Abstract
AIMS
IL-13 is implicated as an important mediator of the pathology of asthma. This first clinical study with GSK679586, a novel humanized anti-IL-13 IgG1 monoclonal antibody, evaluated the safety, pharmacokinetics and pharmacodynamics of escalating single and repeat doses of GSK679586.
METHODS
In this randomized, double-blind study, healthy subjects received single intravenous infusions of GSK679586 (0.005, 0.05, 0.5, 2.5, 10 mg kg−1) or placebo and mild intermittent asthmatics received two once monthly intravenous infusions of GSK679586 (2.5, 10, 20 mg kg−1) or placebo.
RESULTS
GSK679586 displayed approximately linear pharmacokinetics (based on AUC and Cmax) with limited accumulation upon repeat administration. In mild intermittent asthmatics, treatment with GSK679586 produced an increase in serum total IL-13 concentrations, indicative of GSK679586–IL-13 complex formation. Additionally, mean levels of exhaled nitric oxide (FeNO), a marker of pulmonary inflammation, were reduced relative to baseline at 2.5, 10 and 20 mg kg−1 doses of GSK679586 at both 2 weeks (19%, 44% and 52% decreases) and 8 weeks (29%, 55% and 42% decreases) after the second infusion. GSK679586 was well tolerated; the incidence of AEs was comparable across all presumed biologically active doses and there were no treatment-related SAEs.
CONCLUSIONS
GSK679586 demonstrated dose-dependent pharmacological activity in the lungs of mild intermittent asthmatics. These findings, together with the favourable safety profile and advantageous PK characteristics of a monoclonal antibody (e.g. a long half-life supporting less frequent dosing), warrant further investigation of GSK679586 in a broader asthma patient population.
Keywords: asthma, exhaled nitric oxide, IL-13, pharmacodynamics, pharmacokinetics, safety
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
IL-13 can recapitulate the key pathological and clinical features of asthma.
Exhaled NO (FeNO) concentrations are elevated under conditions of pulmonary inflammation, including asthma.
Therapies that inhibit signalling by both IL-13 and the functionally redundant cytokine IL-4 have demonstrated reductions in FeNO, although relationship to dose was not reported.
WHAT THIS STUDY ADDS
This study provides the first report of the safety and pharmacokinetics of GSK679586, a novel humanized monoclonal antibody that prevents IL-13 from binding its two known receptors.
This is the first report of the effects on FeNO of a therapy targeting IL-13 alone. Inhibition of IL-13 signalling by GSK679586 produces dose- and time-dependent reductions in FeNO in mild intermittent asthmatics. Thus, FeNO appears to be a dose- and time-responsive clinical biomarker of IL-13 inhibition in mild asthma.
Introduction
Asthma is characterized by bronchial airway hyper-responsiveness (AHR) to a variety of stimuli, reversible airway obstruction and airway inflammation. Asthma can be classified as extrinsic (atopic) or intrinsic (non-atopic) based on whether or not symptoms are precipitated by allergens. Irrespective of the precipitating factors, the most common pathologic expression of asthma is inflammation of the airways [1]. The allergen-driven inflammatory response is characterized by infiltration of the airway wall with lymphocytes, mast cells, and eosinophils, and expression of Th2 cytokines such as IL-13, IL-4, IL-5 and GM-CSF [2–4]. These cytokines orchestrate the recruitment and activation of mast cells and eosinophils which release pro-inflammatory mediators, causing bronchial submucosal oedema, mucous plugging of airways, collagen deposition under the basement membrane and smooth muscle hypertrophy, which lead to changes in AHR and airway remodelling.
The mainstay of treatment for patients with persistent asthma requiring more than occasional inhaled β-adrenoceptor agonist is regular inhaled corticosteroid (ICS) therapy, with or without a long acting β-adrenoceptor agonist or leukotriene antagonist. Options for patients with more severe disease include high dose ICS [5] and maintenance oral corticosteroids, often in addition to other medications, but these increase the potential for unwanted side effects and may not always achieve clinical control [6]. Therefore, new drugs are sought that may be used in addition to, or in place of, corticosteroids in such patients.
Recently, a number of clinical phenotypes, associated with distinct cellular profiles, have been identified among patients with severe refractory asthma [7–9], suggesting that treatment strategies tailored to specific clinical and/or molecular phenotypes may provide improved clinical benefit. For instance, some asthmatic patients exhibit a molecular signature consistent with Th2 cytokine-induced gene expression. In these patients, therapeutics targeting the IL-13 signalling pathway may provide additional improvements in asthma control [10].
IL-13 is a member of the Th2 cytokine family, the gene for which is located on human chromosome 5 (5q31), a locus linked to the development of atopic diseases. IL-13 can be produced by both haematopoietic and non-haematopoietic cells (most notably CD4+ T cells, mast cells and basophils) and signals through both the type II IL-4 receptor (composed of IL13Rα1 and IL4Rα) and IL13Rα2 [11–13]. IL-13 is closely related to the canonical Th2 cytokine IL-4 and these cytokines exhibit some functional redundancy due to shared receptor usage at the type II IL-4 receptor. There are extensive preclinical data demonstrating that IL-13 is able to mediate the key features of asthma pathogenesis, such as IgE production, mucus hyper-secretion, AHR and airway inflammation, and although some of these functions can be recapitulated by IL-4, studies in rodent models of asthma suggest that IL-13 is the dominant effector cytokine acting in the lung [14, 15]. Antagonism of IL-13 may therefore be a useful therapeutic approach in the treatment of asthma.
GSK679586 is a humanized IgG1-type monoclonal antibody that binds to human IL-13 with an affinity of 300–400 pm and high specificity. GSK679586 targets an epitope known to contain residues that are important for IL-13 interaction with both IL13Rα1 and IL13Rα2 [11, 16], thereby competing with both IL-13 receptors for binding to IL-13 and resulting in neutralization of IL-13 bioactivity in in vitro cell-based bioassays. GSK679586 also binds to cynomolgus macaque IL-13 and intravenous (i.v.) treatment with GSK679586 in a cynomolgus macaque model of asthma has been shown to improve lung function and reduce pulmonary inflammation following Ascaris suum antigen challenge (unpublished data, GlaxoSmithKline).
The first clinical study of GSK679586, which is described herein, evaluated the safety, tolerability and pharmacokinetics (PK) of escalating single doses of GSK679586 in healthy subjects and escalating repeat doses of GSK679586 in subjects with mild intermittent asthma for whom evidence of target engagement in the lung was also assessed.
