Abstract
AIMS
Intraglomerular pressure is one of the main drivers of progression of renal failure. Experimental data suggest that there are important differences between calcium channel blockers (CCBs) in their renal haemodynamic effects: manidipine reduces, whereas amlodipine increases intraglomerular pressure. The aim of this study was to investigate the effects of manidipine and amlodipine treatment on intragomerular pressure (Pglom) in patients with mild to moderate essential hypertension.
METHODS
In this randomized, double-blind, parallel group study, hypertensive patients were randomly assigned to receive manidipine 20 mg (n= 54) or amlodipine 10 mg (n= 50) for 4 weeks. Renal plasma flow (RPF) and glomerular filtration rate (GFR) were determined by constant-infusion input-clearance technique with p-aminohippurate (PAH) and inulin. Pglom and resistances of the afferent (RA) and efferent (RE) arterioles were calculated according to the model established by Gomez.
RESULTS
Pglom did not change in the manidipine group (P= 0.951), whereas a significant increase occurred in the amlodipine group (P= 0.009). There was a significant difference in the change of Pglom by 1.2 mmHg between the manidipine and amlodipine group (P= 0.042). In both treatment arms, RA was reduced (manidipine P= 0.018; amlodipine P < 0.001). The reduction of RA was significantly more pronounced with amlodipine compared with manidipine treatment (P < 0.001). RE increased in both treatment arms (manidipine P= 0.012; amlodipine P= 0.002), with no difference between the treatment arms. Both CCBs significantly reduced systolic and diastolic blood pressure (BP) (both P < 0.001). However, amlodipine treatment resulted in a significantly greater decrease of BP compared with manidipine (P < 0.001).
CONCLUSIONS
In accordance with experimental data after antihypertensive treatment of 4 weeks, intraglomerular pressure was significantly lower with the CCB manidipine than with amlodipine, resulting and explaining their disparate effects on albuminuria.
Keywords: antihypertensive drugs, Ca-channels, cardiovascular pharmacology, human pharmacology
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Dihydropyridine calcium channel blockers (CCBs) are widely used antihypertensive drugs. Evidence from animal studies indicates there are differences between CCBs in their renal haemodynamic effects suggesting that manidipine reduces, whereas amlodipine increases intraglomerular pressure but data from humans are scarce.
WHAT THIS STUDY ADDS
In this study amlodipine was found to increase significantly intraglomerular pressure whereas manidipine decreased intraglomerular pressure in people with essential hypertension. This difference was attributed to significant vasodilation of the afferent arteriole with amlodipine compared with manidipine. This study for the first time has confirmed in humans data from experimental models on the different action of CCBs on dilatory capacity on efferent and afferent arterioles.
Introduction
Hypertension is a major determinant of renal disease progression. Although tight blood pressure (BP) control is an established goal in the treatment of patients with renal disease, it is also well recognized that some antihypertensive drugs have additional effects on renal haemodynamics, potentially affecting the progression of renal disease. Dihydropyridine calcium channel blockers (CCBs) are widely used as both first choice treatment and in combination with other antihypertensive drugs [1]. However, there is still a matter of debate about CCBs and their effects on intrarenal haemodynamics. Repeatedly, experimental models have shown that the potent blood pressure lowering actions of conventional CCBs (e.g. amlodipine) reduce glomerular hyperfiltration and afford renal protection [2, 3]. However, there are also data to demonstrate that the preferential activity of amlodipine on preglomerular vessels, causing mainly dilation of the afferent arteriole, with only modest effects on the efferent arteriole, could also cause an increase in glomerular hyperfiltration and glomerular pressure that could promote renal disease progression [4–6]. In accordance, we have recently evaluated the effect of the conventional CCB amlodipine on renal haemodynamics in hypertensive humans and demonstrated that amlodipine led to glomerular hyperfiltration and an increase in glomerular pressure [7].
