Abstract
AIM
Fruit juice reduces the plasma concentrations of several β-adrenoceptor blockers, likely by inhibiting OATP2B1-mediated intestinal absorption. The aim of this study was to investigate the effects of apple juice on the pharmacokinetics of atenolol.
METHODS
Twelve healthy Korean volunteers with genotypes of SLCO2B1 c.1457C> T (*1/*1 (n= 6) and *3/*3 (n= 6)) were enrolled in this study. In a three-phase, one-sequence crossover study, the pharmacokinetics (PK) of atenolol was evaluated after administration of 50 mg atenolol. Subjects received atenolol with either 300 ml water, 1200 ml apple juice or 600 ml apple juice.
RESULTS
Apple juice markedly reduced the systemic exposure to atenolol. The geometric mean ratios (95% confidence intervals) of apple juice : water were 0.18 (0.13, 0.25, 1200 ml) and 0.42 (0.30, 0.59, 600 ml) for the AUC(0,tlast). In this study, the PK parameters of atenolol responded in a dose-dependent manner to apple juice.
CONCLUSIONS
Apple juice markedly reduced systemic exposure to atenolol. The genetic variation of SLCO2B1 c.1457C>T had a minimal effect on the pharmacokinetics of atenolol when the drug was administered with water or apple juice.
Keywords: apple juice, atenolol, pharmacokinetics, SLCO2B1
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Atenolol is an antihypertensive drug, of which negligible amounts are metabolized.
Fruit juices may decrease the oral absorption of drugs by inhibiting intestinal drug transporters, as demonstrated in vitro and in vivo.
WHAT THIS STUDY ADDS
The pharmacokinetic characteristics of atenolol were determined according to the SLCO2B1 genotype after apple juice administration in healthy Korean volunteers.
Apple juice ingestion markedly reduced the systemic exposure to atenolol, but genetic variations in SLCO2B1 were unlikely to contribute substantial variability to the pharmacokinetics of atenolol.
Introduction
Atenolol is a selective β1-receptor blocking agent and is one of the most widely prescribed drugs used in the treatment of angina pectoris and hypertension [1–4]. As a first line therapy for hypertension, β-adrenoceptor blockers have proven to be safe and effective in reducing mortality and morbidity [3, 5]. The bioavailability of atenolol is approximately 40–50%. Less than 5% of the drug is bound to plasma proteins and renal excretion eliminates approximately 90% of the absorbed drug in its unchanged form [6, 7]. Atenolol is a hydrophilic compound, and only negligible amounts of the drug are metabolized [7]. The pharmacokinetic parameters of atenolol show 26–38% inter-individual variability [8].
Organic anion-transporting polypeptides OATP2B1 and OATP1A2 are influx transporters located in the luminal membranes of small intestine enterocytes [9]. Fruit juices may decrease the oral absorption of drugs by inhibiting intestinal drug transport, as demonstrated by in vitro and in vivo studies [10–15]. Orange juice reduced the mean peak plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) of atenolol by 49% and 40%, respectively [16]. Apple juice reduced the exposure of fexofenadine to a mean of 75%, and grapefruit juice decreased the bioavailability of celiprolol, in part due to the OATP2B1-mediated absorption process [17, 18]. At present, OATP2B1 and OATP1A2 are considered to be related to this interaction [19]. Recently, genetic variants with non-synonymous single nucleotide polymorphisms (SNPs), SLCO2B1*3 (Ser→Phe (c.1457C>T)) have been shown to alter the systemic exposure of substrate drugs compared with the wild-type genotype [17, 18, 20]. In a Japanese population, an allele frequency of 30.9% of the SLCO2B1*3 was reported [21]. In a Korean population, the allele frequency of the variant was 25.5% in healthy volunteers (our unpublished data), which is similar to that of the Japanese population and greater than that of the Finnish population (2.1%) [22]. Atenolol is thought to be a substrate of OATP1A2 [10]. However the frequency of nonsynonymous SNPs of SLCO1A2 has been reported to be very low in the Asian population [23]. Although atenolol is one substrate of OATP1A2, we hypothesized that atenolol could also be transported by OATP2B1, as is observed for hydrophilic β-adrenoceptor blockers that are only metabolized weakly (e.g. celiprolol or talinolol) [18, 24]. Therefore, we investigated whether atenolol absorption is affected by (i) apple juice ingestion and (ii) genetic variants in the gene coding for OATP2B1 (SLCO2B1) in humans.