Methods
Study design
This two-part, randomized, double-blind, placebo-controlled, dose-escalation study (clinicaltrials.gov identifier NCT00411814) investigated the safety, PK and pharmacodynamics (PD) of GSK679586, administered as single i.v. infusions of 0.005, 0.05, 0.5, 2.5 and 10 mg kg−1 in five sequential cohorts of healthy subjects (part 1) and as repeat i.v. infusions (two infusions administered 4 weeks apart) of 2.5, 10 and 20 mg kg−1 in three sequential cohorts of mild intermittent asthmatic subjects (part 2) (Figure 1). The sample size for each cohort was selected to allow adequate evaluation of safety and tolerability while minimizing exposure to a new molecular entity. Four subjects were enrolled in the initial two cohorts in part 1 (0.005 and 0.05 mg kg−1). These dose levels were considered unlikely to have any appreciable biological activity against the target (IL-13), as predicted human Cmax values were ≤1.06 times the in vitro concentration resulting in 50% neutralization of bioactivity, and were chosen as test doses to exclude the risk of unexpected, acute, non-target related adverse effects. Eight subjects were enrolled in each remaining cohort with the exception of the final cohort in part 2 (20 mg kg−1), which enrolled 12 subjects. In each cohort, subjects were randomly allocated in a 3:1 ratio to receive treatment with GSK679586 (supplied by GlaxoSmithKline, King of Prussia, PA) or placebo (0.9% weight/volume sodium chloride), in accordance with a randomization schedule generated using a web-based validated randomization software system (Rand All) at GlaxoSmithKIine. The investigator enrolled subjects and assigned them to blinded interventions upon confirmation of eligibility. Study medication was prepared by an unblinded site pharmacist and blinded using a syringe cover and foil-wrapped infusion lines. In this way, the study subjects and all sponsor and site personnel involved in the conduct of the study remained blinded to treatment assignment until study completion. All infusions were administered over approximately 60 min, with the exception of the lowest dose in part 1, which was administered over approximately 6 min. Dosing was staggered within each cohort and dosing in part 2 commenced after completion of dosing in part 1. All subjects were evaluated for at least 56 days and up to 192 days after their last infusion, depending on the dose and dosing regimen of GSK679586 administered. The study was conducted at Nucleus Network (Melbourne, Victoria, Australia, Site 1) and the GlaxoSmithKline Medicines Research Unit (Sydney, New South Wales, Australia, Site 2) in accordance with the revised Declaration of Helsinki (1996), Good Clinical Practice and all applicable regulatory requirements during the period from November 2006 to September 2008. The study protocol and informed consent form were approved by the ethics research board of the respective institutions and written informed consent was obtained from each subject prior to the performance of any study procedures.
Figure 1.
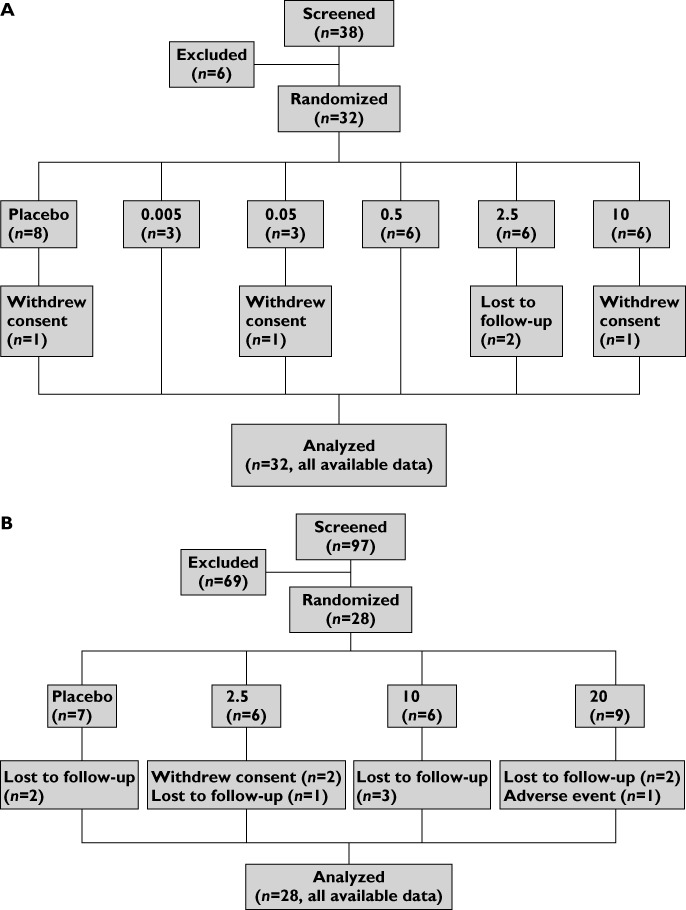
CONSORT diagram of the sequential enrollment and treatment of (A) healthy subjects in part 1 followed by (B) mild intermittent asthmatic subjects in part 2. Dose of GSK679586 in mg kg–1. Subjects were lost to follow-up for the final visit for PK and immunogenicity testing
Study population
All study subjects were non-smoking males, 18–65 years of age, with a BMI of 19–29.9 kg m−2. While females of non-childbearing potential were also eligible to participate in the study, none was enrolled. Part 1 of the study enrolled healthy subjects who had not recently received any medications or supplements. Part 2 of the study enrolled subjects with mild bronchial asthma diagnosed at least 6 months prior to the screening visit, but otherwise healthy. These subjects were skin prick test positive for a common inhaled allergen (ryegrass) and not currently using any asthma medication with the exception of an intermittent inhaled short-acting β2-adrenoceptor agonist. Subjects were excluded if they had suffered a prior life-threatening asthmatic episode, recent respiratory tract infection or asthma exacerbation, were undergoing desensitization therapy, or had received any of the following medications as indicated prior to screening: anti-IgE therapy (6 months), oral or injectable corticosteroids (8 weeks), inhaled, intranasal or topical steroids (4 weeks), leukotriene antagonists (2 weeks), xanthines excepting caffeine, anticholinergics, cromoglycates and long acting β-adrenoceptor agonists (1 week). Following commencement of the trial, eligibility criteria were amended to require a screening pre-bronchodilator FEV1 >70% and <90% of predicted normal with ≥12% reversibility upon β2-adrenoceptor agonist inhalation or, for subjects not meeting these criteria, a bronchial provocation test to confirm AHR (the test was considered positive if, after inhalation of increasing doses of mannitol from 0 mg (baseline) up to a maximum of 160 mg, FEV1 decreased ≥15% from baseline or ≥10% from the previous dose). In addition, subjects with a prior confirmed or active parasitic infection or intending to travel to a country with a high prevalence of such infections were excluded because of a theoretical risk that blockade of IL-13 signalling by GSK679586 may increase a subject's susceptibility to parasitic infections.
