in children may continue, but a vigorous and challenging regulatory environment may help to alleviate these fears.
Clinical trials in children are essential to develop the best treatment strategies in a safe environment [76]. Avoidance of polypharmacy in children is desirable, due to interactions and the potential for long-term, sometimes irreversible, side-effects. It may be that further paediatric studies will confirm a steroid-sparing effect of MMF that is observed in clinical practice, perhaps bringing about the use of MMF as a dual therapy or monotherapy for some conditions.
As demonstrated in this review, MMF has been used in children, particularly off-label, for several years. It is possible that the new European regulations may have an influence on future paediatric clinical trials. However, although MMF is still under patent for paediatric use in the UK, its commercial value may be limited because the market in children is small. As the overall commercial value of MMF has been drastically reduced, due to expiration of the adult patent, it can be predicted that paediatric trials might not be pursued by the manufacturer. Notwithstanding these considerations, with increasing knowledge of the phramacodynamics, pharmacokinetics and pharmacogenomics demonstrating the clinical benefits of MMF, new, formal, investigator-led, paediatric clinical trial proposals might attract funding, and the vital aspects specific to children will be addressed.
Acknowledgments
H.J.D. is an NIHR academic clinical fellow in Paediatric Clinical Pharmacology. Both M.P. and R.L.S. are NIHR Senior Investigators. The authors acknowledge the support of the Department of Health, NIHR, MRC, AR-UK and Wellcome Trust in their research.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Bentley R. Mycophenolic acid: a one hundred year odyssey from antibiotic to immunosuppressant [Review][201 refs] Chem Rev. 2000;100:3801–25. doi: 10.1021/cr990097b. [DOI] [PubMed] [Google Scholar]
- 2.Alsberg CL, Black F. Contribution to the study of maize deterioration; biochemical and toxicological investigations of Penicillium puberulum and Penicillium stoloniferum. U S Dept Agr, Bureau Plant Ind, Bull. 1913;270:1–47. [Google Scholar]
- 3.Carter SB, Franklin TJ, Jones DF, Leonard BJ, Mills SD, Turner RW, Turner WB. Mycophenolic acid: an anti-cancer compound with unusual properties. Nature. 1969;223:848–50. doi: 10.1038/223848a0. [DOI] [PubMed] [Google Scholar]
- 4.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(Suppl. 1):s2–8. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 5.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. [Review][130 refs] Immunopharmacology. 2000;47:215–45. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 6.Chong CR, Qian DZ, Pan F, Wei Y, Pili R, Sullivan DJ, Jr, Liu JO. Identification of type 1 inosine monophosphate dehydrogenase as an antiangiogenic drug target. J Med Chem. 2006;49:2677–80. doi: 10.1021/jm051225t. [DOI] [PubMed] [Google Scholar]
- 7.Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165:2374–81. doi: 10.4049/jimmunol.165.5.2374. [DOI] [PubMed] [Google Scholar]
- 8.Haug C, Schmid-Kotsas A, Linder T, Jehle PM, Bachem MG, Gruenert A, Rozdzinski E. The immunosuppressive drug mycophenolic acid reduces endothelin-1 synthesis in endothelial cells and renal epithelial cells. Clin Sci. 2002;103:76S–80S. doi: 10.1042/CS103S076S. [DOI] [PubMed] [Google Scholar]
- 9.Roche. Summary of Product Characteristics Data Sheet Cellcept 500 mg Tablets. Welwyn Garden City, Hertfordshire: Datapharm Communications Ltd; 2009. Available at: http://www.medicines.org.uk/emc/document.aspx?documentId=1680&doctype=SPC (last accessed October 2011) [Google Scholar]
- 10.Roche. Cellcept US label. 2009. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050722s024,050723s023,050758s022,050759s028lbl.pdf (last accessed October 2011)