Methods
Subjects
Korean male volunteers aged 20 to 50 years with a body mass index in the range of 17 to 28 kg m−2 were enrolled in this study if deemed healthy by physical examination, medical history, laboratory results, 12-lead electrocardiogram and vital signs. The subjects who had ingested fruit juice within 7 days prior to drug administration were excluded. Written informed consent was obtained from all subjects prior to the study. Twelve healthy male volunteers were selected based on genotyping for SLCO2B1 (SLCO2B1*1/*1 (n= 6) and *3/*3 (n= 6)).
Study design
The study was a single-dose, randomized, open-label, one-sequence, three-period crossover design. All subjects were hospitalized the night before drug administration. After overnight fasting, each individual received a single oral dose of atenolol (Tenormin, AstraZeneca Japan, Osaka, Japan). The study drug was administered with water or apple juice (100% Martinelli's Gold Medal apple juice, CA, USA). In period 1, 50 mg atenolol was administered with 300 ml soft mineral water. In period 2, the drug was taken with 300 ml apple juice, and the participants consumed an additional 150 ml apple juice every 0.5 h for 3 h (for a total of 1200 ml), which was identical to the protocol used for the previous juice–drug interaction study [11]. In period 3, 300 ml apple juice was administered as in period 2 and the participants also consumed an additional 100 ml every 0.5 h for 1.5 h (for a total of 600 ml). Each period was separated by a wash-out interval of at least 7 days. For 4 h after drug administration, subjects were required to remain in a seated position. After this time period, standardized lunches were provided and the subjects were allowed to drink water. Blood samples were collected before dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36 and 48 h after dosing. Each sample was collected in a heparinized tube, centrifuged at 2000 g for 10 min and stored below −70°C until the assay was performed. For safety evaluation, systolic and diastolic blood pressures were measured after the subjects had been sitting for a minimum of 5 min using an automatic oscillometric blood pressure monitor prior to each pharmacokinetic sampling (Dash 5000, General Electric Healthcare, Finland). This study was approved by the Institutional Review Board of Seoul National University Hospital and registered at ClinicalTrials.gov (identifier: NCT01445964).
Genotyping
Blood samples were collected from each subject and DNA was extracted using a purification kit (QIAamp DNA blood mini kit, QIAGEN, Hilden, Germany). SLCO2B1*3 (1457C>T, rs2306168, assay ID: C_16193013_20). The allele detection of three other common variants (935G>A, rs12422149, assay ID: C_3101331_10; 282 G>A, rs2712807, assay ID: C_1786365_10; 1175 C>T, rs1621378, assay ID: C_8750223_30) was performed by TaqMan allelic discrimination assays on an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA).
For the 12 enrolled subjects, genetic variants regarding pharmacokinetics were evaluated using the DMET plus panel (Affymetrix, Santa Clara, California, USA). The DMET plus panel is a targeted single nucleotide polymorphism (SNP) chip that is useful in identifying genetic biomarker candidates and drug responders [25]. The polymorphisms that were chosen for the panel are known as drug-metabolizing enzymes and transporters. The DMET plus chip utilizes molecular-inversion probe technology to develop a multiplex genotyping assay that can simultaneously test more than 1936 markers in 225 genes associated with drug metabolizing enzymes and transporters [26, 27].
Quantification of plasma concentrations of atenolol
Plasma atenolol concentration was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The high performance liquid chromatography system (HPLC, Agilent 1100 series, Agilent Technologies, Wilmington, DE, USA) was connected to a mass spectrometer (API 4000 Quadrupole, Applied Biosystems, Foster City, USA) equipped with a Turbo Spray. The chromatographic column was a Luna Phenylhexyl 3 µm analytical column (100 × 2.0 mm inner diameter, Phenomenex, Torrance, USA). Atenolol was monitored in the positive mode and quantifications were conducted in the multiple reaction monitoring (MRM) mode. The mass transitions were m/z 267.288→145.100 for atenolol and m/z 260.190→116.200 for the internal standard.