Nowadays novel types of CCBs (e.g. manidipine) have been developed, and experimental data suggest that these novel CCBs differ in particular with respect to their effects on intrarenal haemodynamics, demonstrating a relatively greater vasodilatory effect on efferent arterioles [8]. In the hydronephrotic kidney model it was shown that manidipine dilates both afferent and efferent arterioles, although the magnitude on efferent arterioles was less than on afferent arterioles in this particular model of renal injury [9]. Using microdissected renal arterioles, Arima et al. have demonstrated that manididpine has dilatory actions on both afferent and efferent arterioles [10]. Another experimental study has clearly demonstrated that manidipine causes vasodilation of the afferent as well as efferent arterioles, thereby leading to a fall of intraglomerular pressure [11].
In contrast to the relative abundance of data derived from animal studies, data in humans are scarce. Studies have focused on effects of CCBs on changes in albuminuria or creatinine clearance, reflecting renal function [12, 13]. To date, no data are available on the effects of different CCBs on intrarenal haemodynamics, in particular on intraglomerular pressure. The objective of the current study was therefore to test whether the experimental data hold true in the clinical setting and the two CCB, manidipine and amlodipine, exert disparate effects on intrarenal haemodynamics and intraglomerular pressure in hypertensive patients.
Methods
Study population
This investigator-initiated trial followed a randomized, double-blind, parallel group design in 104 patients with arterial hypertension grade 1–2. Subjects were recruited by advertising in local newspapers in the area of Erlangen-Nürnberg, Germany, and eligible subjects were enrolled consecutively. Written informed consent was obtained prior to study inclusion.
Main inclusion criteria were age between 18 and 65 years, arterial hypertension grade 1 or 2 according to ESH guidelines 2003 and no evidence of significant cardiovascular disease other than hypertension. Main exclusion criteria were secondary arterial hypertension, arterial hypertension grade 3, uncontrolled diabetes mellitus or requiring insulin, concomitant treatment with other antihypertensive drugs or drugs known to affect blood pressure.
The study protocol was approved by the Local Ethics Committee (University of Erlangen-Nürnberg) and the study was conducted in accordance with the Declaration of Helsinki and the principles of ‘good clinical practice’ (GCP) guidelines.
The study was registered at http://www.clincialtrials.gov (ID: NCT00627952).
Study design
Participants on antihypertensive medication underwent a wash-out phase for 4 weeks and inclusion and exclusion criteria were rechecked again. Thereafter, baseline measurement of renal haemodynamics was performed using the constant infusion input clearance technique. Then study participants were randomly assigned to receive manidipine 20 mg or amlodipine 10 mg for 28 days and repeated measurements of renal haemodynamics were performed thereafter.
The primary objective of this study was to investigate the effects of a once daily oral dose of manidipine 20 mg, compared with once daily oral dose of amlodipine 10 mg over a 4 week treatment period on intraglomerular pressure in patients with mild to moderate essential hypertension.
Determination of renal haemodynamics
All clearances were performed at the same time in the morning in a quiet and temperature-controlled room. Renal haemodynamics were determined by the constant infusion input clearance technique with inulin (Inutest, Fresenius, Linz, Austria) and sodium p-aminohippurate (PAH) (Clinalfa, Basel, Switzerland) for glomerular filtration rate (GFR) and renal plasma flow (RPF), respectively, as suggested by Cole et al. [14] and repeatedly reported from our laboratory (http://www.crc-erlangen.de). In brief, after a bolus infusion of inulin and PAH over 15 min and a subsequent constant infusion over 105 min, a steady-state between input and renal excretion of the tracer substances was reached. Duplicate blood samples were collected for the assessment of RPF and GFR. PAH was measured according to previously described methods [15]. Inulin was measured indirectly by converting inulin to fructose and subsequently measuring fructose by an enzymatic method (Boehringer Mannheim, Mannheim, Germany). Each blood sample was measured in duplicate with a coefficient variation of <5%.