Study assessments
Safety and tolerability were the primary outcome measures of the study, and were evaluated from the first dose of study medication through the final follow-up visit by assessment of AEs, standard clinical laboratory tests, physical examinations, vital sign measurements and electrocardiograms (ECGs). Anti-GSK679586 antibodies (ADAs) and neutralizing antibodies, secondary outcome measures, were analyzed over the same time period in serum samples using a bridging electrochemiluminescent immunoassay (ECLIA) and a ligand-binding assay, respectively, each utilizing the MesoScale Discovery (MSD) platform (Gaithersburg, MD, US).
Blood samples for determination of GSK679586 plasma concentrations, a secondary outcome measure, were collected for all subjects in part 1 (pre dose and at 0.25 [or 6 min for cohort 1], 0.5, 1, 2, 4, 8, 12, 24 and 48 h after the start of the infusion [day 1] and at days 5, 7, 14, 28, 42 and 56) and part 2 (pre dose and at 0.25, 0.5, 1, 2, 6, 12 and 24 h after the start of each infusion [days 1 and day 28] and on days 3, 7, 14, 30, 34, 41, 55, 70 and 84). Samples collected post 24 h dosing were obtained within a pre-specified time window. A final PK sample was also collected concurrent with the final immunogenicity sample. Plasma samples were analyzed using a chemiluminescent immunoassay validated over the range 100 to 2500 ng ml−1 based on 100 µl of human plasma diluted 10-fold in assay buffer. Within- and between-run coefficients of variation for the assay were ≤17.8% and ≤13.2%, respectively, and % bias ranged from −18.1% to 7.5%. Derived individual PK parameters were obtained using non-compartmental analysis method (WinNonlin, version 5.2) and included: area under the plasma concentration time curve (AUC) from time zero extrapolated to infinity (AUC(0,∞)), AUC from time zero to week 4 (AUC(0,week 4)), maximum observed plasma concentration (Cmax), time to maximum observed plasma concentration (tmax), systemic clearance (CL), volume of distribution at steady-state (Vss), and terminal phase half-life (t1/2) in part 1 and AUC over the dosing interval (AUC(0,τ)), Cmax, tmax and, after the second dose, t1/2 in part 2.
Exploratory endpoints in mild intermittent asthmatics included levels of free and total IL-13 and total IgE in serum samples obtained at screening and days 0, 7, 14, 28, 30, 34, 41, 55, 70 and 84, and the fractional concentration of exhaled nitric oxide (FeNO) measured at screening, day 41 and day 84. Free and total serum IL-13 were measured using sandwich ECLIAs on the MSD platform, and had an assay LLQ of 4.88 pg ml−1 and 30.86 pg ml−1, respectively. Total IgE was measured using a commercially available sandwich ELISA (AlerCHEK Inc, Portland ME, US), and had an assay LLQ of 50 IU ml−1. FeNO was measured in seated, resting subjects following single breath exhalations at a flow rate of 50 ml s−1 using the CLD 77 AM chemiluminescent nitric oxide analyzer (Eco Physics, Duernten, Switzerland). Measurements were obtained prior to spirometry and at least 24 h after any strenuous activity and nitrite/nitrate-containing foods. At site 1, subjects underwent a nasal allergen challenge (NAC) with ryegrass allergen at baseline and day 81, followed by assessment of nasal symptom scores (to confirm that the challenge had elicited an appropriate response) and measurement of inflammatory mediators in nasal secretions absorbed on filter paper, pSTAT6 levels (a downstream marker of IL13 signalling) in epithelial cells obtained by nasal scrapings and inflammatory cell numbers in nasal lavages. These non-invasive sample collection methods and assays have been described previously [17, 18].
Statistical methods
Statistical sample size calculations were not performed. Subject numbers were chosen to permit an adequate assessment of safety and tolerability at each dose while minimizing the number of subjects exposed to this new molecular entity. There was no formal interim analysis. However, blinded summary safety and PK data from preceding dose cohorts were reviewed prior to each dose escalation, and blinded summary data from the first infusion were reviewed prior to administration of the second infusion for each dose cohort in part 2. All final study data were listed, summarized and/or plotted, as appropriate. Data for placebo subjects from each cohort were pooled for the purpose of summary statistics. Preliminary investigation of GSK679586 dose proportionality was carried out by analysis of variance (anova) on dose-normalized AUC and Cmax, using a reference dose of 0.05 mg kg−1 in part 1 and 2.5 mg kg−1 in part 2. No other formal statistical analyses were planned or conducted.
Results
Subject disposition, demographics, and exposure data
Thirty-two healthy subjects were randomized in part 1 of the study, of whom 27 subjects completed the study as planned and five subjects were either lost to follow-up or withdrew consent (Figure 1A). In part 2, 28 mild intermittent asthmatic subjects were randomized and 17 subjects completed the study as planned. One subject was discontinued from blinded treatment (GSK679586 20 mg kg−1) due to a non-treatment-related SAE of vasovagal syncope, eight subjects were lost to follow-up after day 84 (missing only the extended follow-up visit for PK and immunogenicity) and two subjects withdrew consent (Figure 1B).
Table 1A and Table 1B summarize demographic and/or baseline disease characteristics for subjects in part 1 and part 2 of the study, respectively. All subjects were male and the majority of subjects (87%) were Caucasian. The treatment groups were similar with respect to demographics and, in part 2, baseline pulmonary function.
1A.