Stock solutions of atenolol and propranolol (internal standard, Sigma-Aldrich, Buchs, Switzerland) were extracted from the samples by protein precipitation using acetonitrile and prepared in methanol at approximately 1 mg ml−1. The solutions were diluted and used to spike blank matrices to obtain calibration standard solutions. The standard curve for atenolol was 5–2000 ng ml−1 and each standard solution was prepared by adding a working standard solution to a blank matrix. Quality control samples were prepared by adding separate stock solutions of atenolol to the blank matrix. The internal standard (propranolol) solution was added to 1 ml plasma. After centrifugation for 5 min at 9900 g, 4 µl of supernatant was injected into the LC-MS/MS. The concentrations were quantified by the internal standard method. The calibration curve was obtained using least square linear regression analysis by fitting the peak area ratio against the internal standard and nominal concentration (x) of the analyte to the equation y = ax + b (weighting 1/x2). The quantification limit of atenolol was 5 ng ml−1. The accuracy of the analysis ranged from 99–102%, and the precision was lower than 5%.
Pharmacokinetic analysis
Pharmacokinetic parameters (Cmax, AUC(0,tlast), tmax and half-life (t1/2)) were calculated using Phoenix 6.1 (Pharsight Corporation, Mountain View, CA, USA) for non-compartmental analysis. The maximal plasma concentration (Cmax) and the time to reach Cmax (tmax) were identified from the observed values. The area under the plasma concentration–time curve (AUC) from dosing to the last measurable concentration (AUC(0,tlast)) was estimated using the linear up/log down method. The elimination rate constant (λz) was estimated by linear regression of the log-linear portion of the plasma concentration-time curve. t1/2 was calculated by ln 2/λz.
Statistical analysis
The values of pharmacokinetic parameters, except tmax, are presented as geometric means with 95% confidence intervals. tmax is given as a median with a range. The pharmacokinetic parameters (Cmax, AUC(0,tlast) and t1/2) were logarithmically transformed before statistical analysis. Statistical comparisons were conducted using a mixed model with period and genotype as fixed effects and subject as a random effect. Haemodynamic values (systolic and diastolic blood pressures) were compared using a repeated-measures analysis of variance (anova). P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Twelve healthy male Korean volunteers participated in the study (six with SLCO2B1 c.1457C> T *1/*1 and six with *3/*3). Three subjects withdrew consent before the third period (one in the SLCO2B1*1/*1 group and two in the SLCO2B1*3/*3 group). The systemic exposure of atenolol was inversely proportional to the total amount of apple juice received (Figures 1 and 2, and Table 1).
Figure 2.
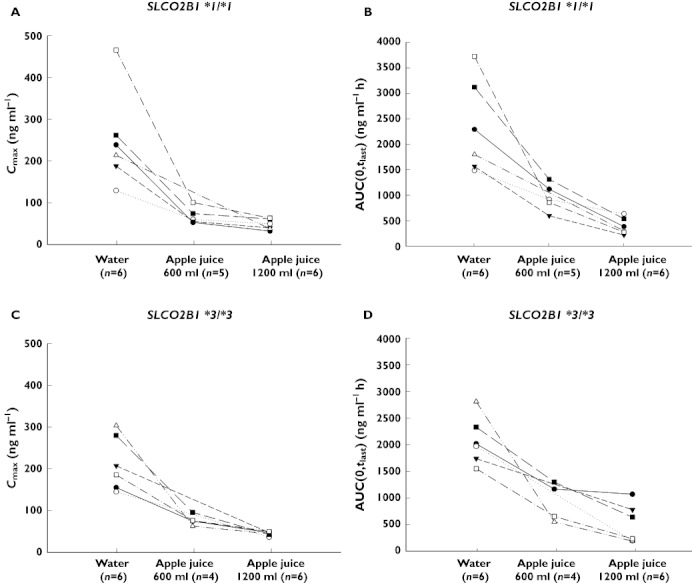
Individual Cmax (A, C) and AUC(0,tlast) (B, D) values of atenolol in 12 healthy volunteers according to SLCO2B1 genotype (*1/*1 A, B and *3/*3 C, D). The volunteers ingested 300 ml soft mineral water, a total of 600 ml, and 1200 ml apple juice with 50 mg atenolol
Table 1.