Calculation of renal and intraglomerular haemodynamics
Filtration fraction (FF) was calculated as GFR/RPF and renal blood flow (RBF) as RPF/(1 – haematocrit).
Intraglomerular pressure (Pglom) and resistances of the afferent (RA) and efferent (RE) arterioles were calculated according to the model originally established by Gomez [16], which has been discussed by Guidi et al. [17] and applied in previous studies [7, 18], as follows:
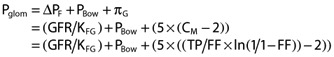 |
In the above equation, KFG (gross filtration coefficient) is estimated as 0.0406 ml s−1 per kidney. PBow (hydrostatic pressure in the Bowman's space) is estimated as 10 mmHg. πG (oncotic pressure within glomerular capillaries) can be obtained from CM (plasma protein concentration within the glomerular capillaries), and calculated from TP (total protein concentration) and FF.
From Ohm's law:
Measurement of urinary albumin and creatinine
All samples were measured centrally at the biochemistry laboratory of the University of Erlangen-Nürnberg according to established methods. In brief, urinary albumin concentration was measured by a turbidimetric method. The inter-assay coefficient of variation was 3.44%. Creatinine concentration in urine was measured photometrically by the Jaffé method. The inter-assay coefficient of variation was 2.03%.
Statistical analysis
Normal distribution of data was confirmed by Kolmogorov–Smirnov tests before further analysis. Normally distributed data were compared by paired and unpaired Student's t-tests and are expressed as mean ± standard deviation (SD). Data of UACR were not normally distributed. Therefore, median and interquartile range are reported. UACR values were log-transformed before statistical analysis. Where indicated, a multiple stepwise regression analysis with significance levels of 0.05 for entry and 0.10 for removal of a variable at each forward step was conducted. Two-tailed values of P < 0.05 were considered statistically significant. All analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Baseline characteristics of participants randomized to the respective CCB (manidipine or amlodipine) are given in Table 1. There were no differences in any of the clinical characteristics between the two groups.
Table 1.
Clinical characteristics (mean ± SD) of participants stratified according to assigned calcium channel blocker
| Manidipine | Amlodipine | P value | |
|---|---|---|---|
| Age (years) | 50.5 ± 12 | 50.7 ± 9 | NS |
| Gender (M/F) | 44/10 | 41/9 | NS |
| BSA (m2) | 2.05 ± 0.2 | 2.03 ± 0.2 | NS |
| BMI (kg m−2) | 27.7 ± 3.5 | 27.0 ± 3.3 | NS |
| Systolic BP (mmHg) | 150 ± 10 | 148 ± 9 | NS |
| Diastolic BP (mmHg) | 93 ± 8 | 93 ± 9 | NS |
| Heart rate (beats min−1) | 71 ± 10 | 72 ± 11 | NS |
| UACR (mg g−1 creatinine) | 12.7 ± 17 | 14.3 ± 33 | NS |
BSA, body surface area; BMI, body mass index; UACR, urinary albumin : creatinine ratio; NS, not significant.
Intrarenal haemodynamics
The primary endpoint of our study, Pglom, did not change in the manidipine group (69.5 ± 3.9 vs. 69.5 ± 3.6 mmHg, P= 0.951), whereas a significant increase was noted in the amlodipine group (68.6 ± 4.4 vs. 70.2 ± 4.0 mmHg, P= 0.009). There was a significant difference in the change of Pglom by 1.2 mmHg between the manidipine and amlodipine group (P= 0.042, Figure 1).
Figure 1.
