Abstract
Adenovirus (Ad) vaccine vectors have been used for many applications due to the capacity of the Ad capsid proteins to evoke potent immune responses, but these vectors are often ineffective in the context of pre-existing anti-Ad immunity. Leveraging the knowledge that E1−E3− Ad gene transfer vectors are potent immunogens, we have developed a vaccine platform against small molecules by covalently coupling analogs of small molecules to the capsid proteins of disrupted Ad (dAd5). We hypothesized that the dAd5 platform would maintain immunopotency even in the context of anti-Ad neutralizing antibodies. To test this hypothesis, we coupled cocaine and nicotine analogs, GNE and AM1, to dAd5 capsid proteins to generate dAd5GNE and dAd5AM1, respectively. Mice were pre-immunized with Ad5Null, resulting in high titer anti-Ad5 neutralizing antibodies comparable to those observed in the human population. The dAd5GNE and dAd5AM1 vaccines elicited high anti-cocaine and anti-nicotine antibody titers, respectively, in both naive and Ad5-immune mice, and both functioned to prevent cocaine or nicotine from reaching the brain of anti-Ad immune mice. Thus, disrupted Ad5 evokes potent humoral immunity that is effective in the context of pre-existing neutralizing anti-Ad immunity, overcoming a major limitation for current Ad-based vaccines.
De and colleagues examine the efficacy of adenovirus-based vaccines against cocaine and nicotine. They report that each respective vaccine elicits high anti-cocaine or anti-nicotine antibody titers in mice irrespective of preexisting neutralizing anti-adenovirus immunity.
Introduction
Human adenovirus (Ad) gene transfer vectors were initially used as a vehicle to transfer genes in vivo (Rosenfeld et al., 1991; Jaffe et al., 1992; Rosenfeld et al., 1992; Davidson et al., 1993; Li et al., 1993). While highly effective, it was soon recognized that the Ad capsid was very immunogenic (Wilson, 1995; Tatsis and Ertl, 2004), and the resulting anti-Ad response limited the duration of gene expression and prevented effective readministration of the vectors (Setoguchi et al., 1994; Yang et al., 1994; Dai et al., 1995; Gilgenkrantz et al., 1995; van Ginkel et al., 1995; Yang et al., 1995; Harvey et al., 1999a; Harvey et al., 1999b). This property of the Ad capsid led to the use of Ad vectors for vaccines where the protein/peptide target of the vaccine was expressed as a transgene, incorporated into the sequence of a capsid gene, or exogenously attached to the capsid (Shiver et al., 2002; Baez-Astua et al., 2005; Hashimoto et al., 2005; Worgall et al., 2005; Chiuchiolo et al., 2006; Matthews et al., 2010; Palma et al., 2011). However, while highly effective in Ad-naive recipients, these Ad5-based vaccines are usually ineffective in the presence of pre-existing anti-Ad immunity, a common occurrence in the human population (D'Ambrosio et al., 1982; Piedra et al., 1998; Nwanegbo et al., 2004; Mast et al., 2010). One solution is to use the Ad capsid proteins, which we hypothesize would maintain immunopotency even in the context of pre-existing anti-Ad neutralizing antibodies, instead of live, intact Ad vectors.
We have recently extended the concept of the Ad-based vaccine to use the Ad capsid as a platform for vaccines against small addictive molecules by coupling the haptenic representation of the addictive molecule to the capsid proteins of a disrupted E1−E3− serotype 5 adenovirus (Hicks et al., 2011; Wee et al., 2012). Because these molecules are small, they are not normally recognized by the immune system and must be coupled to a protein to induce immunity against the addictive molecule (Carrera et al., 1995; Fox et al., 1996; Kinsey et al., 2009). Using cocaine as a model for addictive small molecules, we demonstrated that covalent coupling of the cocaine haptens GNC or GNE to the disrupted Ad5 capsid results in a highly effective anti-cocaine vaccine (Hicks et al., 2011; Wee et al., 2012).
Because this vaccine platform is based on Ad capsid proteins rather than live, intact Ad vectors, we hypothesized that they would maintain immunopotency even in the context of pre-existing anti-Ad neutralizing antibodies. As a test of this hypothesis, we assessed two vaccines based on the disrupted Ad5 platform, one against cocaine and one against nicotine, and compared the ability of these vaccines to evoke functional anti-cocaine or anti-nicotine immunity in naive and anti-Ad5 immunized mice. The anti-cocaine vaccine was constructed by covalently coupling the cocaine hapten GNE to the disrupted Ad5 capsid proteins, and the anti-nicotine vaccine was constructed by covalently coupling the nicotine hapten AM1 to the disrupted Ad5 capsid proteins (Moreno et al., 2010; Wee et al., 2012). In contrast to the inability of live Ad vector vaccines to function in the context of anti-Ad immunity, both of the dAd5GNE and dAd5AM1 vaccines functioned equally well in naive mice as well as those pre-immunized with Ad5.