Pharmacokinetic variables of a single 50 mg dose of atenolol in 12 healthy volunteers during the water, 1200 ml apple juice and 600 ml apple juice phases
| Geometric mean ratio | ||||||
|---|---|---|---|---|---|---|
| Water | Apple juice (1200 ml) | Apple juice (600 ml) | Apple juice 1200 ml/water | Apple juice 600 ml/water | Apple juice 1200 ml/apple juice 600 ml | |
| Total (n=12) | ||||||
| tmax (h) | 2.50 [1.00–4.00] | 1.50 [1.00–2.00] | 2.00 [1.00–6.00] | – | – | – |
| Cmax (ng ml−1) | 216 (183, 254) | 44.3 (37.6, 52.2) | 68.0 (56.7, 81.5) | 0.21 (0.17, 0.25)** | 0.32 (0.26, 0.38)** | 0.65 (0.54, 0.79)** |
| AUC(0,tlast) (ng ml−1 h) | 2,110 (1,635, 2,723) | 389.7 (302.0, 503.0) | 885.3 (662.6, 1183) | 0.18 (0.13, 0.25)** | 0.42 (0.30, 0.59)** | 0.44 (0.31, 0.62)** |
| t1/2 (h) | 9.93 (7.33, 13.5) | 10.5 (7.73, 14.2) | 12.8 (9.14, 17.9) | – | – | – |
| SLCO2B1*1/*1 (n=6) | ||||||
| tmax (h) | 2.50 [1.00–4.00] | 1.50 [1.00–2.00] | 2.00 [1.00–4.00] | – | – | – |
| Cmax (ng ml−1) | 228 (168, 310) | 44.9 (33.1, 61.1) | 64.2 (46.7, 88.3) | 0.20 (0.15, 0.26)** | 0.28 (0.21, 0.38)** | 0.70 (0.52, 0.94)* |
| AUC(0,tlast) (ng ml−1 h) | 2,182 (1,568, 3,036) | 359.0 (258.0, 499.4) | 902.9 (633.7, 1,287) | 0.16 (0.11, 0.24)** | 0.41 (0.27, 0.63)* | 0.40 (0.26, 0.60)** |
| t1/2 (h) | 9.64 (6.66, 13.9) | 9.48 (6.55, 13.7) | 11.6 (7.76, 17.3) | – | – | – |
| SLCO2B1*3/*3 (n=6) | ||||||
| tmax (h) | 2.50 [1.50-4.00] | 1.50 [1.00–2.00] | 2.25 [1.50-6.00] | – | – | – |
| Cmax (ng ml−1) | 204 (168, 247) | 43.6 (36.0, 52.9) | 75.2 (59.4, 95.2) | 0.21 (0.16, 0.29)** | 0.37 (0.27, 0.51)** | 0.58 (0.42, 0.79)* |
| AUC(0,tlast) (ng ml−1 h) | 2,040 (1,284, 3,241) | 423.1 (266.2, 672.2) | 859.1 (493.3, 1496) | 0.21 (0.11, 0.38)** | 0.42 (0.21, 0.83)* | 0.49 (0.25, 0.97)* |
| t1/2 (h) | 10.2 (5.78, 18.1) | 11.5 (6.52, 20.4) | 14.1 (7.43, 26.7) | – | – | – |
The data are presented as the geometric mean (95% confidence interval) except for tmax, which is given as the median [minimum-maximum].
Apple juice 600 ml: In total, nine subjects completed the study. (SLCO2B1*1/*1 (n= 5), SLCO2B1*3/*3 (n= 4)).
P < 0.05.
P < 0.001.
Genetic variation (SLCO2B1*3/*3) did not affect the pharmacokinetic parameters of atenolol in any of the three periods (Figures 2 and 3, Table 1). During period 1 (water phase), lower Cmax and AUC(0,tlast) were observed in the *3/*3 group, but the difference between the two genotypes was not significant (P= 0.870 and 0.909, respectively). The mean t1/2 was 10.2 h in the *3/*3 group, which was similar to that in the *1/*1 group (9.6 h). The median tmax was equal to that observed in the *3/*3 group: 2.5 h during the water period (Table 1). The pharmacokinetic parameters of atenolol exhibited moderate inter-individual variability in that the Cmax and AUC(0,tlast) of atenolol ranged from 100 to 400 ng ml−1 and 1500 to 3500 ng ml−1 h, respectively (Figure 2). There were no significant differences in t1/2 among periods (Figure 1). Interestingly, there were no remarkable relationships between certain genotypes and systemic exposure to atenolol. Therefore, we chose to explore other common variants, SLCO2B1 G>A (rs12422149, R312Q) and SLCO2B1 282 G>A (rs2712807, −546A/G), alone or in combination. However, there was no meaningful genetic factor that affected the pharmacokinetics of atenolol.
Figure 3.
