Abstract
AIM
The aim of this study was to determine the ciprofloxacin serum concentrations in hospitalized patients and to determine which percentage reached the efficacy target of AUC : MIC > 125. Additionally, the influence of demographic anthropomorphic and clinical parameters on the pharmacokinetics and pharmacodynamics of ciprofloxacin were investigated.
METHODS
In serum of 80 hospitalized patients ciprofloxacin concentrations were measured with reverse phase high performance liquid chromatography with fluorescence detection. The ciprofloxacin dose was 400–1200 mg day−1 i.v. in two or three doses depending on renal function and causative bacteria. Pharmacokinetic parameters were calculated with maximum a posteriori Bayesian estimation (MW\PHARM 3.60). A two compartment open model was used.
RESULTS
Mean (± SD) age was 66 (± 17) years, the mean clearance corrected for bodyweight was 0.24 l h−1 kg−1 and the mean AUC was 49 mg l−1 h. Ciprofloxacin clearance and thus AUC were associated with both age and serum creatinine. Of all patients, 21% and 75% of the patients, did not reach the proposed ciprofloxacin AUC : MIC > 125 target with MICs of 0.25 and 0.5 mg l−1, respectively. A computer simulated increase in the daily dose from 800 mg to 1200 mg, decreased these percentages to 1% and 37%, respectively.
CONCLUSION
A substantial proportion of the hospitalized patients did not reach the target ciprofloxacin AUC : MIC and are suboptimally dosed with recommended doses. Taking into account the increasing resistance to ciprofloxacin worldwide, a ciprofloxacin dose of 1200 mg i.v. daily in patients with normal renal function is necessary to reach the targeted AUC : MIC > 125.
Keywords: AUC, ciprofloxacin, elderly, pharmokokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The efficacy target of AUC : MIC > 125 is based on the study of Forrest et al. in 1993.
Recent studies have shown that in ICU patients the ciprofloxacin efficacy target of AUC : MIC > 125 is often not reached.
WHAT THIS STUDY ADDS
The efficacy targets of ciprofloxacin in patients in general wards are often not reached. Most patients have low AUC with current i.v. dosing regimens. We suggest increasing the standard dose of ciprofloxacin to 1200 mg intravenously 24 h–1.
Patients in general wards have high interindividual variability of pharmacokinetic parameters and therapeutic drug monitoring could be useful to support dosing.
Introduction
Ciprofloxacin, a broad-spectrum antimicrobial drug, has been extensively used in inpatient and outpatient settings. The probability of cure of an infection has been shown to be dependent on the 24 h area under the curve (AUC) of ciprofloxacin and the minimal inhibitory concentration (MIC) for specific bacteria [1–5]. The quotient AUC : MIC needed for a high probability of cure varies in different studies. AUC : MIC > 125 was found to be predictive of microbiological and clinical cure in patients with serious Gram-negative infections and in Intensive Care Unit (ICU) patients [3, 5]. Microbiological cure was reached in 86% above and in 26% below this breakpoint and clinical cure in 82% above and in 42% below this breakpoint [5]. Using a clinical outcome-based Monte Carlo simulation, it was shown that in patients with Enterobacteriaceae bacteraemia an AUC : MIC > 250 was associated with cure rates of 91% in patients with values above and of 29% below this breakpoint [4]. Moreover in nosocomial pneumonia dose alterations based on plasma drug concentration and bacterial MIC value improved the probability of good clinical outcome and pathogen eradication [6].
In the nineties, it was shown that 80% of treatment failures during ciprofloxacin treatment were due to drug resistance [7]. The MIC predicted clinical response, especially in patients with infections caused by organisms for which the MICs were at the marginal points of susceptibility. Development of resistance to ciprofloxacin was largely confined to marginally susceptible organisms such as Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa. Recently Enterobacteriaceae were added to this marginally susceptible group based on a Monte Carlo simulation [4]. Both MIC and resistance for ciprofloxacin are steadily increasing. In the Netherlands ciprofloxacin resistance increased from 1 to 15% in Escherichia coli and from 1 to 13% in Klebsiella pneumoniae from 2001 until 2009, and in susceptible bacteria MICs increased [8, 9]. In Greece ciprofloxacin resistance for E. coli increased from 9% in 2001 to 23% in 2009 and ciprofloxacin resistance for K. pneumoniae increased from 54% to 66% from 2005 to 2009% [10].