Methods
dAd5GNE and dAd5AM1 vaccines
A recombinant serotype 5 E1a−, partial E1b−, E3− Ad vector (“Ad5”) with either β-galactosidase, luciferase, or nontranslatable polylinker in the expression cassette in the E1− region was propagated and purified to produce Ad5LacZ, Ad5Luc, or Ad5Null, respectively (Jaffe et al., 1992; Rosenfeld et al., 1992; Sprangers et al., 2003). Disruption of Ad5LacZ vector was carried out (56°C, 45 sec) in 0.5% sodium dodecyl sulfate. The cocaine analog GNE (6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carboxoamido-hexanoic acid) or the nicotine analog AM1 (rac 6-((trans-1-methyl-2-(pyridin-3-yl)pyrrolidin-3-yl)methoxy)hexanoic acid), 300 μg each, were activated overnight at 4°C after the addition of 7.2 μl charging solution, made by dissolving 2.4 mg of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and 2 mg of N-hydroxysulfosuccinimide in 4 μl H2O and 40 μl dimethylformamide (Hicks et al., 2011). Conjugation of 200 μg of disrupted Ad vector (“dAd5”) with charged cocaine or nicotine analog (300:1 hapten to Ad capsomere molar ratio) was carried out by incubating overnight at 4°C in 200 μl of phosphate-buffered saline (PBS), pH 7.4. The conjugated dAd5-based vaccines were purified from excess unreacted small molecules by dialysis against 100 mM Tris-HCl buffer (pH 7.8) containing 20% sucrose. The amount of Ad vector protein was quantified by the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL).
Western analysis
Polyclonal antibody to cocaine and nicotine were produced using the haptens conjugated separately to keyhole limpet hemocyanin (KLH) at a ratio of 2:1 (Carrera et al., 1995) and 0.1 mg formulated in Freund's adjuvant administered intramuscularly to BALB/c mice (CFA; Sigma-Aldrich, St. Louis, MO). Sera derived from 10-week bleeds were used for Western analysis of the conjugated dAd5GNE and dAd5AM1. Each dAd5 construct was resolved on a 4 to 12% gradient polyacrylamide sodium dodecyl sulfate (SDS) gel under reducing conditions and transferred to a polyvinylidene fluoride (PVDF) membrane, probed with the corresponding anti-drug sera or, to assess for the anti-Ad capsid components, anti-adenovirus antibody (Abcam, Cambridge, MA). The membranes were developed with horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and enhanced chemiluminescence (ECL) reagent (GE Healthcare, Piscataway, NJ).
Immunization with Ad5 vector and assessment of anti-Ad5 titers
Female BALB/c mice were immunized by three intramuscular injections, 4 weeks apart, of Ad5Null (2×1010 particle units) in 50 μl of PBS. Neutralizing antibody titers were determined by an in vitro assay with A549 cells in 96-well plates. Ad5LacZ (3×106 particle units; lacZ- β-galactosidase) was incubated with serial dilutions of sera from Ad5Null administered mice or naive control serum at 37°C for 45 min and then used to infect cells at a multiplicity of infection of 100. At 48 hours post-infection, β-galactosidase activity was assayed (Stratagene, La Jolla, CA). The neutralizing antibody titer was expressed as the reciprocal of serum dilution at which 50% inhibition of Ad5LacZ was observed. All animal studies were conducted under protocols reviewed and approved by the Weill Cornell Institutional Animal Care and Use Committee.
Immunization with dAd5 conjugate vaccines
The Ad5-immune and naive control mice were immunized by intramuscular injection to the quadriceps with 4 μg of dAd5 conjugate vaccines (dAd5GNE or dAd5AM1) in 50 μl volume, formulated in 20% Adjuplex™ (Advanced BioAdjuvants, LLC, Omaha, NE) at 3 and 6 weeks. Quadriceps muscle was located by palpating the anterior portion of the femur and was used for both the prime and boost vaccine administrations. Blood was collected from the transected tail vein, allowed to clot, centrifuged at 10,000 g for 20 min, and the resulting serum was stored at −20°C. To assess the effectiveness of the anti-Ad5 neutralizing antibodies generated by immunization with Ad5Null in vivo, Ad5Luc (5×1010 particle units) was administered intravenously to the Ad5-immune or naive control mice, and 3 days later mice were sacrificed, organs were collected, and luciferase activity was assessed in tissue homogenates (Promega Corp, Madison, WI).
In vitro assessment of anti-cocaine and anti-nicotine antibody titers
Wells of flat-bottomed 96-well enzyme immunoassays/radioimmunoassays plates (Corning, New York, NY) were coated with 100 μl of 1 mg/ml GNE or AM1-conjugated bovine serum albumin (BSA, ratio of 1:2) in carbonate buffer, pH 9.4, overnight at 4°C. Anti-Ad5 antibody titers were similarly assessed by ELISA using Ad5LacZ (109 particle units/well). The plates were washed with 0.05% Tween 20 in PBS (PBS-Tween) and blocked with 5% dry milk in PBS for 30 min at 23°C. Two-fold serial dilutions of serum were added to each well and incubated for 90 min at 23°C. The plates were washed four times with PBS-Tween. For total immunoglobulin G (IgG), 100 μl of 1:2000 diluted horseradish peroxidase–conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) in 1% dry milk in PBS was added to each well and incubated for 90 min at 23°C. Peroxidase substrate (100 μl/well; Bio-Rad, Hercules, CA) was added and incubated for 15 min at 23°C. For the rabbit anti-mouse IgG isotype-specific antibody, 100 μl of anti-IgG1, IgG2a, or IgG2b (Bio-Rad) was added to each independent well and incubated in 1% dry milk in PBS for 90 min at 23°C. The plates were washed four times, goat anti-rabbit horseradish peroxidase conjugate was added, incubated for 90 min at 23°C, and the plates washed again. Peroxidase reactions were stopped by addition of 2% oxalic acid (100 μl/well). Absorbance was measured at 415 nm. Anti-small molecule hapten antibody titers were calculated by interpolation of the log(OD)-log(dilution) with a cutoff value equal to two-fold the absorbance of background.