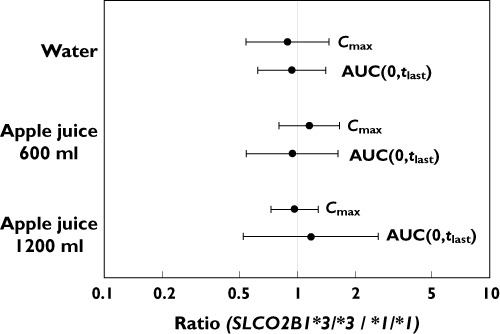
Geometric mean ratios and 95% confidence intervals (SLCO2B1*3/*3 to *1/*1) for Cmax and AUC(0,tlast) of atenolol after the ingestion of water, 600 ml apple juice, and 1200 ml apple juice
Figure 1.

Plasma atenolol concentration–time profiles (arithmetic mean ± standard deviation) after the oral administration of 50 mg atenolol with water or apple juice. (inset shows log-linear scale).  , water (n= 12);
, water (n= 12);  , apple juice (total 600 ml) (n= 9);
, apple juice (total 600 ml) (n= 9);  , apple juice (total 1200 ml) (n= 12)
, apple juice (total 1200 ml) (n= 12)
Using the DMET Plus, a commercial SNP chip, pharmacokinetic-related genetic polymorphisms were screened in each participant to identify the effect of other influx or efflux transporters. The nonsynonymous SNPs of several genes demonstrating more than 0.05 minor allele frequencies were studied due to the translation of these genes to transporter proteins, such as OATP1A2, peptide transporter 1 (PEPT1, SLC15A1), apical sodium-dependent bile acid cotransporter (ASBT, SLCO10A2) and multi-drug resistance protein 1 (MDR1, ABCB1) [28–31]. However, there was no obvious relationship between these SNPs and atenolol pharmacokinetic parameters, possibly due to the relatively small number of subjects (n= 12, Table 2). All subjects were wild-type for OATP1A2, based on an analysis of the SLCO1A2*2, *3, *5, *6, *7 variants, and two more nonsynonymous SNPs (data not shown) [23].
Table 2.
SLC15A1, SLC10A2 and ABCB1 genotypes of the volunteers
| SLC15A1 | SLC10A2 | ABCB1 | |||
|---|---|---|---|---|---|
| rs2274828 | rs4646227 | rs2297322 | rs188096 | rs2032582 | |
| c.1348G>A | c.1256G>C | c.350G>A | c.511T>G | c.2677T>G | |
| Participant | p.Val450Ile | p.Gly419Ala | p.Ser117Asn | p.Ala171= | p.Ser893Ala |
| R002 | G/G | G/G | A/A | G/T | G/G |
| R003 | G/G | C/G | G/G | G/T | G/G |
| R004 | A/G | G/G | A/G | G/T | G/T |
| R005 | G/G | G/G | G/G | G/T | G/G |
| R006 | G/G | G/G | A/G | G/G | T/T |
| R007 | A/G | G/G | G/G | G/G | G/T |
| R008 | G/G | G/G | A/G | G/G | G/G |
| R010 | G/G | G/G | G/G | G/T | G/T |
| R011 | G/G | G/G | A/G | G/T | G/G |
| R012 | G/G | G/G | G/G | G/G | No |
| R013 | G/G | G/G | A/G | G/T | G/T |
| R014 | G/G | G/G | G/G | G/T | G/T |
Systolic and diastolic blood pressures decreased with treatment and recovered to pre-dose levels approximately 24 h after drug treatment was discontinued. When comparing each period using repeated-measures analysis of variance (anova), apple juice had an effect on systolic blood pressure but not on diastolic blood pressure. For systolic blood pressure, the difference between water and apple juice 1200 ml phases was only 2.8 mmHg. It was therefore considered not to have clinical meaning (Figure 4). Treatment-related adverse events were all mild in intensity, with diarrhoea being the most frequently reported (12 of 33 total adverse events).
Figure 4.