With increasing MICs, the targeted AUC : MIC > 125 may be less often reached, resulting in increasing ciprofloxacin resistance and higher clinical failure rates. Indeed, Perreiter et al. calculated with a population model from the literature that 66% reached the minimum AUC : MIC target of >100 [11], Neef et al. found in 2002 from an in vitro model that 1200 mg was the optimal dose to be given if the MIC was 0.25 mg l−1[12] and recent studies on critically ill patients on ICUs have shown that the AUC : MIC target of >125 is reached in only 31–84% of the patients [1, 3]. In these studies AUC of ciprofloxacin was usually low, respectively 42 ± 36 [1] and 57 ± 33.5 mg l−1 h [3]. In a recent study in 25 critically ill patients with chronic obstructive pulmonary disease low AUC values and high variability of kinetic parameters were found with ciprofloxacin 1200 mg [13]. However, ciprofloxacin is also widely used in patients admitted to the general hospital wards and only sparse data regarding ciprofloxacin AUC : MIC are available in this particular population. Therefore, we have measured ciprofloxacin serum concentrations in patients admitted to such wards that were given ciprofloxacin intravenously (i.v.) and evaluated the AUC : MIC ratio. Additionally, the influence of demographic anthropomorphic and clinical parameters on the pharmacokinetics and pharmacodynamics of ciprofloxacin were investigated.
Methods
Study design
Patients above 18 years of age with i.v. ciprofloxacin treatment hospitalized at the Maastricht University Medical Centre (MUMC), a 715 bed university hospital, admitted to general wards were included from April 2009 until April 2010. Ciprofloxacin serum concentrations were measured in samples drawn for routine assays. Patients with minimal two serum concentrations available were included. Ciprofloxacin Kabi (Fresenius Kabi, Bad Homburg, Germany) was started at the discretion of the attending physician, usually when an infection was suspected or cultured pathogens proved susceptible to ciprofloxacin. Ciprofloxacin was dosed from 400 to 1200 mg day−1 in two or three doses; the standard dose was 800 mg day−1, 400 mg day−1 was prescribed when the creatinine clearance was less than 30 ml min−1 and 1200 mg day−1 was prescribed for patients with a P. aeruginosa infection and normal renal function. A solution for infusion of 2 mg ml−1 (400 mg 200 ml–1 and 200 mg 100 ml–1) was used. The 400 mg 200 ml−1 infusion was run in 60 min and the 200 mg 100 ml−1 was run in 30 min. Demographic and clinical data, i.e. age, gender, weight, co-medication, ciprofloxacin dose, times of ciprofloxacin doses and blood collections, serum creatinine, admission days, C-reactive protein (CRP) and culture results were retrieved from the electronic patient file (iSoft, the Netherlands). Creatinine clearance was calculated with the Modification of Diet in Renal Disease formula (MDRD). Cultures were performed and ciprofloxacin MICs were determined as standard clinical care with the Becton Dickinson Phoenix™ Automated Microbiology System (Franklin Lakes, New Jersey, USA) using the ID/AST Combo panels UNMIC/ID53 and NMIC/ID75 for Gram negative bacteria and the AST panel PMIC-58 for Gram positive bacteria. Clinical outcome was defined by CRP decrease, number of admission days and clinical cure (defeveresence, microbiological cure and discharge from the hospital).
This study was registered at the Dutch Trial Register (NTR 1725) and was approved by the Medical Ethics Committee of the MUMC (MEC 08-4-063).
Hplc analysis
A simple, fast and specific method for measuring ciprofloxacin serum concentrations has been validated for linearity, precision, accuracy and stability, following the guidelines for industry bioanalytical method validation recommended by the Food and Drug Administration (FDA) [14]. In short, serum samples were precipitated with acetonitrile. A reverse phase high pressure liquid chromatography method with fluorescence detection was used (excitation and emission wave lengths for ciprofloxacin were 278 and 440 nm, respectively). The calibration range was 0.5 to 10.0 mg l−1. Three quality controls (0.8, 4 and 8 mg l−1) were tested. The intra- and inter-assay variability was within 7.5%. The lower limit of quantification was 0.2 mg l−1. The freeze-and-thaw, the short term, long term, the stock solution and post preparation stability were all determined and adequate. The extraction recovery was 82%, 87% and 88% at, respectively, 0.8, 4 and 8 mg l−1[15].