- 11.Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schutz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol. 1999;126:1075–82. doi: 10.1038/sj.bjp.0702399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipkova M, Armstrong VW, Weber L, Niedmann PD, Wieland E, Haley J, Tonshoff B, Oellerich M German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant R. Pharmacokinetics and protein adduct formation of the pharmacologically active acyl glucuronide metabolite of mycophenolic acid in pediatric renal transplant recipients. Ther Drug Monit. 2002;24:390–9. doi: 10.1097/00007691-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Shipkova M, Schutz E, Besenthal I, Fraunberger P, Wieland E, Shipkova M, Schutz E, Besenthal I, Fraunberger P, Wieland E. Investigation of the crossreactivity of mycophenolic acid glucuronide metabolites and of mycophenolate mofetil in the Cedia MPA assay. Ther Drug Monit. 2010;32:79–85. doi: 10.1097/FTD.0b013e3181cc342a. [DOI] [PubMed] [Google Scholar]
- 14.Pranzatelli MR, Tate ED, Travelstead AL, Baumgardner CA, Gowda NV, Halthore SN, Kerstan P, Kossak BD, Mitchell WG, Taub JW. Insights on chronic-relapsing opsoclonus-myoclonus from a pilot study of mycophenolate mofetil. J Child Neurol. 2009;24:316–22. doi: 10.1177/0883073808324217. [DOI] [PubMed] [Google Scholar]
- 15.Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol. 2008;23:2013–20. doi: 10.1007/s00467-008-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De S, Al-Nabhani D, Thorner P, Cattran D, Piscione TD, Licht C. Remission of resistant MPGN type I with mycophenolate mofetil and steroids. Pediatr Nephrol. 2009;24:597–600. doi: 10.1007/s00467-008-1023-7. [DOI] [PubMed] [Google Scholar]
- 17.Aw MM, Brown NW, Itsuka T, Gonde CE, Adams JE, Heaton ND, Tredger JM, Mieli-Vergani G, Dhawan A. Mycophenolic acid pharmacokinetics in pediatric liver transplant recipients. Liver Transpl. 2003;9:383–8. doi: 10.1053/jlts.2003.50022. [DOI] [PubMed] [Google Scholar]
- 18.David-Neto E, Pereira Araujo LM, Sumita NM, Mendes ME, Ribeiro Castro MC, Alves CF, Kakehashi E, Romano P, Yagyu EM, Queiroga M, Nahas WC, Ianhez LE. Mycophenolic acid pharmacokinetics in stable pediatric renal transplantation. Pediatr Nephrol. 2003;18:266–72. doi: 10.1007/s00467-002-1057-1. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, Morris E, Tallamy B, van deVen C, Ayello J, Baxter-Lowe L, Satwani P, George D, Bradley MB, Garvin J, Cairo MS. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–43. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Sagcal-Gironella ACP, Fukuda T, Wiers K, Cox S, Nelson S, Dina B, Sherwin CMT, Klein-Gitelman MS, Vinks AA, Brunner HI. Pharmacokinetics and pharmacodynamics of mycophenolic acid and their relation to response to therapy of childhood-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2011;40:307–13. doi: 10.1016/j.semarthrit.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M, Heaton N. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004;10:492–502. doi: 10.1002/lt.20124. [DOI] [PubMed] [Google Scholar]
- 22.Aigrain EJ, Shaghaghi EK, Baudouin V, Popon M, Loirat C. Pharmacokinetics of mycophenolate mofetil in eight pediatric renal transplant patients. Transplant Proc. 2000;32:388–90. doi: 10.1016/s0041-1345(99)00989-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Elie V, Baudouin V, Bensman A, Andre JL, Brochard K, Broux F, Cailliez M, Loirat C, Jacqz-Aigrain E. Population pharmacokinetics and Bayesian estimator of mycophenolic acid in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol. 2010;69:358–66. doi: 10.1111/j.1365-2125.2010.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Fakhoury M, Deschenes G, Roussey G, Brochard K, Niaudet P, Tsimaratos M, Andre JL, Cloarec S, Cochat P, Bensman A, Azougagh S, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol. 2010;50:1280–91. doi: 10.1177/0091270009357429. [DOI] [PubMed] [Google Scholar]
- 25.Antoniadis A, Papachristou F, Gakis D, Takoudas D, Sotiriou I. Comparison between mycophenolate mofetil and azathioprine based immunosuppression in pediatric renal transplantation from living related donors. Transplant Proc. 1998;30:4085–6. doi: 10.1016/s0041-1345(98)01350-5. [DOI] [PubMed] [Google Scholar]