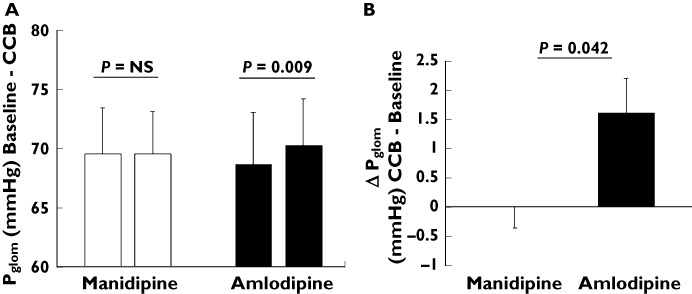
A) Pglom at baseline and after 4 weeks of treatment with CCBs manidipine (white columns) and amlodipine (black columns), a comparison within the CCB groups. B) Change of Pglom between baseline and 4 weeks of treatment with CCBs manidipine (white column) and amlodipine (black column), a comparison between the CCB groups
In both treatment arms RA was reduced (manidipine 3389 ± 1383 vs. 3055 ± 1192 dyn s−1 cm−5, P= 0.018; amlodipine 2987 ± 925 vs. 2320 ± 739 dyn s−1 cm−5, P < 0.001). The reduction of RA was significantly more pronounced with amlodipine compared with manidipine treatment (P < 0.001). RE increased in both treatment arms (manidipine 2557 ± 494 vs. 2701 ± 524 dyn s−1 cm−5, P= 0.012; amlodipine 2488 ± 505 vs. 2737 ± 590 dyn s−1 cm−5, P= 0.002), with no difference between the treatment arms.
The intraglomerular pressure is mainly the result of the interplay between the efferent and afferent arteriolar resistance. Hence, the ratio of the resistances of the efferent to the afferent arterioles (RE : RA) are given in Figure 2. Baseline levels did not differ between manidpine and amlodipine groups. In both groups there was an increase of this ratio (manidipine 0.83 ± 0.3 vs. 1.0 ± 0.3, P= 0.001; amlodipine 0.9 ± 0.3 vs. 1.27 ± 0.4, P < 0.001). However, the change of the RE :RA was more pronounced with amlodipine compared with manidipine (0.37 ± 0.04 vs. 0.17 ± 0.05, P= 0.002).
Figure 2.
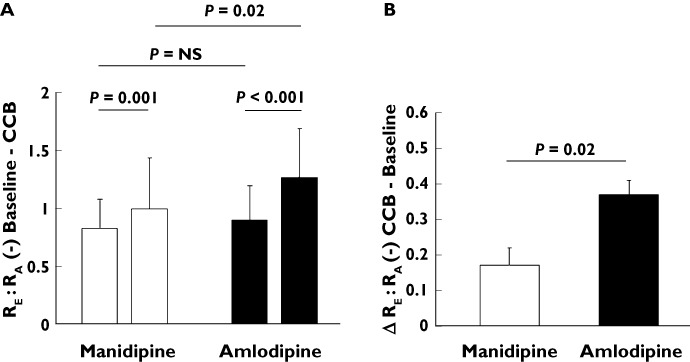
A) Ratio of the resistances of the efferent and afferent arteriole (RE : RA) at baseline and after 4 weeks of treatment with CCBs manidipine (white columns) and amlodipine (black columns), a comparison within the CCB groups. B) Change of RE : RA between baseline and 4 weeks of treatment with CCBs manidipine (white column) and amlodipine (black column), a comparison between the CCB groups
Renal haemodymanics
The RPF was maintained in both treatment groups (manidipine 616 ± 141 vs. 597 ± 146 ml min−1, P= 0.169; amlodipine 621 ± 130 vs. 603 ± 115 ml min−1, P= 0.318). Consistent with the findings on intraglomerular haemodynamics, GFR was numerically more increased with amlodipine (140 ± 18 vs. 144 ± 16 ml min−1, P= 0.081), than with manidipine (141 ± 18 vs. 142 ± 19 ml min−1, P= 0.467).