Pharmacokinetic analysis
Pharmacokinetic parameters of ciprofloxacin in individual patients were calculated with maximum a posteriori Bayesian estimation program (MW/PHARM 3.60, Mediware, the Netherlands). We used a two compartment open pharmacokinetic model based on a previous clinical study [5]. The AUC was calculated from 48 to 72 h after start of therapy, assuming a steady-state was reached.
Statistical analysis
Metric variables were tested for normality of distribution by the Shapiro-Wilk test. If normally distributed, means and SDs are used to characterize the univariate distributions. If not, score ranges are also given. Categorical variables are presented as frequencies and percentages. Scores for main outcome parameter (CL weight−1 of ciprofloxacin) turned out to be non-normally distributed and had to be log10 transformed to perform valid parametric tests. Univariate analysis on log10 CL weight−1 with categorical variables is either done by Student's t-test or by one way anova (using the Bonferroni correction for polychotomous categories). Pearson correlation coefficients (r) for this outcome with the dichotomous predictor (gender) and metric variables are also presented. All data analysis was done with SPSS-pc version 16.0. A P value of <0.05 was considered to be statistically significant.
Results
Study group
A total of 80 patients with a median of 4 (range 2−7) blood samples were included. The average age was 66 years and 60% were male (Table 1). One third was diagnosed as having pneumonia and 10% had sepsis (Table 1). Patients were admitted throughout all wards of the hospital. Co-medication did not interfere with pharmacokinetic parameters of ciprofloxacin (data not shown).
Table 1.
Patient characteristics of 80 hospitalized patients
| Median (range) | |
|---|---|
| Age (years) | 68 (19–101) |
| Weight (kg) | 70 (32–110) |
| Ciprofloxacin i.v. (days) | 8 (2–45) |
| Admission (days) | 20 (5–122) |
| CRP before therapy (mg l−1) | 203 (1–469) |
| CRP after therapy (mg l−1) | 67 (3–405) |
| Gender | Frequency (percentage) |
| Male | 48 (60%) |
| Female | 32 (40%) |
| Infection | Frequency (percentage) |
| Pneumonia | 26 (33%) |
| Wound infection | 18 (23%) |
| UTI | 18 (23%) |
| Sepsis | 8 (10%) |
| Abdominal infection | 6 (6%) |
| Other | 4 (5%) |
| Ciprofloxacin combination with | Frequency (percentage) |
| Monotherapy | 18 (23%) |
| ß-lactam antibiotics | 45 (56%) |
| Clindamycin | 9 (11%) |
| Other | 8 (10%) |
| Comedication | Frequency (percentage) |
| None | 13 (16%) |
| Cardiovascular | 35 (43%) |
| Immunosupressive medication | 20 (25%) |
| Diabetes mellitus | 10 (13%) |
| Other | 15 (19%) |
UTI, urinary tract infection.
At least one culture was taken from all patients: 21 urine cultures, 20 blood cultures, 15 sputum cultures, 15 wound cultures and nine cultures of drains and/or abdominal fluid. Forty-five of the 80 patients (58%) had a positive culture. Of the positive cultures 35 (78%) were Gram-negative bacteria: E. coli (n= 12), Klebsiella spp. (n= 10), Enterobacteriaceae (n= 6), Proteus spp. (n= 4) and P. aeruginosa (n= 3). In urine cultures MICs were not determined lower than 0.5 mg l−1 by the UNMIC/ID53 panel (standard clinical care). Therefore MIC values were available in 27/35 (77%) in patients with a Gram negative bacterium cultured. Gram positive bacterium were cultured in 10/80 patients (12%): S. aureus 4/80 (5%), Enterococcus spp. 4/80 (5%), CNS 1/80 (1%) and S. pneumoniae 1/80 (1%).