Cocaine and nicotine pharmacokinetics
Naive or dAd5 hapten conjugate vaccinated mice (with and without pre-existing Ad5-immunity) were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) 2 min prior to tail-vein administration of 2.5 μg cocaine or 0.4 μg nicotine with 1.0 μCi [3H]cocaine or [3H]nicotine (PerkinElmer, Waltham, MA). One min later, the mice were sacrificed and brain and trunk blood were collected separately. Brain tissue was homogenized in PBS, and 300 μl of brain homogenate and 50 μl of serum were added to separate 5-ml liquid scintillant (Ultima Gold™; PerkinElmer), assayed in triplicate for tritium, and normalized with a standard quenching curve. For the blood compartment, cocaine or nicotine was normalized to serum volume and brain was normalized to brain wet weight.
Statistics
All data are expressed as means±standard error. Comparisons between groups were conducted by one-way or two-way paired t-test.
Results
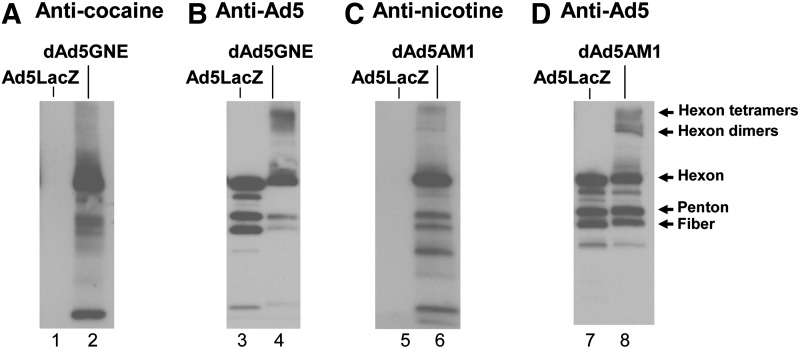
To assess the capacity of the disrupted Ad vaccine platform to evoke immunity against small molecules in the presence of pre-existing Ad5 immunity, two distinct vaccines were created by covalently conjugating either a nicotine or a cocaine hapten to a disrupted serotype 5 E1−E3− Ad5LacZ gene transfer vector. Western analysis demonstrated that the disrupted adenovirus was covalently coupled to each of the two drug analogs, the cocaine hapten or the nicotine hapten, to create dAd5GNE or dAd5AM1, respectively (Fig. 1).
FIG. 1.
Characterization of disrupted Ad5-based anti-haptenic conjugate vaccines. Conjugation of haptens cocaine or nicotine to disrupted E1−E3− Ad5. (A) Anti-cocaine Western analysis of dAd5GNE. Lane 1, Ad5LacZ; lane 2, dAd5GNE. (B) Anti-Ad5 Western of dAd5GNE. Lane 3, Ad5LacZ; lane 4, dAd5GNE. (C) Anti-nicotine Western analysis of dAd5AM1. Lane 5, Ad5LacZ; lane 6, dAd5AM1. (D) Anti-Ad5 Western of dAd5AM1. Lane 7, Ad5LacZ; lane 8, dAd5AM1.
Anti-Ad5 immunity in mice
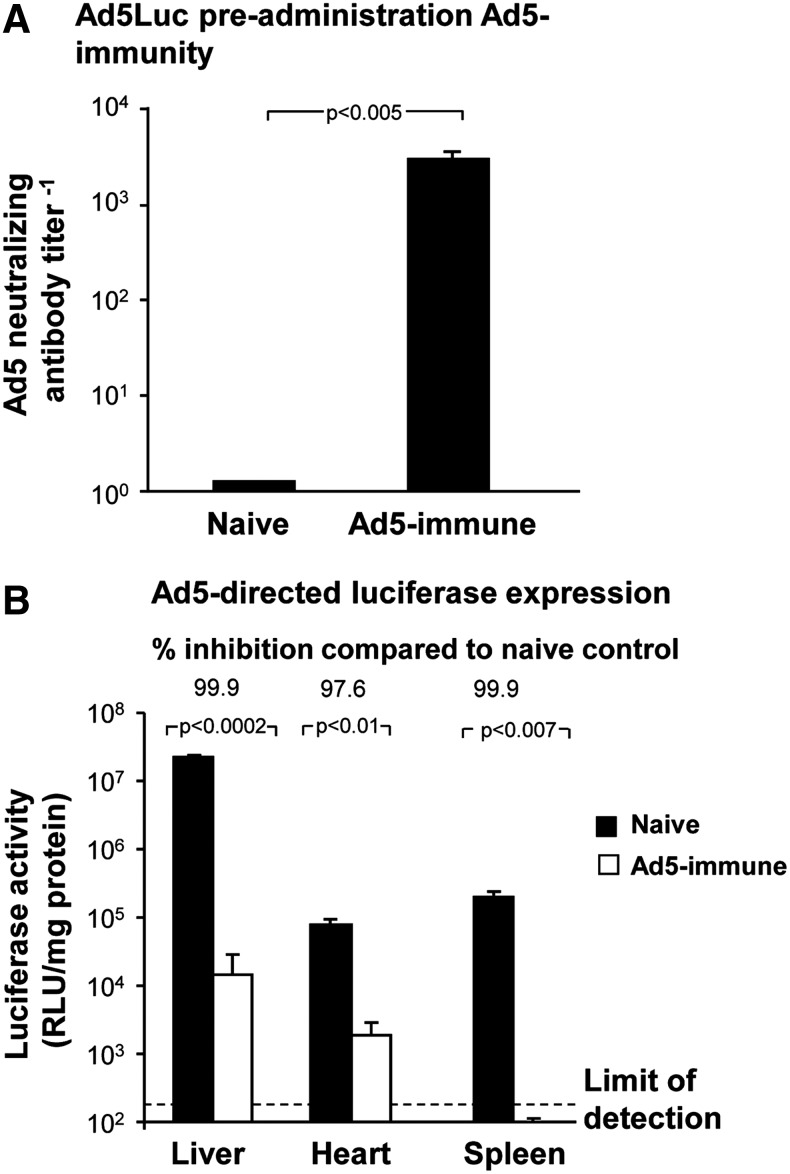
Anti-Ad5 immunity was first generated in mice (Fig. 2A). Administration of the Ad5Luc vector in naive control mice showed high expression of the luciferase transgene in the liver, heart, and spleen, but at markedly reduced expression of luciferase in these organs in Ad5-immune mice (Fig. 2B). This reduced efficacy of Ad5 vectors in the context of pre-existing Ad5 immunity is the dilemma for Ad5-based vaccines in the human population (D'Ambrosio et al., 1982; Piedra et al., 1998; Chirmule et al., 1999; Harvey et al., 1999a; Harvey et al., 1999b; Nwanegbo et al., 2004; Sumida et al., 2004; Mast et al., 2010).