Systemic haemodynamics
Baseline systolic and diastolic BP did not differ between manidipine and amlodipine groups. Both CCBs decreased systolic (manidipine 150 ± 10 vs. 143 ± 12 mmHg, P < 0.001; amlodipine 148 ± 9 vs. 133 ± 10 mmHg, P < 0.001) and diastolic BP (manidipine 93 ± 8 vs. 90 ± 7 mmHg, P < 0.001; amlodipine 93 ± 9 vs. 85 ± 9 mmHg, P < 0.001). However, BP reduction was significantly less pronounced with manidipine treatment compared with amlodipine treatment (systolic −7 ± 11 vs.−16 ± 13 mmHg, P < 0.001; diastolic −3 ± 6 vs.−8 ± 7 mmHg, P < 0.001).
Urinary albumin : creatinine ratio (UACR)
In accordance with the data on intraglomerular pressure and RE : RA, manidipine treatment resulted in lower UACR compared with baseline levels (6.0 (4.0–10.0) vs. 4.0 (3.3–9.0) mg g−1 creatinine, P= 0.053), which, however, failed to reach our prespecified statistical significance level. In contrast, amlodipine treatment resulted in a significant increase of UACR compared with baseline levels (5.0 (3.0–10.0) vs. 7.0 (4.0–14.0) mg g−1 creatinine, P= 0.003). There was a significant difference in the change of UACR between the treatment groups, in favour of manidipine (P < 0.001).
Since blood pressure changes due to antihypertensive treatment may also lead to alterations in renal perfusion pressure and thereby albumin excretion, we performed further analyses of our data. To assess the influence of MAP changes due to antihypertensive treatment as a potential confounding factor, multiple linear regression analyses were performed. MAP change in response to antihypertensive treatment was not related to the change in log-transformed UACR (β= 0.164, P= 0.167), whereas assigned antihypertensive medication was related to change in log-transformed UACR (β= 0.541, P < 0.001).
Adverse events
Incidence of peripheral oedema, the most common side effect of antihypertensive therapy with dihydropyridine-type CCBs, was significantly lower with manidipine compared with amlodipine treatment (2 (3.6%) vs. 10 (17.5%), P= 0.03). Other typical side effects of antihypertensive therapy with CCBs (e.g. palpitations or flushing) did not differ between manidipine and amlodipine.
Discussion
The main result of the present study is that in contrast to antihypertensive treatment with manidipine, amlodipine increases Pglom significantly, resulting in a significant difference in the change of Pglom between these two CCBs in favour of manidipine. The intraglomerular pressure is mainly the result of the interplay between the efferent and the afferent arteriole, and in line with our data on Pglom we found a significant increase in the RE : RA ratio, resulting from a more prominent vasodilation of the afferent arteriole with amlodipine compared with manidipine. Therefore, we could translate published data from experimental models that action of CCBs regarding dilatory capacity on efferent and afferent arterioles varies depending on the used agents [19] for the first time to humans.
The detrimental consequences of an increased glomerular pressure on the progression of renal disease are well known [20]. Conversely, a reduction in Pglom prevents glomerular hyperfiltration and reduces albuminuria, since the driving force of albuminuria (i.e. the filtration pressure) is reduced. Indeed, we could demonstrate that manidipine treatment resulted in a lower UACR whereas amlodipine significantly increased UACR, with a significant difference in the change of UACR between the two CCB.
This is in line with previous findings in hypertensive patients with metabolic syndrome randomly assigned to manidipine (20 mg) or amlodipine (10 mg). Despite a similar blood pressure reduction, albuminuria was only significantly reduced with manidipine, but not with amlodipine, with a significant difference between treatment arms [21]. Often monotherapy is not sufficient to achieve recommended BP targets, and patients require combination therapy. Recently, a study focusing on combination therapy with CCBs and renin-angiotensin system (RAS)-modulating agents reported disparate effects between different CCBs on albuminuria despite similar effects on BP. In hypertensive diabetic patients with microalbuminuria, despite a maximum dose of a RAS blocker, urinary albumin excretion (UAE) was significantly more reduced with manidipine vs. amlodipine [22].