Pharmacokinetic and pharmacodynamic analysis
The average (SD) CL weight−1 was 0.24 (0.13) l h−1 kg−1, (median 0.23, range 0.04−0.57 l h−1 kg−1), the average (SD) volume of distribution corrected for weight (V weight−1) was 2.5 (0.9) l kg−1, (median 2.5, range 0.9−5.9 l kg−1), average (SD) AUC was 49 (23) mg l−1 h, (median 43, range15–128 mg l−1 h), the average (SD) creatinine was 148 (125) µmol l−1, median 91, range 40–625 µmol l−1) and 23 (29%) of the patients had a 400 mg dose, one (1%) a dose of 600 mg, 51 (64%) a 800 mg dose and five (6%) one 1200 mg dose. The interindividual variability of pharmacokinetic parameters was large (Table 2). Patients above 80 years had lower CL weight−1 (P= 0.001) and higher AUC compared with younger patients (P= 0.04) (Table 2). The ciprofloxacin efficacy target (AUC : MIC > 125) was reached in 100% of the patients with the current dosing regimens when Gram negative bacteria have a MIC ≤ 0.125 µg ml−1, 79% of the patients reached this target with an MIC ≤ 0.25 mg l−1, 25% with an MIC ≤ 0.5 mg l−1 and only 1% of the patients reached this target when the MIC ≥ 1 mg l−1 (Figure 1). In our study, 16/27 (59%) of all Gram negative bacteria had a MIC ≤ 0.125 mg l−1, 3/27 (11%) had a MIC of 0.5 mg l−1 and 8/27 (30%) had a MIC of 2 mg l−1.
Table 2.
CLcr, CL weight−1, V weight−1 and AUC broken down for ciprofloxacin 24 h dose categories and age group
| CLcr (ml min−1) | CL weight−1 (l h−1 kg−1) | V weight−1 (l kg−1) | AUC (mg l−1 h) | ||
|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | ||
| n | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| 24 h dose (mg) | |||||
| 400–600 | 24 | 24 (8–184) | 0.15 (0.06–0.45) | 2.4 (0.9–4.4) | 39 (15–78) |
| 39 (43) | 0.17 (0.09) | 2.3 (0.8) | 41 (16) | ||
| 800 | 51 | 81 (12–162) | 0.25 (0.04–0.51) | 2.5 (1.2–5.9) | 45 (23–128) |
| 81 (39) | 0.26 (0.11) | 2.6 (1.0) | 52 (25) | ||
| 1200 | 5 | 131 (37–173) | 0.52 (0.12–0.57) | 2.7 (1.3–4.4) | 44 (29–89) |
| 113 (52) | 0.39 (0.21) | 2.9 (1.2) | 53 (27) | ||
| Age group (years) | |||||
| <70 | 41 | 79 (12–184) | 0.27 (0.07–0.57) | 2.1 (1.3–4.4) | 38 (15–125) |
| 85 (49) | 0.29 (0.13) | 2.6 (0.7) | 43 (20) | ||
| 70–80 | 24 | 55 (8–131) | 0.20 (0.04–0.45) | 2.4 (1.0–6.0) | 47 (23–96) |
| 58 (40) | 0.21 (0.11) | 2.4 (1.2) | 52 (22) | ||
| >80 | 15 | 37 (12–119) | 0.13 (0.06–0.37) | 2.4 (0.9–5.7) | 63 (24–128) |
| 51 (33) | 0.16 (0.09) | 2.3 (1.1) | 59 (27) |
CLcr, creatinine clearance; CL weight−1, ciprofloxacin clearance corrected for bodyweight; V weight−1, volume of distribution corrected for bodyweight; AUC, area under the curve in 24 h.
Figure 1.
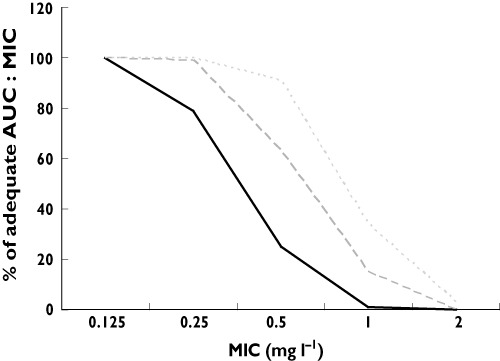
Calculated percentage of patients with an adequate AUC : MIC ratio > 125 at MIC 0.125, 0.25, 0.5, 1.0 and 2.0 mg l−1 for increasing ciprofloxacin dosages based on the pharmacokinetics obtained at current dosage regimens.  , 800 mg;
, 800 mg;  , 1200 mg;
, 1200 mg;  , 1600 mg
, 1600 mg
Analysis of influence of covariates on pharmacokinetic and pharmacodynamic parameters
To determine which covariates have an effect on pharmacokinetic and pharmacodynamic parameters of ciprofloxacin, a univariate analysis was done using a predetermined set of predictors (Table 3). In the univariate analysis CL weight−1 was related to age, gender, V weight−1 and creatinine (Table 3). No significant associations were found between AUC : MIC (and AUC) and CRP decrease or increase, admission days and clinical outcome (data not shown).