- 26.Grenda R. Effects of steroid avoidance and novel protocols on growth in paediatric renal transplant patients. Pediatr Nephrol. 2010;25:747–52. doi: 10.1007/s00467-009-1318-3. [DOI] [PubMed] [Google Scholar]
- 27.Olney RC. Mechanisms of impaired growth: effect of steroids on bone and cartilage. Horm Res. 2009;72(Suppl. 1):30–5. doi: 10.1159/000229761. [DOI] [PubMed] [Google Scholar]
- 28.Birkeland SA, Larsen KE, Rohr N. Pediatric renal transplantation without steroids. Pediatr Nephrol. 1998;12:87–92. doi: 10.1007/s004670050410. [DOI] [PubMed] [Google Scholar]
- 29.Silverstein DM, Aviles DH, LeBlanc PM, Jung FF, Vehaskari VM. Results of one-year follow-up of steroid-free immunosuppression in pediatric renal transplant patients. Pediatr Transplant. 2005;9:589–97. doi: 10.1111/j.1399-3046.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 30.Nematalla AH, Bakr MA, Gheith OA, Elagroudy AE, Elshahawy M, Aghoneim M. Steroid-avoidance immunosuppression regimen in live-donor renal allotransplant recipients: a prospective, randomized, controlled study. Exp Clin Transplant. 2007;5:673–9. [PubMed] [Google Scholar]
- 31.Hocker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, Pohl M, Zimmering M, Frund S, Klaus G, Wuhl E, Tonshoff B. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation. 2009;87:934–41. doi: 10.1097/TP.0b013e31819b6d4a. [DOI] [PubMed] [Google Scholar]
- 32.Hocker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, Pohl M, Zimmering M, Frund S, Klaus G, Wuhl E, Tonshoff B. Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant. 2010;25:617–24. doi: 10.1093/ndt/gfp506. [DOI] [PubMed] [Google Scholar]
- 33.Chavers BM, Chang YC, Gillingham KJ, Matas A. Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation. 2009;88:237–41. doi: 10.1097/TP.0b013e3181ac6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, Murer L, Vondrak K, Maxwell H, van Damme-Lombaerts R, Loirat C, Mor E, Cochat P, Milford DV, Brown M, Webb NJA. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. 2010;10:828–36. doi: 10.1111/j.1600-6143.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 35.Krischock L, Gullett A, Bockenhauer D, Rees L, Trompeter RS, Marks SD. Calcineurin-inhibitor free immunosuppression with mycophenolate mofetil and corticosteroids in paediatric renal transplantation improves renal allograft function without increasing acute rejection. Pediatr Transplant. 2009;13:475–81. doi: 10.1111/j.1399-3046.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 36.Cransberg K, Cornelissen M, Lilien M, Van Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation. 2007;83:1041–7. doi: 10.1097/01.tp.0000260146.57898.9c. [DOI] [PubMed] [Google Scholar]
- 37.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, Pasquini M, Goldstein SC, Ho VT, Hayes-Lattin B, Wingard JR, Horowitz MM, Levine JE Blood, Marrow Transplant Clinical Trials N. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinclair A, Appel G, Dooley MA, Ginzler E, Isenberg D, Jayne D, Wofsy D, Solomons N. Mycophenolate mofetil as induction and maintenance therapy for lupus nephritis: rationale and protocol for the randomized, controlled Aspreva Lupus Management Study (ALMS) Lupus. 2007;16:972–80. doi: 10.1177/0961203307084712. [DOI] [PubMed] [Google Scholar]
- 39.Hocker B, van Gelder T, Martin-Govantes J, Machado P, Tedesco H, Rubik J, Dehennault M, Garcia Meseguer C, Tonshoff B, Group FS. Comparison of MMF efficacy and safety in paediatric vs. adult renal transplantation: subgroup analysis of the randomised, multicentre FDCC trial. Nephrol Dial Transplant. 2011;26:1073–9. doi: 10.1093/ndt/gfq450. [DOI] [PubMed] [Google Scholar]
- 40.Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, Kohaut E. Mycophenolate mofetil in pediatric renal transplantation. Pediatr Transplant. 1999;3:33–7. doi: 10.1034/j.1399-3046.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 41.GlaxoSmithKline. 2009. Imuran Summary of Product Characteristics.