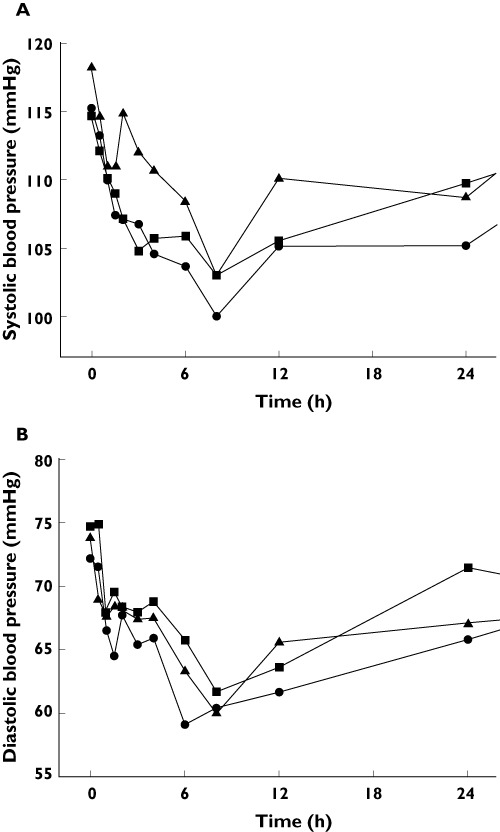
Mean systolic (A) and diastolic (B) blood pressure (mmHg) after atenolol administration.  , water;
, water;  , apple juice 600 ml;
, apple juice 600 ml;  , apple juice 1200 ml
, apple juice 1200 ml
Discussion
Membrane transporters, which are mainly expressed in the intestines, can affect the uptake process of many endogenous and exogenous anions, including many small molecular drugs. Because the transport of β-adrenoceptor blockers across the intestinal epithelium may be mediated by the solute carrier OATP2B1, we evaluated the effect of apple juice and genetic polymorphisms of SLCO2B1, the gene that encodes OATP2B1 [18, 24]. In a previous study, the ingestion of orange juice three times a day for 4 days decreased the systemic exposure to atenolol by 40% compared with administration of the drug with water [32]. In the present study, the ingestion of 600 ml and 1200 ml apple juice reduced the systemic exposure to atenolol in a dose-dependent manner. To the best of our knowledge, this is the first study to report that the AUC of atenolol decreased by 82% after the ingestion of 1200 ml apple juice. Due to the apple juice–atenolol interaction observed in patients consuming either 600 or 1200 ml apple juice, no obvious changes in pharmacodynamic outcomes were observed in healthy volunteers.
With minimal biotransformation and without prolonging the t1/2 of atenolol, apple juice seems to affect intestinal drug uptake rather than the efflux transporters [28]. Although fruit juices are known to be associated with diminished oral bioavailability through the inhibition of intestinal uptake transporters, there was no obvious effect of SLCO2B1 genotypes on the pharmacokinetics of atenolol, in contrast to the results reported for celiprolol and fexofenadine [17, 18]. Therefore, we expect that the minimal effect of SLCO2B1 on transport activity was due to the substrate specificity of OATP2B1. One possible mechanism of the reduced exposure to atenolol is a potential indirect effect on OATP function resulting from enhanced intestinal fluid volume due to the non-specific osmotic effects of solutes in apple juice [11]. The pharmacokinetic parameters of atenolol in the apple juice phase displayed less inter-individual variability than those in the water phase, regardless of the SLCO2B1 genotype that was present. The temporary ingestion of excessive amounts of sugar water may alter OATP function or the acidity of the gastrointestinal environment, which can influence the absorption of a drug [24]. Therefore, further evaluation regarding the effects of variable pH and the amount of apple juice ingested on the transport activity of OATPs ex vivo is needed to confirm this hypothesis.
In previous studies, systemic exposure to aliskiren, fexofenadine and montelukast, which are substrates of OATP2B1 or other uptake transporters, were decreased by the ingestion of grapefruit juice and apple juice [10, 33, 34]. The molecular weight of atenolol (266.3) is less than that of fexofenadine (501.7), aliskiren (551.8) and montelukast (586.2). The smaller size would allow for paracellular absorption and transport through other membrane carriers as well [35].
Another possible mechanism for decreased atenolol concentrations with the co-administration of apple juice relies on the indirect effect on uptake transporters mediated by the apple juice itself. For example, atenolol may form a molecular complex with apple juice in the intestine [11, 36]. In the present study we conducted a candidate gene approach as part of a pharmacogenomic study to examine the effect of SLCO2B1 c.1457C>T and apple juice on the pharmacokinetics of atenolol. Further research is needed to confirm which transporters are related to the absorption of atenolol.
Decreases in mean systolic and diastolic blood pressures were observed at all time points after atenolol administration. The intervals of change did not differ significantly among the various treatment conditions. Notably, the results of this study are limited by the relatively small sample size and all participating subjects were healthy volunteers.
In conclusion, our results indicate that apple juice ingestion markedly reduced the systemic exposure to atenolol, but genetic variations in SLCO2B1 are unlikely to contribute substantial variability to the pharmacokinetics of atenolol. Further research is needed to identify the specific mechanism relating apple juice ingestion and its influence on the systemic exposure to atenolol.