FIG. 2.
Anti-Ad5 pre-existing immunity blocks Ad5 vector-directed transgene expression. Pre-existing Ad5-immunity was evoked in BALB/c mice (n=3/group) by intramuscular immunization with 2×1010 particle units Ad5Null at 0, 4, and 8 weeks. Ad5 neutralizing antibody titers were assessed by Ad5LacZ-based neutralizing assay at 10 weeks. To evaluate Ad5 vector-directed transgene expression, Ad5Luc (5×1010 particle units) was administered intravenously to Ad5-immune or naive control mice. After 3 days, luciferase activity was measured in liver, heart, and spleen homogenates and normalized to total protein concentration. (A) Ad5 neutralizing antibody titers before Ad5Luc administration in naive mice and Ad5Null treated mice. (B) Ad5 vector-mediated luciferase expression in naive and Ad5-immune mice.
Efficacy of disrupted Ad5-based small molecule vaccines in the context of Ad5 immunity
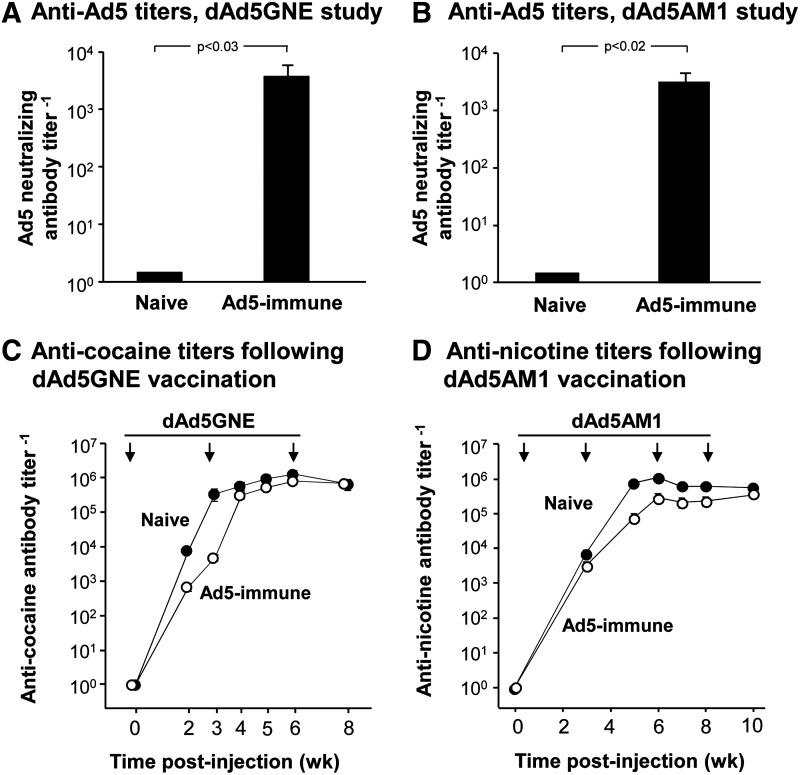
The serum from two groups of mice (n=10/group) 2 weeks following the last of three intramuscular administrations of Ad5Null had high titer anti-Ad5 neutralizing antibodies, significantly greater than the naive control group, and comparable to titers observed in humans (both p<0.03; Fig. 3A and B). Serum from naive mice had no detectable Ad5 neutralizing antibodies.
FIG. 3.
Antibody titers from dAd5-based vaccines in the context of Ad5 immunity. Ad5-preimmune and naive BALB/c mice (n=10/group) were assessed for neutralizing anti-Ad5 antibodies using an Ad5LacZ-based neutralizing assay at 10 weeks. Ad5-immune or naive control mice (n=10/group) were vaccinated intramuscularly with 4 μg dAd5GNE or dAd5AM1 at 0, 3, and 6 weeks. Antibody titers against cocaine or nicotine were assessed by ELISA using bovine serum albumin (BSA)-conjugated GNC or AM1 at 0, 2, 3, 5, 6, and 8 weeks. (A) Ad5-neutralizing antibody titers for animals used in the Ad5GNE study. (B) Ad5-neutralizing antibody titers for animals used in the Ad5AM1 study. (C) Anti-cocaine antibody titers in naive and anti-Ad5 preimmune mice. (D) Anti-nicotine antibody titers in naive and anti-Ad5 preimmune mice. Antibody titers are mean values±SEM.