Table 3.
Univariate Pearson correlation coefficients between ciprofloxacin, log10 CL weight−1 and predictors used in this study
| Univariate 10log CL weight−1 | ||
|---|---|---|
| r | P value | |
| Creatinine | −0.547 | <0.001 |
| Age | −0.468 | <0.001 |
| Gender | −0.165 | 0.072 |
| V weight−1 | 0.416 | <0.001 |
| 24 h dose/1000 | 0.453 | <0.001 |
CL weight−1, ciprofloxacin clearance corrected for bodyweight. V weight−1, volume of distribution corrected for bodyweight.
Dosing simulations
To determine whether increasing the dose of ciprofloxacin would lead to sufficiently high AUC : MIC values the AUC was calculated with MW/PHARM 3.60 for all patients. When increasing the daily dose of all patients by 400 mg (i.e. 1200 mg 24 h−1 in patients with normal renal function), 99% reached the AUC : MIC target with a MIC ≤ 0.25 mg l−1 and 63% reached the AUC : MIC target with a MIC ≤ 0.5 mg l−1. When increasing the daily dose of all patients by 800 mg (i.e. 1600 mg 24 h−1 in patients with normal renal function), 100% reached the AUC : MIC target with a MIC ≤ 0.25 mg l−1 and 91% reached the AUC : MIC target with a MIC ≤ 0.5 mg l−1 (Figure 1).
Discussion
This study shows that the majority of hospitalized patients using ciprofloxacin have low AUC with current i.v. dose regimens. A substantial number (21–75%) of hospitalized patients on general wards did not reach the target AUC : MIC ratio when the MIC is between 0.25−0.5 mg l−1. A ciprofloxacin daily dose of 1200 mg may be more effective, but fear for adverse drug events (ADEs) might hamper the use of this dosage. In general ciprofloxacin is considered safe and is well tolerated [16–18], even in higher doses [5, 19]. Most common ADEs are mild; gastrointestinal reactions (nausea and diarrhoea), but CNS toxicity (anxiety, restlessness and seizures) occurs, as do skin reactions and hepatotoxicity [16–18, 20]. However, even at high dose seizures are rarely reported [18]. The pharmacodynamic better dose of 1600 mg day−1 cannot yet be recommended, although to our knowledge no increase in frequency or severity of ADEs has been described. The variability in pharmacokinetic parameters is high, therefore dosing should be optimized individually. The ciprofloxacin dose is adjusted to the individual renal clearance to prevent high ciprofloxacin concentrations. However, other covariates, such as age also influence the ciprofloxacin clearance independently. Adjusting for all co-variates might not be feasible and therapeutic drug monitoring (TDM) could further prevent high and low ciprofloxacin serum concentrations and could be useful in reaching the target AUC : MIC ratio. Although TDM for ciprofloxacin has been advocated [1, 3–6], it is not the standard of care in most hospitals. Taken together, with increasing MICs of Gram negative bacteria, higher daily doses of ciprofloxacin seem necessary and feasible with regard to ADEs and TDM may be the appropriate tool to decrease development of ciprofloxacin resistance.
A limitation of this study is that no effect of low AUC : MIC ratio on clinical outcome could be demonstrated. Although cultures were taken in all patients, in only 44% Gram negative bacteria could be cultured. Furthermore, the group that used ciprofloxacin monotherapy was small. A different study and much larger numbers of patients are needed for this endpoint. On the other hand, low AUCs were shown in our study and low AUC : MIC ratios and worse clinical outcome has been shown in several studies [4–6].
In conclusion, the majority of hospitalized patients did not reach the target AUC : MIC ratio with the current iv doses. Taking into account the increasing resistance for ciprofloxacin worldwide, TDM and subsequent dose adjustment may decrease development of ciprofloxacin resistance. A large randomized clinical trial of ciprofloxacin treatment is needed to confirm the AUC : MIC > 125 or higher AUC : MIC ratios (>250) are needed for good clinical and microbiological outcome.
Acknowledgments
We acknowledge the support provided by department of Clinical Chemistry and the Pharmacy laboratory. We also acknowledge the assistance of Dr Bekers and the excellent technical assistance of Jeroen Welzen, Inez Widow and Pauline Vinken.