Acknowledgments
This work was supported by a grant of the National Project for Personalized Genomic Medicine, Ministry for Health & Welfare, Republic of Korea (A111218-PG01). We thank Ms Hwa-Suk Kim and Geum-Gwa Ryu for conducting the quantification of atenolol and genotyping. We also acknowledge Ms Jong-Hwa Lee for supporting the clinical conduct of the study.
Hyewon Jeon and SeungHwan Lee received a training programme grant from the Korea National Enterprise for Clinical Trials (KoNECT, A070001).
Competing Interests
The authors do not have any conflicts of interest to disclose.
REFERENCES
- 1.Blackburn DF, Lamb DA, Eurich DT, Johnson JA, Wilson TW, Dobson RT, Blackburn JL. Atenolol as initial antihypertensive therapy: an observational study comparing first-line agents. J Hypertens. 2007;25:1499–505. doi: 10.1097/HJH.0b013e328136bd21. [DOI] [PubMed] [Google Scholar]
- 2.Tabacova SA, Kimmel CA. Atenolol: pharmacokinetic/dynamic aspects of comparative developmental toxicity. Reprod Toxicol. 2002;16:1–7. doi: 10.1016/s0890-6238(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 3.Navare HA, Frye RF, Cooper-Dehoff RM, Shuster JJ, Hall K, Schmidt SO, Turner ST, Johnson JA. Atenolol exposure and risk for development of adverse metabolic effects: a pilot study. Pharmacotherapy. 2010;30:872–8. doi: 10.1592/phco.30.9.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAinsh J. Clinical pharmacokinetics of atenolol. Postgrad Med J. 1977;53(Suppl. 3):74–8. [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Wan SH, Koda RT, Maronde RF. Pharmacokinetics, pharmacology of atenolol and effect of renal disease. Br J Clin Pharmacol. 1979;7:569–74. doi: 10.1111/j.1365-2125.1979.tb04644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald JD, Ruffin R, Smedstad KG, Roberts R, McAinsh J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. Eur J Clin Pharmacol. 1978;13:81–9. doi: 10.1007/BF00609750. [DOI] [PubMed] [Google Scholar]
- 8.Blomqvist I, Westergren G, Sandberg A, Jonsson UE, Lundborg P. Pharmacokinetics and pharmacodynamics of controlled-release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33(Suppl.):S19–24. doi: 10.1007/BF00578408. [DOI] [PubMed] [Google Scholar]
- 9.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70:645–55. doi: 10.1111/j.1365-2125.2010.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 12.Lilja JJ, Backman JT, Laitila J, Luurila H, Neuvonen PJ. Itraconazole increases but grapefruit juice greatly decreases plasma concentrations of celiprolol. Clin Pharmacol Ther. 2003;73:192–8. doi: 10.1067/mcp.2003.26. [DOI] [PubMed] [Google Scholar]
- 13.Lilja JJ, Juntti-Patinen L, Neuvonen PJ. Orange juice substantially reduces the bioavailability of the beta-adrenergic-blocking agent celiprolol. Clin Pharmacol Ther. 2004;75:184–90. doi: 10.1016/j.clpt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–23. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 15.Lilja JJ, Raaska K, Neuvonen PJ. Effects of grapefruit juice on the pharmacokinetics of acebutolol. Br J Clin Pharmacol. 2005;60:659–63. doi: 10.1111/j.1365-2125.2005.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilja JJ, Raaska K, Neuvonen PJ. Effects of orange juice on the pharmacokinetics of atenolol. Eur J Clin Pharmacol. 2005;61:337–40. doi: 10.1007/s00228-005-0930-9. [DOI] [PubMed] [Google Scholar]
- 17.Imanaga J, Kotegawa T, Imai H, Tsutsumi K, Yoshizato T, Ohyama T, Shirasaka Y, Tamai I, Tateishi T, Ohashi K. The effects of the SLCO2B1 c.1457C > T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011;21:84–93. doi: 10.1097/fpc.0b013e32834300cc. [DOI] [PubMed] [Google Scholar]