The dA5GNE or dAd5AM1 vaccines evoked high levels of anti-cocaine or anti-nicotine antibody titers in naive mice (Fig. 3C and D). This was also true in Ad5-preimmune mice, indicating no significant effect of the anti-Ad5 immunity on the efficacy of the dAd5GNE and dAd5AM1 vaccines (p>0.9, naive vs. Ad5-immune vaccinated with dAd5GNE at 8 weeks; p>0.08, naive vs. Ad5-immune vaccinated with dAd5AM1 at 10 weeks).
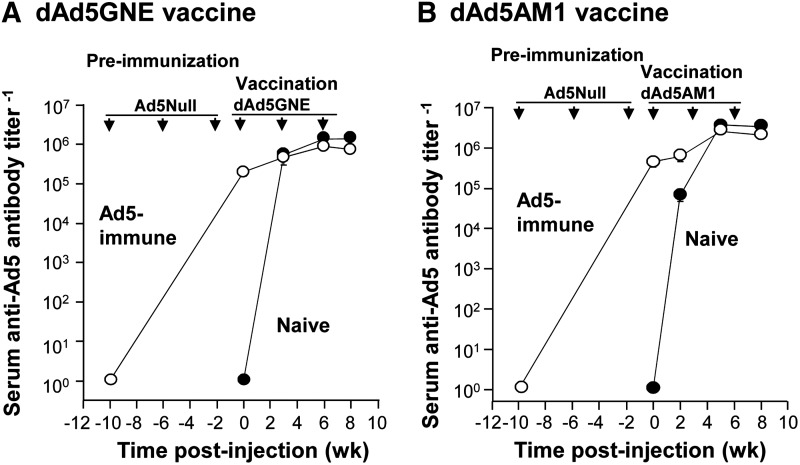
Vaccination with dAd5 maintains existing Ad5 immunity and evokes anti-Ad5 in naive mice
Although designed to evoke high-titer response to target molecules (cocaine and nicotine, respectively), vaccination with the disrupted Ad5 small molecule vaccines simultaneously maintained or boosted primary immunity against Ad5 (Fig. 4). dAd5GNE vaccination evoked high levels of anti-Ad5 antibodies in naive control mice, similar to levels observed in Ad5-immune mice (p>0.3 at 6 weeks; p>0.1 at 8 weeks). Likewise, dAd5AM1 evoked higher anti-Ad5 antibody titers in both naive control and Ad-immune mice, although the naive mice were slightly higher at 8 weeks (p>0.1 at 5 weeks; p<0.008 at 8 weeks).
FIG. 4.
Anti-Ad5 antibody titers before and after dAd5 haptenic vaccine administered. BALB/c mice (n=10/group) were immunized intramuscularly with 2×1010 particle units Ad5Null at −10, −6 and −2 weeks, followed by vaccination with dAd5GNE or dAd5AM1 (4 μg each) at 0, 3, and 6 weeks compared to naive control mice (n=10/group) vaccinated with dAd5GNE or dAd5AM1 at 0, 3, and 6 weeks. (A) Serum anti-Ad5 antibody titers in dAd5GNE vaccine-administered mice, assessed by ELISA at −10, 0, 3, 6, and 8 weeks in naive and Ad5-preimmune mice. (B) Serum anti-Ad5 antibody titers in dAd5AM1 vaccine-administered mice, assessed by ELISA at −10, 0, 2, 5, and 8 weeks in naive and Ad-preimmune mice. Antibody titers are mean values±SEM.
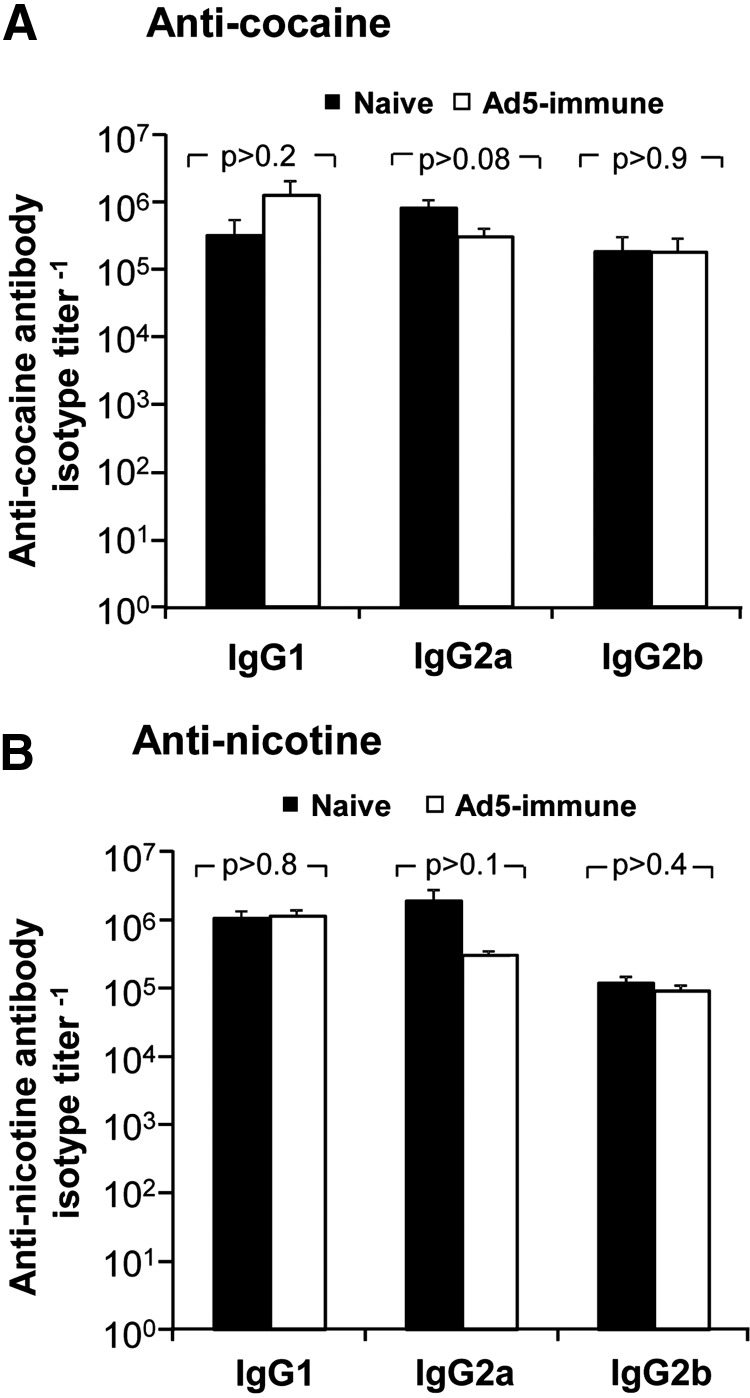
Antibody isotypes evoked by the dAd5 vaccines
Both disrupted Ad5 small-molecule vaccines evoked similar levels of each IgG isotype in naive and Ad5-preimmune mice (Fig. 5A and B). For both vaccines, the small molecule antibody titers were similar for IgG1 and IgG2a, which were both higher than IgG2b titers. There was no significant difference in naive and Ad5-preimmune groups (p>0.09).