This work was supported by the Care and Public Health Research Institute (CAPHRI), Maastricht, the Netherlands and the Medical University Centre Maastricht, the Netherlands.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Conil JM, Georges B, de Lussy A, Khachman D, Seguin T, Ruiz S, Cougot P, Fourcade O, Houin G, Saivin S. Ciprofloxacin use in critically ill patients: pharmacokinetic and pharmacodynamic approaches. Int J Antimicrob Agents. 2008;32:505–10. doi: 10.1016/j.ijantimicag.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Schentag JJ, Nix DE, Adelman MH. Mathematical examination of dual individualization principles (I): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP. 1991;25:1050–7. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 3.van Zanten AR, Polderman KH, van Geijlswijk IM, van der Meer GY, Schouten MA, Girbes AR. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care. 2008;23:422–30. doi: 10.1016/j.jcrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Zelenitsky SA, Ariano RE. Support for higher ciprofloxacin AUC 24/MIC targets in treating Enterobacteriaceae bloodstream infection. J Antimicrob Chemother. 2010;65:1725–32. doi: 10.1093/jac/dkq211. [DOI] [PubMed] [Google Scholar]
- 5.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–81. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaglione F, Esposito S, Leone S, Lucini V, Pannacci M, Ma L, Drusano GL. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur Respir J. 2009;34:394–400. doi: 10.1183/09031936.00149508. [DOI] [PubMed] [Google Scholar]
- 7.Fish DN, Piscitelli SC, Danziger LH. Development of resistance during antimicrobial therapy: a review of antibiotic classes and patient characteristics in 173 studies. Pharmacotherapy. 1995;15:279–91. [PubMed] [Google Scholar]
- 8.Oudhuis GJ, Verbon A, Hoogkamp-Korstanje JA, Stobberingh EE. Antimicrobial resistance in Escherichia coli and Pseudomonas aeruginosa from Intensive Care Units in The Netherlands, 1998-2005. Int J Antimicrob Agents. 2008;31:58–63. doi: 10.1016/j.ijantimicag.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Nethmap. 2010. Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands.
- 10.ECDC. 2009. European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe in 2009.
- 11.Perreiter AP, Nix DE, Matthias KR. Appropriateness of ciprofloxacin dosing based on a population pharmacokinetic model. Hosp Pharm. 2010;45:237–43. [Google Scholar]
- 12.Neef C, van Gils SA, IJzerman WL. Analogy between temperature-dependent and concentration-dependent bacterial killing. Comput Biol Med. 2002;32:529–49. doi: 10.1016/s0010-4825(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 13.Kontou P, Chatzika K, Pitsiou G, Stanopoulos I, Argyropoulou-Pataka P, Kioumis I. Pharmacokinetics of ciprofloxacin and its penetration into bronchial secretions of mechanically ventilated patients with chronic obstructive pulmonary disease. Antimicrob Agents Chemother. 2011;55:4149–53. doi: 10.1128/AAC.00566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. 2001. Guidance for industry. Bioanalytical method validation. U.S. Department of Health and Food Science, Rockville, MD, USA, Food and Drug Administration (FDA)
- 15.Haeseker MB, Verbon A, Welzen J, Neef C, Bruggeman CA, Stolk LML. A simple and rapid RP-HPLC method to determine ciprofloxacin level In human serum. Asian J Pharm Biol Res. 2011;10092011:350–4. [Google Scholar]
- 16.Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;1:193–209. doi: 10.2165/11531490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Bertino J, Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798–817. doi: 10.1016/S0149-2918(00)80053-3. discussion 797. [DOI] [PubMed] [Google Scholar]
- 18.Stahlmann R. Safety profile of the quinolones. J Antimicrob Chemother. 1990;26:31–44. doi: 10.1093/jac/26.suppl_d.31. [DOI] [PubMed] [Google Scholar]
- 19.Lipman J, Scribante J, Gous AG, Hon H, Tshukutsoane S. Pharmacokinetic profiles of high-dose intravenous ciprofloxacin in severe sepsis. The Baragwanath Ciprofloxacin Study Group. Antimicrob Agents Chemother. 1998;42:2235–9. doi: 10.1128/aac.42.9.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alan C, Kocoglu H, Ersay AR, Ertung Y, Kurt HA. Unexpected severe hepatotoxicity of ciprofloxacine: two case reports. Drug Chem Toxicol. 2011;34:189–91. doi: 10.3109/01480545.2010.495392. [DOI] [PubMed] [Google Scholar]