- 42.Al-Akash S, Al-Makdama A. Mycophenolate mofetil in children with steroid-dependent and/or frequently relapsing nephrotic syndrome. Ann Saudi Med. 2005;25:380–4. doi: 10.5144/0256-4947.2005.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Mello VR, Rodrigues MT, Mastrocinque TH, Martins SPL, de Andrade OVB, Guidoni EBM, Scheffer DK, Martini Filho D, Toporovski J, Benini V. Mycophenolate mofetil in children with steroid/cyclophosphamide-resistant nephrotic syndrome. Pediatr Nephrol. 2010;25:453–60. doi: 10.1007/s00467-009-1356-x. [DOI] [PubMed] [Google Scholar]
- 44.Chardot C, Nicoluzzi JE, Janssen M, Sokal E, Lerut J, Otte JB, Reding R. Use of mycophenolate mofetil as rescue therapy after pediatric liver transplantation. Transplantation. 2001;71:224–9. doi: 10.1097/00007890-200101270-00009. [DOI] [PubMed] [Google Scholar]
- 45.Dipchand AI, Benson L, McCrindle BW, Coles J, West L. Mycophenolate mofetil in pediatric heart transplant recipients: a single-center experience. Pediatr Transplant. 2001;5:112–8. doi: 10.1034/j.1399-3046.2001.005002112.x. [DOI] [PubMed] [Google Scholar]
- 46.BNF for Children 2011–2012. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Ltd; 2011. [Google Scholar]
- 47.Filler G. Value of therapeutic drug monitoring of MMF therapy in pediatric transplantation. [Review][46 refs] Pediatr Transplant. 2006;10:707–11. doi: 10.1111/j.1399-3046.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 48.Pape L, Ehrich JH, Offner G. Long-term follow-up of pediatric transplant recipients: mycophenolic acid trough levels are not a good indicator for long-term graft function. Clin Transplant. 2004;18:576–9. doi: 10.1111/j.1399-0012.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 49.Filler G, Foster J, Berard R, Mai I, Lepage N. Age-dependency of mycophenolate mofetil dosing in combination with tacrolimus after pediatric renal transplantation. Transplant Proc. 2004;36:1327–31. doi: 10.1016/j.transproceed.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 50.Rousseau A, Laroche M-L, Venisse N, Loichot-Roselmac C, Turcant A, Hoizey G, Compagnon P, Hary L, Debruyne D, Saivin S, Jacqz-Aigrain E, Buchler M, Villeneuve C, Vergnenegre A, Le Meur Y, Marquet P. Cost-effectiveness analysis of individualized mycophenolate mofetil dosing in kidney transplant patients in the APOMYGRE trial. Transplantation. 2010;89:1255–62. doi: 10.1097/TP.0b013e3181d75952. [DOI] [PubMed] [Google Scholar]
- 51.Tonshoff B, David-Neto E, Ettenger R, Filler G, van Gelder T, Goebel J, Kuypers DRJ, Tsai E, Vinks AA, Weber LT, Zimmerhackl LB. Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transplant Rev. 2011;25:78–89. doi: 10.1016/j.trre.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Fukuda T, Goebel J, Thogersen H, Maseck D, Cox S, Logan B, Sherbotie J, Seikaly M, Vinks AA. Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol. 2011;51:309–20. doi: 10.1177/0091270010368542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levesque E, Benoit-Biancamano MO, Delage R, Couture F, Guillemette C. Pharmacokinetics of mycophenolate mofetil and its glucuronide metabolites in healthy volunteers. Pharmacogenomics. 2008;9:869–79. doi: 10.2217/14622416.9.7.869. [DOI] [PubMed] [Google Scholar]