Since there is a continuous relation between albuminuria and cardiovascular (CV) and renal risk, even at levels of albumin excretion within the normal range [23–26], and its reduction is associated with an improved prognosis [27], it can be postulated that the effect of antihypertensive medication on albuminuria is of importance even in hypertensive patients without concomitant renal disease.
There was a significantly lower incidence of peripheral oedema with manidipine compared with amlodipine treatment (2 vs. 10, P= 0.03). This finding is in line with previous studies [22, 28]. Due to its lipophilicity and vasoselective action, it its postulated that manidipine has a lower degree of baroreflex-induced activation of the sympathetic system, and hence causes a lesser increase of the capillary pressure gradient [29]. This finding has an important clinical impact, since antihypertensive management remains suboptimal in western civilization [30]. One of the reasons is side-effects of antihypertensive medication, a crucial determinant of adherence to prescribed therapy. Therefore, reduction of side effects may translate into better therapy adherence and improved long term BP control, resulting in better CV and renal outcomes.
There are several limitations of our study. In contrast to experimental models, the human renal microcirculation (resistances of the afferent and efferent arteriole and glomerular hydrostatic pressure) cannot be examined directly. Therefore the physiologist Gomez had already in the 1950s developed a model to calculate glomerular haemodynamic parameters from RPF, GFR and total protein concentration (with some assumptions such as the presence of filtration disequilibrium along the glomerular capillaries) [16]. Although based on several assumptions, the Gomez calculations have been repeatedly applied and appear to be reliable in humans without renal disease and normal renal function [7, 18]. In particular, comparisons of intrarenal haemodynamics within one individual, and within short periods of time are considered to be reliable in humans.
Secondly, despite clinical studies [21, 28] showing a comparable BP lowering effects of manidpine 20 mg and amlodipine 10 mg, in our study amlodipine treatment resulted in a significantly greater decrease of BP compared with manidipine treatment. Therefore, multiple regression analysis was performed to determine the impact of BP change on renal haemodynamic parameters. MAP change in response to antihypertensive treatment was not related, whereas assigned antihypertensive medication was related to the change in UACR. This is also strengthened by findings of previous published studies. In the Ramipril Efficacy in Nephropathy 2 (REIN-2) trial, the CCB felodipine was added to ramipril. In contrast to the further blood pressure reduction with the add-on therapy of felodipine, throughout the study urinary protein excretion was similar in both arms [31]. In hypertensive patients with albuminuria, despite maximum dose of RAS blockers, manidipine decreased UAE significantly. There was no correlation between SBP reduction and UAE [32]. Another study compared the effect of manidipine vs. hydrochlorothiazide (HCTZ) in addition to candesartan in hypertensive patients with type 2 diabetes and microalbuminuria. There was no significant difference in achieved BP levels between the treatment arms, whereas UAE rate was further reduced only with manidipine in contrast with no significant change with HCTZ, indicating that the antiproteinuric effect of manidipine is independent of BP reduction [33].
Nevertheless, we cannot rule out that effects seen in this study may have been influenced by the sizable difference in blood pressure reduction between the treatment groups.
We have to consider that multiple comparisons (both within and between the groups) increase the possibility of findings by chance, but may also provide additional exploratory information and in our statistical analysis the primary objective was clearly prespecified.
In summary, we could demonstrate beneficial effects of manidipine compared with amlodipine on the renal microcirculation, specifically a lower intraglomerular pressure and reduced urinary albumin excretion, which may translate into better renal protection.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Ortrun Alter, Dorothea Bader-Schmieder, Sadhana Duhme, Ingrid Fleischmann, Ulrike Heinritz and Simone Pejkovic.
The study was in part supported by a grant from Chiesi Farmaceutici S.p.A., Parma, Italy.