- 18.Ieiri I, Doi Y, Maeda K, Sasaki T, Kimura M, Hirota T, Chiyoda T, Miyagawa M, Irie S, Iwasaki K, Sugiyama Y. Microdosing clinical study: pharmacokinetic, pharmacogenomic (SLCO2B1), and interaction (grapefruit juice) profiles of celiprolol following the oral microdose and therapeutic dose. J Clin Pharmacol. 2011 doi: 10.1177/0091270011408612. doi: 10.1177/0091270011408612. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Hu M, Li X. Oral Bioavailability : Basic Principles, Advanced Concepts, and Applications. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 20.Fahrmayr C, Fromm MF, Konig J. Hepatic OATP and OCT uptake transporters: their role for drug-drug interactions and pharmacogenetic aspects. Drug Metab Rev. 2010;42:380–401. doi: 10.3109/03602530903491683. [DOI] [PubMed] [Google Scholar]
- 21.Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302:804–13. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- 22.Laitinen A, Niemi M. Frequencies of single-nucleotide polymorphisms of SLCO1A2, SLCO1B3 and SLCO2B1 genes in a Finnish population. Basic Clin Pharmacol Toxicol. 2011;108:9–13. doi: 10.1111/j.1742-7843.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–7. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, Kim RB, Bailey DG, Kirch W. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005;77:291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, King CR, Brower A, Schmelzer JR, Glurich I, Vidaillet HJ, Yale SH, Qi Zhang K, Berg RL, Burmester JK. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111:4106–12. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly TM, Dumaual CM, Miao X, Farmen MW, Njau RK, Fu DJ, Bauer NL, Close S, Watanabe N, Bruckner C, Hardenbol P, Hockett RD. Multiplex assay for comprehensive genotyping of genes involved in drug metabolism, excretion, and transport. Clin Chem. 2007;53:1222–30. doi: 10.1373/clinchem.2007.086348. [DOI] [PubMed] [Google Scholar]
- 27.Ieiri I, Nishimura C, Maeda K, Sasaki T, Kimura M, Chiyoda T, Hirota T, Irie S, Shimizu H, Noguchi T, Yoshida K, Sugiyama Y. Pharmacokinetic and pharmacogenomic profiles of telmisartan after the oral microdose and therapeutic dose. Pharmacogenet Genomics. 2011;21:495–505. doi: 10.1097/FPC.0b013e3283489ce2. [DOI] [PubMed] [Google Scholar]
- 28.Tamai I. Oral drug delivery utilizing intestinal OATP transporters. Adv Drug Deliv Rev. 2012;64:508–14. doi: 10.1016/j.addr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1562–9. doi: 10.1002/ibd.20963. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Song IS, Shin HJ, Kim MH, Choi YL, Lim SJ, Kim WY, Lee SS, Shin JG. Genetic polymorphisms in Na+-taurocholate co-transporting polypeptide (NTCP) and ileal apical sodium-dependent bile acid transporter (ASBT) and ethnic comparisons of functional variants of NTCP among Asian populations. Xenobiotica. 2011;41:501–10. doi: 10.3109/00498254.2011.555567. [DOI] [PubMed] [Google Scholar]
- 31.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Mougey EB, Lang JE, Wen X, Lima JJ. Effect of citrus juice and SLCO2B1 genotype on the pharmacokinetics of montelukast. J Clin Pharmacol. 2011;51:751–60. doi: 10.1177/0091270010374472. [DOI] [PubMed] [Google Scholar]
- 33.Tapaninen T, Neuvonen PJ, Niemi M. Grapefruit juice greatly reduces the plasma concentrations of the OATP2B1 and CYP3A4 substrate aliskiren. Clin Pharmacol Ther. 2010;88:339–42. doi: 10.1038/clpt.2010.101. [DOI] [PubMed] [Google Scholar]
- 34.Tapaninen T, Neuvonen PJ, Niemi M. Orange and apple juice greatly reduce the plasma concentrations of the OATP2B1 substrate aliskiren. Br J Clin Pharmacol. 2011;71:718–26. doi: 10.1111/j.1365-2125.2010.03898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennernas H, Ahrenstedt O, Ungell AL. Intestinal drug absorption during induced net water absorption in man; a mechanistic study using antipyrine, atenolol and enalaprilat. Br J Clin Pharmacol. 1994;37:589–96. doi: 10.1111/j.1365-2125.1994.tb04309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Ghazawi MA, Tutunji MS, AbuRuz SM. The effects of pummelo juice on pharmacokinetics of sildenafil in healthy adult male Jordanian volunteers. Eur J Clin Pharmacol. 2010;66:159–63. doi: 10.1007/s00228-009-0738-0. [DOI] [PubMed] [Google Scholar]