Demographics in healthy subjects
| Placebo | 0.005 | 0.05 | 0.5 | 2.5 | 10 | |
|---|---|---|---|---|---|---|
| (n = 8) | (n = 3) | (n = 3) | (n = 6) | (n = 6) | (n = 6) | |
| Age (years), mean (SD) | 26 (7) | 20 (1) | 24 (1) | 29 (6) | 22 (4) | 27 (11) |
| Males, n (%) | 8 (100) | 3 (100) | 3 (100) | 6 (100) | 6 (100) | 6 (100) |
| BMI (kg m−2), mean (SD), | 23 (2) | 26 (3) | 22 (2) | 25 (2) | 23 (2) | 22 (2) |
| White/Caucasian, n (%) | 7 (88) | 2 (67) | 2 (67) | 6 (100) | 5 (83) | 6 (100) |
Doses of GSK679586 are presented in mg kg−1. BMI, body mass index; SD, standard deviation.
1B.
Demographics and baseline disease characteristics in mild intermittent asthmatics
| Placebo | 2.5 | 10 | 20 | |
|---|---|---|---|---|
| (n = 7) | (n = 6) | (n = 6) | (n = 9) | |
| Age (years), mean (SD) | 29 (6) | 25 (4) | 32 (11) | 29 (10) |
| Males, n (%) | 7 (100) | 6 (100) | 6 (100) | 9 (100) |
| BMI (kg m−2), mean (SD) | 24 (2) | 26 (3) | 26 (2) | 25 (3) |
| White/Caucasian, n (%) | 5 (71) | 6 (100) | 6 (100) | 7 (78) |
| FEV1 % predicted, mean (SD) | 102 (21) | 105 (9) | 104 (24) | 105 (14) |
| FVC (l), mean (SD) | 5.6 (0.9) | 6.0 (0.6) | 5.5 (1.0) | 6.0 (0.7) |
| Free IL-13 ≥ LLQ, n (%) | 2 (29) | 2 (33) | 1 (17) | 0 |
| Total IL-13 ≥ LLQ, n (%) | 0 | 1 (17) | 1 (17) | 0 |
| Total IgE (IU l−1), geometric mean (CV%) | 1037 (20645) | 191 (37) | 100 (174) | 385 (1108) |
| Allergen-specific IgE (U l−1), geometric mean (CV%) | 77466 (82) | 61573 (89) | 61090 (87) | 95564 (50) |
| Exhaled nitric oxide (ppb), mean (SD) | 45 (32) | 55 (34) | 48 (27) | 35 (15) |
Doses of GSK679586 are presented in mg kg−1. The lower limit of quantification (LLQ) was 4.88 pg ml−1 for free IL-13 and 30.86 pg ml−1 for total IL-13. BMI, body mass index; SD, standard deviation; FVC, forced vital capacity; FEV1%, percent of predicted normal forced expiratory volume in 1 s.
Pharmacokinetics
In healthy subjects, after single i.v. dose administration, GSK679586 showed a dose-dependent PK profile (Table 2). An exploratory analysis showed no obvious deviation from dose proportionality for Cmax and AUC(0,∞) and, for all doses investigated, the maximum plasma concentration was reached between 0.5 and 12 h after initiation of the i.v. infusion of GSK679586. The variability associated with Cmax and AUC values ranged from 1 to 29%. There were no obvious changes in CL, Vss or elimination t1/2 across the dose range investigated and the variability in these parameters was generally low. The clearance of GSK679586 was slow and ranged from 2.25 to 3.17 ml day−1 kg−1. The observed Vss ranged from 67 to 105 ml kg−1, suggesting that GSK679586 remains mainly in the central compartment (i.e. systemic circulation). The observed elimination half-life was approximately 3 weeks.
Table 2.
GSK679586 plasma pharmacokinetic parameters after single intravenous infusion of GSK679586 in healthy subjects
| 0.005 mg kg−1 | 0.05 mg kg−1 | 0.5 mg kg−1 | 2.5 mg kg−1 | 10 mg kg−1 | |
|---|---|---|---|---|---|
| (n = 3) | (n = 3) | (n = 6) | (n = 6) | (n = 6) | |
| AUC(0,∞) (mg ml−1 h) | – | 0.404 (6.4) | 4.04 (10.5) | 26.6 (10.9) | 76.0 (28.6) |
| n = 2 | n = 5 | n = 6 | n = 6 | ||
| AUC(0,week 4) (mg ml−1 h) | – | 0.232 (1.7) | 2.56 (11.7) | 16.4 (5.0) | 43.0 (14.6) |
| n = 2 | n = 6 | n = 6 | n = 6 | ||
| Cmax (µg ml−1) | 0.130 (7.6) | 0.883 (0.9) | 11.1 (14.4) | 87.4 (15.1) | 197 (22.1) |
| n = 2 | n = 3 | n = 6 | n = 6 | n = 6 | |
| tmax (h) | 1.25 (0.50, 2.00) | 4.00 (2.00, 4.00) | 2.04 (1.00, 12.0) | 3.00 (2.00, 12.0) | 2.00 (1.00, 4.00) |
| n = 2 | n = 3 | n = 6 | n = 6 | n = 6 | |
| t1/2 (h) | – | 540 (8.1) | 467 (20.0) | 591 (24.2) | 578 (39.4) |
| n = 2 | n = 5 | n = 6 | n = 6 | ||
| CL (ml day−1 kg−1) | – | 2.98 (6.3) | 2.98 (10.5) | 2.25 (10.9) | 3.17 (28.6) |
| n = 2 | n = 5 | n = 6 | n = 6 | ||
| Vss (ml kg−1) | – | 94.6 (1.2) | 83.2 (9.7) | 67.3 (13.9) | 105 (19.3) |
| n = 2 | n = 5 | n = 6 | n = 6 |
Values represent median (range) for tmax and geometric mean (between subject coefficient of variation, CVb%) for all other parameters.
For the 0.005 mg kg−1 dose group, AUC(0,∞), AUC(0,week 4), t1/2, CL, and Vss were not determined because none of the patients had sufficient plasma concentration data for calculation of these parameters. % AUC extrapolated was below 20% except in three subjects, two at the 0.05 mg kg−1 dose (20% and 26%) due to LLQ censoring and one subject at the 10 mg kg−1 dose (25%). AUC(0,∞), area under the plasma concentration–time curve (AUC) from time zero extrapolated to infinity; AUC(0,week 4), AUC from time zero to week 4; Cmax, maximum observed plasma concentration; tmax, time of maximum observed plasma concentration; t1/2, terminal phase half-life; CL = systemic clearance; Vss, volume of distribution at steady-state.