FIG. 5.
Antibody IgG isotype titers evoked by dAd5-based vaccines in naive and anti-Ad5 preimmune mice. Antibody isotypes in dAd5GNE or dAd5AM1 vaccinated naive and Ad5-immune mice at 5 weeks were evaluated by isotype-specific secondary antibodies on ELISA for IgG1, IgG2a, and IgG2b. (A) Anti-cocaine IgG isotype titers in dAd5GNE-vaccinated mice. (B) Anti-nicotine IgG isotype titers in dAd5AM1-vaccinated mice. Antibody titers are mean values±SEM.
Vaccine efficacy
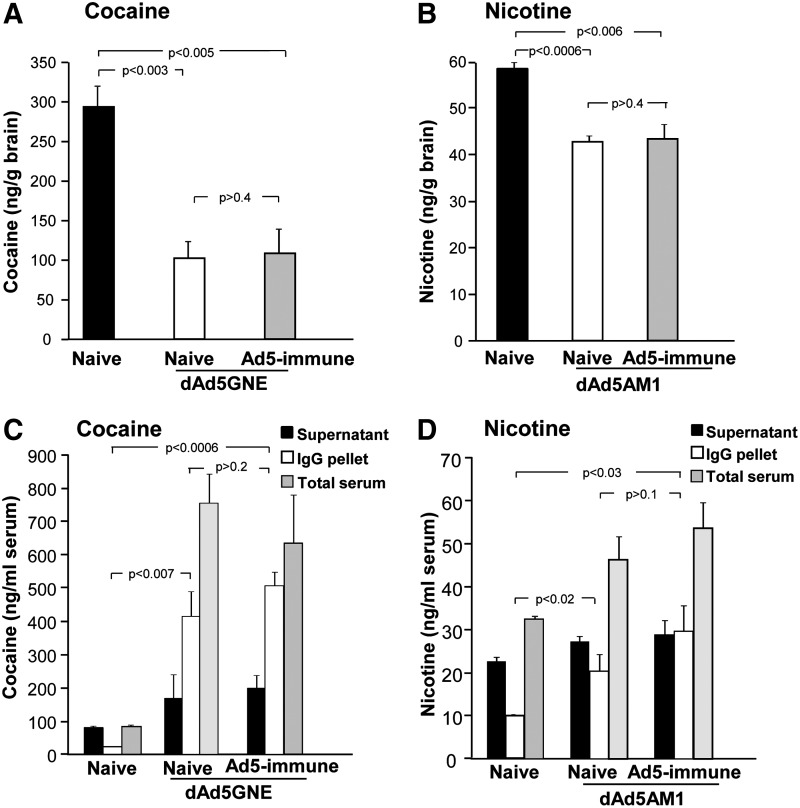
When 3H-labeled cocaine or nicotine was administered intravenously, the levels of each drug were reduced in the brains of vaccinated mice compared to naive mice, even in the context of anti-Ad immunity (Fig. 6).
FIG. 6.
Levels of the addictive drug in brain and serum of dAd5-vaccinated mice with and without pre-existing Ad5 immunity. Mice were challenged with 3H-labeled drug and brain and serum were collected 1 min later, n=4 mice/group, 9 weeks after dAd5GNE or 10 weeks after dAd5AM1 vaccination. (A) Cocaine levels in the brain (ng/g brain) of untreated and dAd5GNE-vaccinated mice naive or with pre-existing Ad5 immunity. (B) Nicotine levels in the brain (ng/g brain) of untreated and dAd5AM1-vaccinated mice, naive or with pre-existing Ad5 immunity. (C) In the same mice as panel (A), serum cocaine levels (ng/ml), IgG-bound, and free cocaine levels (ng/ml). (D) In the same mice as panel (B), serum nicotine levels (ng/ml), IgG-bound, and free nicotine levels (ng/ml).