The sponsor Chiesi Farmaceutici S.p.A. did not contribute to data collection, interpretation of data, or the decision to approve and submit the manuscript.
Competing Interests
M. Alberici is an employee of Chiesi Farmaceutici S.p.A., Parma, Italy. RES has received grants, lectures and consultancy fees from Chiesi Farmaceutici S.p.A., Parma, Italy.
REFERENCES
- 1.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin LD. Effects of calcium channel blockers on experimental glomerular injury. J Am Soc Nephrol. 1990;1:S21–27. [PubMed] [Google Scholar]
- 3.Kanno Y, Suzuki H, Okada H, Saruta T. Renal protective effects of amlodipine on partially nephrectomized spontaneously hypertensive rats fed a high-salt diet. J Cardiovasc Pharmacol. 1994;23:480–4. [PubMed] [Google Scholar]
- 4.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:F1003–1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 5.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int. 1999;55:1849–60. doi: 10.1046/j.1523-1755.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 6.Griffin KA, Picken M, Bakris GL, Bidani AK. Comparative effects of selective T- and L-type calcium channel blockers in the remnant kidney model. Hypertension. 2001;37:1268–72. doi: 10.1161/01.hyp.37.5.1268. [DOI] [PubMed] [Google Scholar]
- 7.Delles C, Klingbeil AU, Schneider MP, Handrock R, Weidinger G, Schmieder RE. Direct comparison of the effects of valsartan and amlodipine on renal hemodynamics in human essential hypertension. Am J Hypertens. 2003;16:1030–5. doi: 10.1016/j.amjhyper.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res. 2007;100:342–53. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- 9.Tojo A, Kimura K, Matsuoka H, Sugimoto T. Effects of manidipine hydrochloride on the renal microcirculation in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1992;20:895–9. doi: 10.1097/00005344-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Arima S, Ito S, Omata K, Tsunoda K, Yaoita H, Abe K. Diverse effects of calcium antagonists on glomerular hemodynamics. Kidney Int Suppl. 1996;55:S132–134. [PubMed] [Google Scholar]
- 11.Rodicio JL. Renal effects of calcium antagonists with special reference to manidipine hydrochloride. Blood Press Suppl. 1996;5:10–5. [PubMed] [Google Scholar]
- 12.Takabatake T, Ohta H, Sasaki T, Satoh S, Ohta K, Ise T, Kobayashi K. Renal effects of manidipine hydrochloride. A new calcium antagonist in hypertensive patients. Eur J Clin Pharmacol. 1993;45:321–5. doi: 10.1007/BF00265948. [DOI] [PubMed] [Google Scholar]
- 13.Bellinghieri G, Mazzaglia G, Savica V, Santoro D. Effects of manidipine and nifedipine on blood pressure and renal function in patients with chronic renal failure: a multicenter randomized controlled trial. Ren Fail. 2003;25:681–9. doi: 10.1081/jdi-120024284. [DOI] [PubMed] [Google Scholar]
- 14.Cole BR, Giangiacomo J, Ingelfinger JR, Robson AM. Measurement of renal function without urine collection. A critical evaluation of the constant-infusion technic for determination of inulin and para-aminohippurate. N Engl J Med. 1972;287:1109–14. doi: 10.1056/NEJM197211302872202. [DOI] [PubMed] [Google Scholar]
- 15.Schlaich MP, Jacobi J, John S, Delles C, Fleischmann I, Schmieder RE. Is l-arginine infusion an adequate tool to assess endothelium-dependent vasodilation of the human renal vasculature? Clin Sci (Lond) 2000;99:293–302. [PubMed] [Google Scholar]
- 16.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest. 1951;30:1143–55. doi: 10.1172/JCI102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidi E, Cozzi MG, Minetti EE, Civati G, Busnach G, Brando B. Effect of familial hypertension on glomerular hemodynamics and tubulo-glomerular feedback after uninephrectomy. Am J Hypertens. 2001;14:121–8. doi: 10.1016/s0895-7061(00)01238-3. [DOI] [PubMed] [Google Scholar]