In mild intermittent asthmatic subjects, after repeat i.v. dose administration (two doses, 4 weeks apart), GSK679586 showed a dose-dependent PK profile (Table 3). An exploratory analysis showed no obvious deviation from dose proportionality for Cmax and AUC(0,τ) either after the first or second infusions of GSK679586. After two i.v. infusions of GSK679586 administered 1 month apart, the maximum plasma concentration was reached between 1 and 13 h across the doses investigated. The variability associated with Cmax, AUC(0,τ) and elimination t1/2 ranged from 5 to 21%. The observed elimination t1/2 was approximately 3 weeks. There was limited accumulation observed for Cmax and AUC(0,τ), with the accumulation ratio between the first and second infusions ranging from 1.0- to 1.8-fold, which is consistent with the observed elimination t1/2. Values of AUC(0,τ) and Cmax obtained after the first infusion of 2.5 or 10 mg kg−1 in mild intermittent asthmatics were similar to values observed for each parameter following single infusions at the same doses in healthy subjects in part 1 of the study.
Table 3.
GSK679586 plasma pharmacokinetic parameters after repeat intravenous infusion of GSK679586 (dose 1 and dose 2) in mild intermittent asthmatic subjects
| 2.5 mg kg−1 | 10 mg kg−1 | 20 mg kg−1 | ||||
|---|---|---|---|---|---|---|
| (n = 6) | (n = 6) | (n = 9) | ||||
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | Dose 1 | Dose 2 | |
| AUC(0,τ) (mg ml−1 h) | 14.0 (12.7) | 25.1 (15.7) | 51.2 (17.0) | 72.4 (12.8) | 107 (14.3) | 156 (14.8) |
| n = 5 | n = 4 | n = 6 | n = 6 | n = 8 | n = 8 | |
| Cmax (µg ml−1) | 52.4 (16.4) | 73.1 (20.9) | 255 (18.2) | 263 (12.4) | 467 (5.3) | 617 (17.8) |
| n = 6 | n = 5 | n = 6 | n = 6 | n = 8 | n = 8 | |
| tmax (h) | 2.00 (2.00–6.00) | 5.83 (1.00–12.67) | 1.50 (1.00–6.00) | 1.00 (1.00–12.00) | 2.00 (1.00–12.00) | 2.00 (2.00–6.02) |
| n = 6 | n = 5 | n = 6 | n = 6 | n = 8 | n = 8 | |
| t1/2 (h) | – | 538 (21.1) | – | 610 (13.3) | – | 561 (11.6) |
| n = 4 | n = 6 | n = 8 | ||||
Values represent median (range) for tmax and geometric mean (between subject coefficient of variation, CVb%) for all other parameters. AUC(0,τ), area under the plasma concentration–time curve from time zero to the end of the dosing interval; Cmax, maximum observed plasma concentration; t1/2, terminal phase half-life.
Pharmacodynamics
Various biomarkers, including circulating total and free IL-13, total IgE, and a clinical measure of asthma, the fractional concentration of exhaled nitric oxide (FeNO), were evaluated in mild intermittent asthmatic subjects to characterize the pharmacology of GSK679586. Free IL-13 concentrations were below the lower limit of quantification (LLQ) of the assay at baseline and after treatment for the majority of subjects in all dose cohorts. Baseline concentrations of total IL-13 (i.e. free IL-13 and IL-13 bound to GSK679586) were also below the LLQ of the assay for all but two subjects (Table 1B). Following infusion of GSK679586 at doses of 10 mg kg−1 or 20 mg kg−1, total IL-13 concentrations increased in all subjects. The geometric mean total IL-13 profile in these two treatment groups was biphasic, and coincident with the administration of each dose of GSK679586 (Figure 2). In contrast, total IL-13 concentrations increased above LLQ in only approximately half of subjects receiving a dose of 2.5 mg kg−1 and remained below LLQ in all placebo subjects. As free IL-13 concentrations were essentially unchanged during treatment, these observations for total IL-13 are consistent with the binding of free IL-13 by GSK679586 to form an antibody-cytokine complex. With a few exceptions, peak concentrations of total IL-13 were generally comparable for subjects in the 10 mg kg−1 and 20 mg kg−1 treatment groups. The inter-subject variability in total IL-13 concentrations was greatest for the 20 mg kg−1 treatment group, with higher peak total IL-13 levels observed for all three subjects treated at study site 1 and one of the six subjects treated at site 2 (range 229–321 pg ml−1) compared with the other five subjects in this treatment group (range 52.8–122 pg ml−1). Of the six subjects in the 10 mg kg−1 treatment group who were all treated at site 1, five subjects had similar total IL-13 concentrations (65.7 to 93.5 pg ml−1) and one subject had a higher peak total IL-13 (232 pg ml−1).
Figure 2.
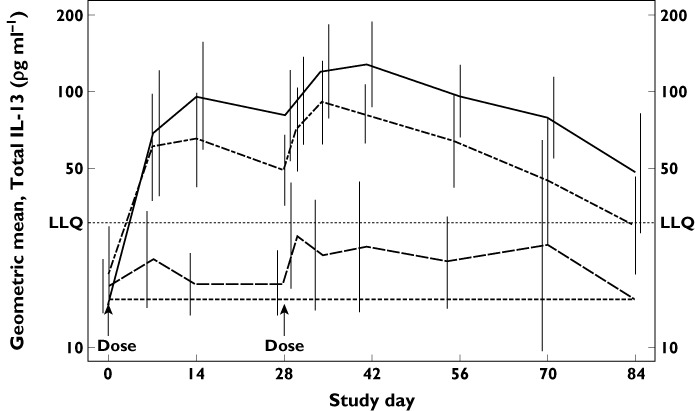
Geometric mean (±95% CI) serum total IL-13 concentrations (log scale) by time and treatment for mild intermittent asthmatic subjects in part 2.  , 20 mg kg–1 (n = 9);
, 20 mg kg–1 (n = 9);  , 10 mg kg–1 (n = 6);
, 10 mg kg–1 (n = 6);  , 2.5 mg kg–1 (n = 6);
, 2.5 mg kg–1 (n = 6);  , placebo (n = 7). LLQ lower limit of quantification (30.86 pg ml–1). Samples with total IL-13 below LLQ were imputed as ½ of LLQ (15.43 pg ml–1)
, placebo (n = 7). LLQ lower limit of quantification (30.86 pg ml–1). Samples with total IL-13 below LLQ were imputed as ½ of LLQ (15.43 pg ml–1)
Circulating concentrations of total IgE, which plays a role in the early asthmatic response, were elevated in the majority of asthmatic subjects at baseline (Table 1B), relative to those typically reported in healthy subjects [19, 20]. Treatment with GSK679586 had little effect on total IgE concentrations.