- 54.Hedstrom L. IMP dehydrogenase: structure, mechanism, and inhibition. [Review][275 refs] Chem Rev. 2009;109:2903–28. doi: 10.1021/cr900021w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winnicki W, Weigel G, Sunder-Plassmann G, Bajari T, Winter B, Herkner H, Sengoelge G. 2009. An inosine 5'-monophosphate dehydrogenase 2 single-nucleotide polymorphism impairs the effect of mycophenolic acid. In, edJournal TP: Nature Publishing Group. [DOI] [PubMed]
- 56.Ohmann EL, Burckart GJ, Brooks MM, Chen Y, Pravica V, Girnita DM, Zeevi A, Webber SA, Ohmann EL, Burckart GJ, Brooks MM, Chen Y, Pravica V, Girnita DM, Zeevi A, Webber SA. Genetic polymorphisms influence mycophenolate mofetil-related adverse events in pediatric heart transplant patients. J Heart Lung Transplant. 2010;29:509–16. doi: 10.1016/j.healun.2009.11.602. [DOI] [PubMed] [Google Scholar]
- 57.Ohmann EL, Burckart GJ, Chen Y, Pravica V, Brooks MM, Zeevi A, Webber SA. Inosine 5′-monophosphate dehydrogenase 1 haplotypes and association with mycophenolate mofetil gastrointestinal intolerance in pediatric heart transplant patients. Pediatr Transplant. 2010;14:891–5. doi: 10.1111/j.1399-3046.2010.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prausa SE, Fukuda T, Maseck D, Curtsinger KL, Liu C, Zhang K, Nick TG, Sherbotie JR, Ellis EN, Goebel J, Vinks AA. UGT genotype may contribute to adverse events following medication with mycophenolate mofetil in pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;85:495–500. doi: 10.1038/clpt.2009.3. [DOI] [PubMed] [Google Scholar]
- 59.NIHR. Medicines for children research network. 2010. Available at: http://www.adric.org.uk/ (last accessed January 2012)
- 60.Gorelik L, Reid C, Testa M, Brickelmaier M, Bossolasco S, Pazzi A, Bestetti A, Carmillo P, Wilson E, McAuliffe M, Tonkin C, Carulli JP, Lugovskoy A, Lazzarin A, Sunyaev S, Simon K, Cinque P. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis. 2011;204:103–14. doi: 10.1093/infdis/jir198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber SC, Uhlenberg B, Raile K, Querfeld U, Muller D. Polyoma virus-associated progressive multifocal leukoencephalopathy after renal transplantation: regression following withdrawal of mycophenolate mofetil. Pediatr Transplant. 2011;15:E19–24. doi: 10.1111/j.1399-3046.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 62.Pape L, Ahlenstiel T, Kreuzer M, Ehrich JH. Improved gastrointestinal symptom burden after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in kidney transplanted children. Pediatr Transplant. 2008;12:640–2. doi: 10.1111/j.1399-3046.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 63.Pape L, Ahlenstiel T. Is EC-MPS effective in reducing GI toxicity of MPA? At what price? Pediatr Transplant. 2009;13:659–60. doi: 10.1111/j.1399-3046.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 64.Reyes H, Hernandez AM, Valverde S, Cataneo A, Mendoza A, Barrera I, Ortiz L, Garcia-Roca P, Lopez-Martinez B, Castaneda-Hernandez G, Medeiros M. Efficacy and safety of conversion of mycophenolate mofetil to enteric-coated mycophenolate sodium in Mexican renal transplant children. Pediatr Transplant. 2010;14:746–52. doi: 10.1111/j.1399-3046.2010.01326.x. [DOI] [PubMed] [Google Scholar]
- 65.Daliphard S, Accard F, Delattre C, Toupance O, Guyot C, Mechinaud F, Chassaing A, Hourmant M, Rousset P, Potron G. Reversible abnormal chromatin clumping in granulocytes from six transplant patients treated with mycophenolate mofetil: a rare adverse effect mimicking abnormal chromatin clumping syndrome. Br J Haematol. 2002;116:726–7. doi: 10.1046/j.1365-2141.2002.3317_2.x. [DOI] [PubMed] [Google Scholar]