- 18.Ott C, Ritt M, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin does not affect intrarenal hemodynamics in patients with hypercholesterolemia. J Nephrol. 2009;22:675–81. [PubMed] [Google Scholar]
- 19.Hayashi K, Ozawa Y, Fujiwara K, Wakino S, Kumagai H, Saruta T. Role of actions of calcium antagonists on efferent arterioles – with special references to glomerular hypertension. Am J Nephrol. 2003;23:229–44. doi: 10.1159/000072054. [DOI] [PubMed] [Google Scholar]
- 20.Chiurchiu C, Remuzzi G, Ruggenenti P. Angiotensin-converting enzyme inhibition and renal protection in nondiabetic patients: the data of the meta-analyses. J Am Soc Nephrol. 2005;16(Suppl. 1):S58–63. doi: 10.1681/asn.2004110968. [DOI] [PubMed] [Google Scholar]
- 21.Martinez Martin FJ. Manidipine in hypertensive patients with metabolic syndrome: the MARIMBA study. Expert Rev Cardiovasc Ther. 2009;7:863–9. doi: 10.1586/erc.09.53. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Martinand FJ, Saiz-Satjes M. Add-on manidipine versus amlodipine in diabetic patients with hypertension and microalbuminuria: the AMANDHA study. Expert Rev Cardiovasc Ther. 2008;6:1347–55. doi: 10.1586/14779072.6.10.1347. [DOI] [PubMed] [Google Scholar]
- 23.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 24.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 25.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–75. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 26.van der Velde M, Halbesma N, de Charro FT, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–62. doi: 10.1681/ASN.2008060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen MH, Wachtell K, Ibsen H, Lindholm LH, Dahlof B, Devereux RB, Kjeldsen SE, Oikarinen L, Okin PM. Reductions in albuminuria and in electrocardiographic left ventricular hypertrophy independently improve prognosis in hypertension: the LIFE study. J Hypertens. 2006;24:775–81. doi: 10.1097/01.hjh.0000217862.50735.dc. [DOI] [PubMed] [Google Scholar]
- 28.Zanchetti A, Omboni S, La Commare P, De Cesaris R, Palatini P. Efficacy, tolerability, and impact on quality of life of long-term treatment with manidipine or amlodipine in patients with essential hypertension. J Cardiovasc Pharmacol. 2001;38:642–50. doi: 10.1097/00005344-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Richyand FF, Laurent S. Efficacy and safety profiles of manidipine compared with amlodipine: a meta-analysis of head-to-head trials. Blood Press. 2010;20:54–9. doi: 10.3109/08037051.2010.518670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramlage P, Thoenes M, Kirch W, Lenfant C. Clinical practice and recent recommendations in hypertension management–reporting a gap in a global survey of 1259 primary care physicians in 17 countries. Curr Med Res Opin. 2007;23:783–91. doi: 10.1185/030079907x182077. [DOI] [PubMed] [Google Scholar]
- 31.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–46. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 32.Galceran J, Plana J, Felip A, Pou G, Vila J, Sobrino J. Manidipine treatment in patients with albuminuria not sufficiently reduced with renin-angiotensin system blockers. Expert Rev Cardiovasc Ther. 2010;8:751–7. doi: 10.1586/erc.10.48. [DOI] [PubMed] [Google Scholar]
- 33.Fogari R, Corradi L, Zoppi A, Lazzari P, Mugellini A, Preti P, Rinaldi A. Addition of manidipine improves the antiproteinuric effect of candesartan in hypertensive patients with type II diabetes and microalbuminuria. Am J Hypertens. 2007;20:1092–6. doi: 10.1016/j.amjhyper.2007.05.012. [DOI] [PubMed] [Google Scholar]