Exhaled NO was assessed in the mild intermittent asthmatic subjects as a marker of the effects of GSK679586 on lung inflammation. At baseline, FeNO levels were above 30 ppb in most subjects, as might be expected in this mild asthmatic population, although there was considerable inter-subject variability (range, 11.8–106 ppb). Exhaled NO levels were reduced relative to baseline in all GSK679586 treatment groups at both 2 weeks (day 41) and 8 weeks (day 84) after the second infusion, whereas FeNO levels remained essentially unchanged over time in the placebo group (Figure 3A). Exhaled NO levels appeared to be suppressed to a similar extent in the 10 and 20 mg kg−1 dose groups, with a pronounced mean reduction in FeNO from baseline to day 41 (decreased by 27 ppb [44%] and 22 ppb [52%], respectively), which was maintained through day 84 (decreased by 36 ppb [55%] and 16 ppb [42%], respectively). A lesser mean reduction from baseline in FeNO levels was observed for the 2.5 mg kg−1 dose at day 41 (16 ppb [19%]) and day 84 (24ppb [29%]).
Figure 3.
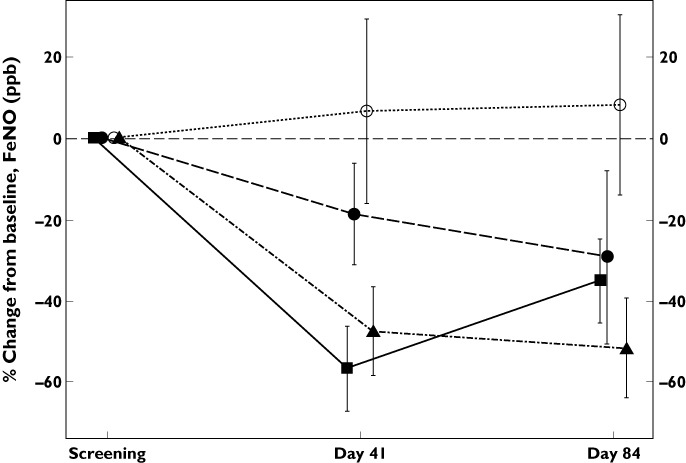
By treatment plot of mean (±SD) % change from baseline in FeNO 2 weeks and 8 weeks after the second infusion of GSK679586.  , placebo;
, placebo;  , 2.5 mg kg–1;
, 2.5 mg kg–1;  , 10 mg kg–1;
, 10 mg kg–1;  , 20 mg kg–1
, 20 mg kg–1
For mild intermittent asthmatic subjects enrolled at site 1, a NAC procedure with ryegrass allergen was also performed to evaluate the effect of GSK697586 on a variety of biomarkers in the nasal compartment. Although nasal symptom scores indicated that the NAC had mediated a robust rhinorhoea response and eosinophilic cationic protein (ECP) concentrations were increased, the concentrations of two Th2 cytokines commonly induced by NAC, IL-4 and IL-5, remained below the assay LLQ for all subjects at all time points. The NAC also failed to consistently elicit an increase in free and total IL13 concentrations, as measured in both nasal secretions and nasal lavages, and there was no change in concentrations of pSTAT6, a downstream marker of IL-13 signalling, in nasal scrapings. Given the absence of a Th2 cytokine response to NAC, analyses of other biomarker data derived from the NAC were not undertaken.
Safety results
No clinically relevant changes were observed in haematology, clinical chemistry, vital signs, or ECG parameters that were attributable to treatment. Overall, GSK679586 was well tolerated by subjects in this study, with the majority of AEs being assessed by the investigators as mild or moderate in intensity and unrelated to treatment with study medication. The incidence of AEs was comparable at all doses of GSK679586 investigated in mild intermittent asthmatic subjects, and at all doses of GSK679586 within the presumed biologically active dose range (0.5 to 10 mg kg−1) in healthy subjects (Table 4). Treatment-related AEs reported by more than one subject receiving GSK679586 (any dose) included nasopharyngitis in healthy subjects, and headache, lethargy and diarrhoea in mild intermittent asthmatic subjects. With the possible exception of headache, no dose-related trends in AEs were noted during the study. One serious adverse event (SAE) was reported during the study. A male subject with a prior history of vagal episodes experienced an extended syncopal episode during dosing with GSK679586 20 mg kg−1. This SAE was considered to be unrelated to study medication. No subject developed treatment-emergent anti-GSK679586 antibodies.
Table 4.
Summary of most frequent adverse events, regardless of causality, reported by more than one subject after administration of GSK679586 or placebo as a single intravenous infusion in healthy subjects and repeat intravenous infusions in mild intermittent asthmatic subjects
| Healthy subjects | ||||||
|---|---|---|---|---|---|---|
| Adverse event, n (%) | Placebo | 0.005 | 0.05 | 0.5 | 2.5 | 10 |
| (n = 8) | (n = 3) | (n = 3) | (n = 6) | (n = 6) | (n = 6) | |
| Nasopharyngitis | 2 (25) | 0 | 0 | 2 (33) | 1 (17) | 1 (17) |
| Muscle spasms | 2 (25) | 0 | 0 | 0 | 0 | 1 (17) |
| URTI | 0 | 0 | 0 | 1 (17) | 1 (17) | 0 |
| Catheter site pain | 0 | 0 | 1 (33) | 0 | 1 (17) | 0 |
| Hyperhidrosis | 1 (13) | 0 | 0 | 1 (17) | 0 | 0 |
| Mild intermittent asthmatics | ||||
|---|---|---|---|---|
| Adverse event, n (%) | Placebo | 2.5 | 10 | 20 |
| (n = 7) | (n = 6) | (n = 6) | (n = 9) | |
| Headache | 0 | 2 (33) | 2 (33) | 7 (78) |
| Lethargy | 1 (14) | 0 | 0 | 2 (22) |
| Diarrhoea | 0 | 0 | 0 | 2 (22) |
| Oropharyngeal pain | 0 | 0 | 0 | 2 (22) |
| Asthma | 1 (14) | 2 (33) | 0 | 1 (11) |
| Nasopharyngitis | 1 (14) | 2 (33) | 0 | 1 (11) |
| Pharyngitis | 0 | 1 (17) | 0 | 1 (11) |
| Eye pruritus | 1 (14) | 0 | 0 | 1 (11) |
| Joint injury | 1 (14) | 0 | 0 | 1 (11) |
| URTI | 0 | 0 | 3 (50) | 0 |
| Influenza | 0 | 0 | 2 (33) | 0 |
| Muscle injury | 1 (14) | 1 (17) | 0 | 0 |
| Myalgia | 1 (14) | 1 (17) | 0 | 0 |
Doses of GSK679586 are presented in mg kg−1. URTI, upper respiratory tract infection.