- 66.Patiroglu T, Ozcan A, Karakukcu M, Ozdemir MA, Poyrazoglu G, Canpolat M, Unal E. Mycophenolate mofetil-induced pseudotumor cerebri in a boy with autoimmune lymphoproliferative disease. Childs Nerv Syst. 2011;27:853–5. doi: 10.1007/s00381-011-1402-4. [DOI] [PubMed] [Google Scholar]
- 67.Phatak UP, Seo-Mayer P, Jain D, Selbst M, Husain S, Pashankar DS. Mycophenolate mofetil-induced colitis in children. J Clin Gastroenterol. 2009;43:967–9. doi: 10.1097/MCG.0b013e3181a8754d. [DOI] [PubMed] [Google Scholar]
- 68.Reynolds BC, Paton JY, Howatson AG, Ramage IJ. Reversible chronic pulmonary fibrosis associated with MMF in a pediatric patient: a case report. Pediatr Transplant. 2008;12:228–31. doi: 10.1111/j.1399-3046.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 69.Pijnenburg MW, Cransberg K, Wolff E, Bouquet J, Merkus PJ. Bronchiectasis in children after renal or liver transplantation: a report of five cases. Pediatr Transplant. 2004;8:71–4. doi: 10.1046/j.1397-3142.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 70.Merkus PJ, Pijnenburg M, Cransberg K. Mycophenolate mofetil and bronchiectasis in pediatric transplant patients. Transplantation. 2006;82:1386. doi: 10.1097/01.tp.0000235912.21172.dd. [DOI] [PubMed] [Google Scholar]
- 71.Koshy AN, Strong D, Earles G, Fassett RG. Congenital malformations with low-dose mycophenolate mofetil after kidney transplantation. Nephrology. 2010;15:133–5. doi: 10.1111/j.1440-1797.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 72.Lin AE, Singh KE, Strauss A, Nguyen S, Rawson K, Kimonis VE. An additional patient with mycophenolate mofetil embryopathy: cardiac and facial analyses. Am J Med Genet. 2011;155A:748–56. doi: 10.1002/ajmg.a.33934. Part A. [DOI] [PubMed] [Google Scholar]
- 73.Schreiner MS. Paediatric clinical trials: redressing the imbalance. [Review][86 refs] Nat Rev Drug Discov. 2003;2:949–61. doi: 10.1038/nrd1253. [DOI] [PubMed] [Google Scholar]
- 74.Sammons HM, Choonara I. What is happening to improve drug therapy in children? Paediatr Child Health. 2007;17:108–10. [Google Scholar]
- 75.Pandolfini C, Bonati M, Rossi V, Santoro E, Choonara I, Naylor C, Sammons H, Jacqz-Aigrain E, Zarrabian S, Arnau JM, Castel JM, Danes I, Fuentes I. The DEC-net European register of paediatric drug therapy trials: contents and context. Eur J Clin Pharmacol. 2008;64:611–7. doi: 10.1007/s00228-007-0458-2. [DOI] [PubMed] [Google Scholar]
- 76.Sammons HM, Gray C, Hudson H, Cherrill J, Choonara I. Safety in paediatric clinical trials – a 7-year review. Acta Paediatr. 2008;97:474–7. doi: 10.1111/j.1651-2227.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 77.Shaddy RE, Fuller TC, Anderson JB, Lambert LM, Brinkman MK, Profaizer T, Hawkins JA. Mycophenolic mofetil reduces the HLA antibody response of children to valved allograft implantation. Ann Thorac Surg. 2004;77:1734–9. doi: 10.1016/j.athoracsur.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 78.Ostronoff F, Ostronoff M, Souto-Maior AP, Domingues M, Sucupira A, Manso DA, de Lima AK, Monteiro PG, Florencio R, Calixto R. Prospective trial of mycophenolate mofetil-cyclosporine A prophylaxis for acute GVHD after G-CSF stimulated allogeneic bone marrow transplantation with HLA-identical sibling donors in patients with severe aplastic anemia and hematological malignancies. Clin Transplant. 2009;23:33–8. doi: 10.1111/j.1399-0012.