Cocaine distribution analysis showed a dAd5GNE vaccine-mediated 65% reduction in the brain of Ad5-immune mice (p<0.005) and 63% reduction in naive control mice (p<0.003; Fig. 6A), both compared to non-vaccinated controls. Importantly, there was no significant difference between the cocaine content in the brain of dAd5GNE-vaccinated Ad preimmune mice and naive controls (p>0.4). The majority of the cocaine in dAd5GNE-vaccinated mice was IgG bound at the same level, regardless of existing pre-Ad5 immunity (dAd5GNE-vaccinated Ad5-immune IgG bound vs dAd5GNE-vaccinated naive controls, p>0.2; Fig. 6C). In contrast, levels of IgG-bound cocaine in non-vaccinated mice were significantly lower (dAd5GNE-vaccinated Ad5-immune vs. non-vaccinated IgG bound, p<0.0006).
In Ad5 naive mice, brain drug levels from administered nicotine were markedly lower in the dAd5AM1-vaccinated group compared to naive controls (vaccinated vs. untreated, p<0.0006) and similarly in Ad5-preimmune mice (vaccinated vs. untreated, p<0.006; Fig. 6B). There was no significant difference between the dAd5AM1-vaccinated cohorts (naive vs. Ad5-preimmune, p>0.4). A large fraction of the nicotine in dAd5AM1-vaccinated mice was IgG bound at the same level, regardless of existing pre-Ad5 immunity (dAd5AM1-vaccinated Ad5-immune, IgG bound vs. dAd5AM1-vaccinated naive controls, p>0.1), whereas IgG bound-nicotine in non-vaccinated controls was significantly lower (dAd5AM1-vaccinated vs. non-vaccinated, IgG bound, p<0.03; Fig. 6D).
Discussion
Adenovirus gene transfer vectors were originally designed to transfer genes in vivo, where they are highly effective in generating expression of the transgene (Rosenfeld et al., 1991; Jaffe et al., 1992; Rosenfeld et al., 1992; Davidson et al., 1993; Li et al., 1993). It was soon recognized, however, that the Ad capsid proteins are highly immunogenic, leading to high levels of anti-Ad capsid humoral and cellular immunity that limits Ad-mediated gene expression to 1 to 2 weeks and prevents effective gene expression upon readministration of the vector (Setoguchi et al., 1994; Yang et al., 1994; Gilgenkrantz et al., 1995; Dai et al., 1995; van Ginkel et al., 1995; Wilson, 1995; Yang et al., 1995; Harvey et al., 1999a; Harvey et al., 1999b; Tatsis and Ertl, 2004). Further, because immunity against human Ad is common in the population, pre-existing anti-Ad immunity reduces the effectiveness of Ad vector-mediated gene transfer (D'Ambrosio et al., 1982; Piedra et al., 1998; Chirmule et al., 1999; Harvey et al., 1999a; Harvey et al., 1999b; Nwanegbo et al., 2004; Sumida et al., 2004; Mast et al., 2010).
Anti-Ad immunity is a challenge for many Ad vectors for gene transfer; although they are highly effective in generating immunity against the capsid proteins, the adenovirus vaccines, including replication-deficient, attenuated, or competent vaccines, are less effective in the context of pre-existing Ad immunity (van Ginkel et al., 1995; Harvey et al., 1999a; Fitzgerald et al., 2003; Tatsis and Ertl, 2004; McCoy et al., 2007; Seregin and Amalfitano, 2009). However, it was soon recognized that the highly immunogenic properties of the Ad capsid could be leveraged to develop effective vaccines, where the Ad capsid proteins themselves act as potent immunogens (Gahery-Segard et al., 1998; Worgall et al., 2005; Matthews et al., 2010; Palma et al., 2011).
Taking advantage of the highly immunogenic properties of the Ad5 capsid proteins, we have developed a platform for evoking immunity against small-molecule addictive drugs. This strategy was achieved by covalently linking an analog (hapten) of the addictive drug to the capsid proteins derived from detergent-disrupted and heat-denatured Ad5 (Hicks et al., 2011; Wee et al., 2012). While highly effective in generating high-titer immunity against the addictive small molecule (Hicks et al., 2011; Wee et al., 2012), the focus of the present study was to ask whether disrupted Ad5 vaccines would also be effective in the context of high levels of pre-existing anti-Ad5 immunity. The data demonstrates that, in the context of anti-Ad5 immunity that blocks Ad5 gene-transfer vectors from effectively transferring genes in vivo, anti-cocaine and anti-nicotine vaccines based on disrupted Ad5 are highly effective in generating anti-cocaine and anti-nicotine. These observations suggest that vaccines comprised of molecules coupled to disrupted Ad5 capsid proteins represent a platform vaccine strategy equally effective in the context of anti-Ad5 naive or anti-Ad5 immunity.
Approaches to circumventing pre-existing Ad-immunity
“Live” Ad are potent immunogens and thus useful as vaccine platforms (Rosenfeld et al., 1991; Jaffe et al., 1992; Rosenfeld et al., 1992; Davidson et al., 1993; Li et al., 1993; Shiver et al., 2002; Tatsis and Ertl, 2004; Baez-Astua et al., 2005; Hashimoto et al., 2005; Worgall et al., 2005; Chiuchiolo et al., 2006; Matthews et al., 2010; Palma et al., 2011). Unfortunately, Ad-based vaccines fail to elicit the same potent immunity in the context of existing anti-Ad immunity. In animal models, it has been possible to partially overcome pre-existing anti-Ad5 immunity by increasing vaccine dosage or by altering prime-boost regimens with plasmid DNA, alternative Ad serotypes, or construction of chimeric capsomeres (Mastrangeli et al., 1996; Casimiro et al., 2003; Worgall et al., 2005; Roberts et al., 2006; Seregin and Amalfitano, 2009; Matthews et al., 2010; Palma et al., 2011). The disrupted adenovirus vaccine platform used in the present study was developed to target small addictive drugs (Hicks et al., 2011; Wee et al., 2012). Importantly, the disrupted Ad platform shows the same efficacy in the context of the pre-existing Ad5 environment as compared to naive controls. Interestingly, an anti-cocaine vaccine made with the intact virion does not circumvent pre-existing Ad5 immunity to the same degree, suggesting that the mechanism of how disruption of the Ad5 proteins negates susceptibility to pre-existing immunity may be that the neutralizing antibodies against the adenovirus recognize conformational epitopes. Although we have not tested peptides, proteins, carbohydrates, or lipids attached to the disrupted Ad capsid proteins, we hypothesize that such vaccines would also be equally effective in both Ad5 naive or preimmune mice (Worgall et al., 2005). However, unlike large molecules where significant memory persists, vaccines against small molecules based on the Ad capsid as a platform may require frequent booster administrations to maintain high levels of immunity.