Discussion
Expression of Th2 cytokines is a consistent feature of the allergen-driven inflammatory response in asthma, and the cytokine IL-13 is thought to be a key Th2 driver of AHR in the lung [14, 21] and other aspects of the disease that are not well controlled by corticosteroids, e.g., mucus secretion and airway remodelling [22–24]. Thus, for asthmatic patients who exhibit a molecular signature consistent with Th2 cytokine-induced gene expression, a targeted therapeutic approach that interferes with IL-13 signalling may provide enhanced efficacy when used in addition to, or in place of, corticosteroids [10]. The current study evaluated the pharmacodynamic (PD) effect of GSK679586, a humanized IgG1 monoclonal antibody to human IL-13, in subjects with mild intermittent asthma, and provided a preliminary characterization of the PK and safety profile of this new biological entity in both healthy subjects and mild intermittent asthmatics.
The PK profile of GSK679586 was similar following a single i.v. infusion in healthy subjects and the first of two i.v. infusions in mild intermittent asthmatics, and was consistent with the PK profiles for other IgG1 monoclonal antibodies that target soluble proteins [25, 26]. The clearance of GSK679586 was slow and the Vss was close to the plasma volume, indicating that there was limited drug distribution into the tissues. An exploratory assessment of dose proportionality did not reveal any obvious deviation from linear PK for Cmax and AUC and there was limited accumulation after administration of two infusions 4 weeks apart, consistent with the observed t1/2 of approximately 3 weeks.
In the mild intermittent asthmatic population, in which pharmacodynamic markers were also measured, serum total IL-13 concentrations increased following each administration of GSK679586 at doses of 10 and 20 mg kg−1, while remaining below the lower limit of quantification for the majority of subjects receiving a dose of 2.5 mg kg−1 and for all placebo subjects. Given that serum free IL-13 concentrations generally remained undetectable at all time points in all subjects, the elevation of serum total IL-13 detected in the 10 and 20 mg kg−1 dose groups most likely represents IL-13 complexed to GSK679586. This increase in total IL-13 is consistent with a reduction in the clearance of IL-13 due to its binding to GSK679586. The magnitude of the total IL-13 response over time at doses of 10 and 20 mg kg−1 was similar and suggestive of saturation of the target in the plasma compartment. Five subjects, including four subjects in the 20 mg kg−1 dose group and one subject in the 10 mg kg−1 dose group, had total IL-13 concentrations that were noticeably higher than those of other subjects in these dose groups. The reason for these higher total IL-13 concentrations, which were comparable in magnitude for all five subjects irrespective of dose group, is not fully understood. However, assay interference or variability in the assay may be contributing factors. Another factor may be the concentration of free IL-13 present in an individual subject and thus available to complex with GSK679586 and contribute to the total IL-13 concentration. It was noted that six of the eight subjects with measurable free IL-13 concentrations at one or more time points in their profile had a higher total IL-13 profile (one subject each at 2.5 and 10 mg kg−1 and four subjects at 20 mg kg−1). Across all dose groups, there was a trend for an indirect relationship between total IL-13 concentrations and GSK679586 plasma concentrations, with the time to maximum concentration of total IL-13 (observed on day 14) being delayed compared with tmax for GSK679586 plasma concentrations, suggesting the possibility of a local tissue site of action for antibody binding.
Most mild intermittent asthmatic subjects in this study had elevated levels of FeNO at baseline, indicating the presence of inflammation in the lung despite normal pulmonary function tests, and repeat administration of GSK679586 produced a marked decrease in FeNO levels at all doses of GSK679586 evaluated in the study. Exhaled NO levels are known to rise in conditions that trigger pulmonary inflammation, such as upper respiratory tract infections or inhalation of aeroallergens, and have been shown to be elevated in the lungs of asthmatics and to be correlated with asthma symptoms and disease severity [27, 28]. Reports of FeNO levels vary, but are in the range of 20 to 30 ppb for healthy subjects and 25 to 50 ppb or higher for individuals with asthma [29]. Based on these ranges, most mild intermittent asthmatic subjects in this study had elevated baseline levels of FeNO, although levels were variable and a few subjects had FeNO levels within the putative normal range. While the source of FeNO in the lung is unclear, there are several mechanisms by which IL-13 may potentiate FeNO production. Ex vivo cultures of differentiated human bronchial epithelial cells have been shown to release gaseous NO in response to treatment with IL-13, which stimulates NO release by upregulating the expression of inducible nitric oxide synthase (iNOS) [30, 31]. Exhaled NO is also produced by other inflammatory cells (e.g. eosinophils) during inflammation [32], and as IL-13 can influence inflammatory cell trafficking to the lung by regulating chemokine concentrations [33], this is another potential mechanism by which IL-13 may affect levels of FeNO in the lung. The reduction in FeNO levels following treatment with GSK679586 in this study was similar for the 10 and 20 mg kg−1 dose groups and appeared to be near maximal by day 41, although the limited sampling time points precluded an accurate determination of the time of peak effect. In the 2.5 mg kg−1 dose group, the reduction in FeNO levels was less pronounced than at the two higher dose groups and, although no statistical analysis was performed, there appeared to be a trend toward further decline in FeNO from day 41 to day 84. These data suggest that while GSK679586 may saturate the target tissue (lung) within 2 weeks of the second infusion at a dose of 10 or 20 mg kg−1, there is continued tissue accumulation of GSK679586 over time at a dose of 2.5 mg kg−1. At day 84, the absolute reduction in FeNO levels from baseline was similar for all dose groups (24 ppb, 36 ppb and 16 ppb at doses of 2.5, 10 and 20 mg kg−1, respectively). This magnitude of treatment effect appears consistent and similar to that of inhaled corticosteroids, a mainstay of asthma therapy, which have also been shown to modulate FeNO in asthmatic subjects. In one such study, FeNO levels were reduced by 19.2–22.5 ppb following 4 weeks of treatment with budesonide 400 µg or 1600 µg [34]. The treatment effect of GSK679586 is also consistent with that reported for other therapeutics that target IL-13 [35, 36]. For example, treatment with pitrakinra, a recombinant IL-4 variant that inhibits binding of IL-4 and IL-13 to IL4Rα receptor complexes, reduced FeNO levels by 28.3 ppb relative to baseline following 4 weeks of treatment at a dose of 60 mg nebulized twice daily compared with placebo [35].