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- 79.Tannuri U, Gibelli NE, Maksoud-Filho JG, Santos MM, Pinho-Apezzato ML, Velhote MC, Ayoub AA, Silva MM, Maksoud JG. Mycophenolate mofetil promotes prolonged improvement of renal dysfunction after pediatric liver transplantation: experience of a single center. Pediatr Transplant. 2007;11:82–6. doi: 10.1111/j.1399-3046.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 80.Tzakis AG, Weppler D, Khan MF, Koutouby R, Romero R, Viciana AL, Raskin J, Nery JR, Thompson J. Mycophenolate mofetil as primary and rescue therapy in intestinal transplantation. Transplant Proc. 1998;30:2677–9. doi: 10.1016/s0041-1345(98)00786-6. [DOI] [PubMed] [Google Scholar]
- 81.Buratti S, Szer IS, Spencer CH, Bartosh S, Reiff A. Mycophenolate mofetil treatment of severe renal disease in pediatric onset systemic lupus erythematosus. J Rheumatol. 2001;28:2103–8. [PubMed] [Google Scholar]
- 82.Falcini F, Capannini S, Martini G, La Torre F, Vitale A, Mangiantini F, Nacci F, Cerinic MM, Cimaz R, Zulian F. Mycophenolate mofetil for the treatment of juvenile onset SLE: a multicenter study. Lupus. 2009;18:139–43. doi: 10.1177/0961203308094999. [DOI] [PubMed] [Google Scholar]
- 83.Rivera TL, Belmont HM, Malani S, Latorre M, Benton L, Weisstuch J, Barisoni L, Tseng CE, Izmirly PM, Buyon JP, Askanase AD. Current therapies for lupus nephritis in an ethnically heterogeneous cohort. J Rheumatol. 2009;36:298–305. doi: 10.3899/jrheum.080335. [DOI] [PubMed] [Google Scholar]
- 84.Wong SN, Chan WK, Hui J, Chim S, Lee TL, Lee KP, Leung LC, Tse NK, Yuen SF. Membranous lupus nephritis in Chinese children – a case series and review of the literature. [Review][24 refs] Pediatr Nephrol. 2009;24:1989–96. doi: 10.1007/s00467-009-1257-z. [DOI] [PubMed] [Google Scholar]
- 85.Posalski JD, Ishimori M, Wallace DJ, Weisman MH. Does mycophenolate mofetil prevent extra-renal flares in systemic lupus erythematosus? Results from an observational study of patients in a single practice treated for up to 5 years. Lupus. 2009;18:516–21. doi: 10.1177/0961203308099471. [DOI] [PubMed] [Google Scholar]
- 86.Aragon E, Chan YH, Ng KH, Lau YW, Tan PH, Yap HK. Good outcomes with mycophenolate-cyclosporine-based induction protocol in children with severe proliferative lupus nephritis. Lupus. 2010;19:965–73. doi: 10.1177/0961203310366855. [DOI] [PubMed] [Google Scholar]
- 87.Kazyra I, Pilkington C, Marks SD, Tullus K. Mycophenolate mofetil treatment in children and adolescents with lupus. Arch Dis Child. 2010;95:1059–61. doi: 10.1136/adc.2009.178608. [DOI] [PubMed] [Google Scholar]
- 88.Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology. 2009;48:1410–3. doi: 10.1093/rheumatology/kep244. [DOI] [PubMed] [Google Scholar]
- 89.Meriggioli MN, Ciafaloni E, Al-Hayk KA, Rowin J, Tucker-Lipscomb B, Massey JM, Sanders DB. Mycophenolate mofetil for myasthenia gravis: an analysis of efficacy, safety, and tolerability. Neurology. 2003;61:1438–40. doi: 10.1212/01.wnl.0000094122.88929.0b. [DOI] [PubMed] [Google Scholar]
- 90.Aw MM, Dhawan A, Samyn M, Bargiota A, Mieli-Vergani G. Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: a 5-year follow-up. J Hepatol. 2009;51:156–60. doi: 10.1016/j.jhep.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 91.Rao VK, Dugan F, Dale JK, Davis J, Tretler J, Hurley JK, Fleisher T, Puck J, Straus SE. Use of mycophenolate mofetil for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome. Br J Haematol. 2005;129:534–8. doi: 10.1111/j.1365-2141.2005.05496.x. [DOI] [PubMed] [Google Scholar]