GSK679586 appeared to be well tolerated at all doses evaluated in both healthy subjects and mild intermittent asthmatics, although the assessment of tolerability was limited by the short duration of treatment and relatively small sample size.
Overall, the PK, PD and safety profiles of GSK679586 were encouraging, and warrant further clinical evaluation of this novel therapeutic in a broader asthma patient population.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline, Research Triangle Park, NC, USA (clinicaltrials.gov identifier NCT00411814). All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individuals for their contributions to the conduct and reporting of this study and/or their critical review during the development of this manuscript: Doug Wicks, Rabia Anselm, Bams Abila, Simon Cozens, Tracey Wright, Patty Wolf and other members of the GlaxoSmithKline study team. Editorial support in the form of development of a draft outline and manuscript first draft, editorial suggestions, assembling tables and figures, copyediting, fact checking and referencing was provided by Lisa Cass, Cass Consulting, Inc. and funded by GlaxoSmithKline.
Competing Interests
GPH received funding from GlaxoSmithKline for the conduct of this study. CA, APC, EHDB, NWL, APS and IJP are employed by GlaxoSmithKline Pharmaceuticals and own company stock but report no other potential conflict of interest.
REFERENCES
- 1.Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broide DH, Firestein GS. Endobronchial allergen challenge in asthma. Demonstration of cellular source of granulocyte macrophage colony-stimulating factor by in situ hybridization. J Clin Invest. 1991;88:1048–53. doi: 10.1172/JCI115366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Granulocyte-macrophage colony-stimulating factor, eosinophils and eosinophil-cationic protein in subjects with and without mild, stable, atopic asthma. Eur Respir J. 1994;7:1576–84. doi: 10.1183/09031936.94.07091576. [DOI] [PubMed] [Google Scholar]
- 5.Heinig HA, Boulet LP, Croonenborghs L, Möllers MJ. The effect of high-dose fluticasone proprionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respir Med. 1999;93:613–20. doi: 10.1016/s0954-6111(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE, for the GOAL Investigators Group Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 7.Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr Opin Allergy Clin Immunol. 2007;7:43–50. doi: 10.1097/ACI.0b013e3280118a32. [DOI] [PubMed] [Google Scholar]
- 8.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradding PB, Green RH. Subclinical phenotypes of asthma. Curr Opin Allergy Clin Immunol. 2010;10:54–9. doi: 10.1097/ACI.0b013e32833489a9. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 13.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator in allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 15.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupardus PJ, Birnbaum ME, Garcia KC. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13 Rα2. Structure. 2010;18:332–42. doi: 10.1016/j.str.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erin EM, Zacharasiewicz AS, Nicholson GC, Tan AJ, Higgins LA, Williams TJ, Murdoch RD, Durham SR, Barnes PJ, Hansel TT. Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge. Clin Exp Allergy. 2005;35:1608–14. doi: 10.1111/j.1365-2222.2005.02381.x. [DOI] [PubMed] [Google Scholar]
- 18.Dreskin SC, Dale SN, Foster SM, Martin D, Buchmeier A, Nelson HS. Measurement of changes in mRNA for IL-5 in non-invasive scrapings of nasal epithelium taken from patients undergoing nasal allergen challenge. J Immunol Methods. 2002;268:189–95. doi: 10.1016/s0022-1759(02)00206-5. [DOI] [PubMed] [Google Scholar]
- 19.Nye L, Merrett TG, Landon J, White RJ. A detailed investigation of circulating IgE levels in a normal population. Clin Allergy. 1975;5:13–24. doi: 10.1111/j.1365-2222.1975.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 20.Wittig HJ, Belloit J, De Fillippi I, Royal G. Age-related serum IgE levels in healthy subjects and in patients with allergic disease. J Allergy Clin Immunol. 1980;66:305–13. doi: 10.1016/0091-6749(80)90026-3. [DOI] [PubMed] [Google Scholar]
- 21.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–90. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, Li L. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–32. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Fulkerson PC, Fischetti CA, Hassman LM, Nikolaidis NM, Rothenberg ME. Persistent effects induced by IL-13 in the lung. Am J Respir Cell Mol Biol. 2006;35:337–46. doi: 10.1165/rcmb.2005-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011;54:215–27. doi: 10.2165/11584340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, Skiepko R, Szmitkowski M. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naïve asthma patients. J Investig Allergol Clin Immunol. 2006;16:239–46. [PubMed] [Google Scholar]
- 28.Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, Barnes PJ. Exhaled NO is elevated in difficult asthma and correlates with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax. 1998;53:1030–4. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner S. Exhaled nitric oxide in the diagnosis and management of asthma. Curr Opin Allergy Clin Immunol. 2008;8:70–6. doi: 10.1097/ACI.0b013e3282f3b4b0. [DOI] [PubMed] [Google Scholar]
- 30.Suresh V, Mih JD, George SC. Measurement of IL-13-induced i-NOS-derived gas phase nitric oxide in human bronchiole epithelial cells. Am J Respir Cell Mol Biol. 2007;37:97–104. doi: 10.1165/rcmb.2006-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, Chu HW, Wenzel SE. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38:936–46. doi: 10.1111/j.1365-2222.2008.02969.x. [DOI] [PubMed] [Google Scholar]
- 32.del Pozo V, de Arruda-Chaves E, de Andres B, Cardaba B, Lopez-Farre A, Gallardo S. Eosinophils transcribe and translate messenger RNA for inducible nitric oxide synthase. J Immunol. 1997;158:859–64. [PubMed] [Google Scholar]
- 33.Matsukura S, Stellato C, Georas SN, Casolaro V, Plitt JR, Miura K, Kurosawa S, Schindler U, Schleimer RP. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24:755–61. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 34.Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–14. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenzel S, Wilbraham D, Fuller R, Getz EB. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–31. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 36.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011 doi: 10.1056/NEJMoa1106469. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]