- 92.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112:1472–7. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 93.Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. 2007;91:180–4. doi: 10.1136/bjo.2006.094698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology. 2008;115:1416–21. doi: 10.1016/j.ophtha.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Chang PY, Giuliari GP, Shaikh M, Thakuria P, Makhoul D, Foster CS. Mycophenolate mofetil monotherapy in the management of paediatric uveitis. Eye. 2011;25:427–35. doi: 10.1038/eye.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trachtman H, Christen E, Frank R, Rini J, Palestro C, Perelstein E, Weiss L, Tarapore F, Fortune S, Horowitz J. Pilot study of mycophenolate mofetil for treatment of kidney disease due to congenital urinary tract disorders in children. Am J Kidney Dis. 2008;52:706–15. doi: 10.1053/j.ajkd.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 97.Rouster-Stevens KA, Morgan GA, Wang D, Pachman LM. Mycophenolate mofetil: a possible therapeutic agent for children with juvenile dermatomyositis. Arthritis Care Res. 2010;62:1446–51. doi: 10.1002/acr.20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barletta GM, Smoyer WE, Bunchman TE, Flynn JT, Kershaw DB. Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome. Pediatr Nephrol. 2003;18:833–7. doi: 10.1007/s00467-003-1175-4. [DOI] [PubMed] [Google Scholar]
- 99.Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R. Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: a report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol CJASN. 2006;1:1173–8. doi: 10.2215/CJN.00550206. [DOI] [PubMed] [Google Scholar]
- 100.Afzal K, Bagga A, Menon S, Hari P, Jordan SC. Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2007;22:2059–65. doi: 10.1007/s00467-007-0617-9. [DOI] [PubMed] [Google Scholar]
- 101.Okada M, Sugimoto K, Yagi K, Yanagida H, Tabata N, Takemura T. Mycophenolate mofetil therapy for children with intractable nephrotic syndrome. Pediatr Int. 2007;49:933–7. doi: 10.1111/j.1442-200X.2007.02487.x. [DOI] [PubMed] [Google Scholar]
- 102.Wong CS, Fink CA, Baechle J, Harris AA, Staples AO, Brandt JR. C1q nephropathy and minimal change nephrotic syndrome. Pediatr Nephrol. 2009;24:761–7. doi: 10.1007/s00467-008-1058-9. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Duan C, He J, Wu T, Xun M, Zhang Y, Yin Y. Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2010;25:883–8. doi: 10.1007/s00467-009-1375-7. [DOI] [PubMed] [Google Scholar]
- 104.Gargah TTG, Lakhoua MR. Mycophenolate mofetil in treatment of childhood steroid-resistant nephrotic syndrome. J Nephrol. 2011;24:203–7. doi: 10.5301/jn.2011.6327. [DOI] [PubMed] [Google Scholar]
- 105.Heller M, Shin HT, Orlow SJ, Schaffer JV. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127–32. doi: 10.1111/j.1365-2133.2007.07947.x. [DOI] [PubMed] [Google Scholar]
- 106.Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133:606–11. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 107.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, Schatz DA, Moran AM, Lachin JM, Skyler JS Type 1 Diabetes TrialNet MMFDZBSG. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010;33:826–32. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]