Small-molecule drugs
Drug addiction has had a significant negative impact on public health, and with many drugs there are still no therapeutic options (Maraj et al., 2010; SAMHSA, 2010). As targets for therapeutic vaccines, addictive drugs pose significant challenges: these are small molecules with poor immunopotency, and therapy requires a high antibody titer to sequester the drug (Kinsey et al., 2009; Moreno and Janda, 2009). The strategy of directing the immune response against a small-molecule target has typically been achieved by conjugation of the hapten to a potent human immunogen such as cholera, tetanus, pseudomonas toxins, or keyhole limpet hemocyanin (Carrera et al., 1995; Fox et al., 1996; Hieda et al., 2000; Kantak et al., 2000; Carrera et al., 2004; Moreno et al., 2010). The goal for each of these approaches is to evoke a robust antibody response in the host such that antibody-mediated sequestering of the administered drug abrogates transfer across the blood–brain barrier. In our prior study with cocaine, and in the present study with nicotine, we show efficacy of the dAd5 vaccine platform to elicit high titer antibodies directed toward drugs of abuse, likely because of the highly immunogenic Ad5 capsid proteins (Gahery-Segard et al., 1998; Worgall et al., 2005; Roberts et al., 2006; Matthews et al., 2010; Palma et al., 2011). Based on this data, it is likely that this platform will also function well as a carrier for other small nonimmunogenic molecules.
Because a large percentage of the population has anti-Ad immunity, a challenge remained to leverage the full potential of Ad-based vaccines in the context of Ad immunity. Our study demonstrates the efficacy of the dAd5 vaccine platform as a potent carrier for haptens, effectively circumventing pre-existing Ad5-immunity and directing a robust immune response toward nonimmunogenic targets. The dAd5-based vaccine against the drugs nicotine and cocaine significantly reduces drug access to the central nervous system even in the context of anti-Ad5 immunity.
Acknowledgments
We thank N. Mohamed and D.N. McCarthy for help in preparing the manuscript. These studies were supported, in part, by NIH grants R01 DA025305 and RC2 DA028847; TRDRP 20XT-0156 (KDJ) and DA008590 (KDJ). The authors thank the National Institute on Drug Abuse (NIDA) drug supply program for the cocaine used in this study.
Author Disclosure Statement
No conflicting interests exist.
References
- Baez-Astua A. Herraez-Hernandez E. Garbi N., et al. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J. Virol. 2005;79:12807–12817. doi: 10.1128/JVI.79.20.12807-12817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera M.R. Ashley J.A. Parsons L.H., et al. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Carrera M.R. Ashley J.A. Hoffman T.Z., et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg. Med. Chem. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Casimiro D.R. Chen L. Fu T.M., et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N. Propert K. Magosin S., et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Chiuchiolo M.J. Boyer J.L. Krause A., et al. Protective immunity against respiratory tract challenge with Yersinia pestis in mice immunized with an adenovirus-based vaccine vector expressing V antigen. J. Infect. Dis. 2006;194:1249–1257. doi: 10.1086/507644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. Schwarz E.M. Gu D., et al. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio E. del G.N. Chicca A., et al. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. (Lond) 1982;89:155–161. doi: 10.1017/s0022172400070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B.L. Allen E.D. Kozarsky K.F., et al. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J.C. Gao G.P. Reyes-Sandoval A., et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- Fox B.S. Kantak K.M. Edwards M.A., et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat. Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- Gahery-Segard H. Farace F. Godfrin D., et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgenkrantz H. Duboc D. Juillard V., et al. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum. Gene Ther. 1995;6:1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- Harvey B.G. Hackett N.R. El-Sawy T., et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J. Virol. 1999a;73:6729–67421. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B.G. Leopold P.L. Hackett N.R., et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Invest. 1999b;104:1245–1255. doi: 10.1172/JCI7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M. Boyer J.L. Hackett N.R., et al. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect. Immun. 2005;73:6885–6891. doi: 10.1128/IAI.73.10.6885-6891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M.J. De B.P. Rosenberg J.B., et al. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol. Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y. Keyler D.E. Ennifar S., et al. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int. J. Immunopharmacol. 2000;22:809–819. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Jaffe H.A. Danel C. Longenecker G., et al. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat. Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Kantak K.M. Collins S.L. Lipman E.G., et al. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kinsey B.M. Jackson D.C. Orson F.M. Anti-drug vaccines to treat substance abuse. Immunol. Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- Li Q. Kay M.A. Finegold M., et al. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum. Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- Maraj S. Figueredo V.M. Lynn M.D. Cocaine and the heart. Clin. Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast T.C. Kierstead L. Gupta S.B., et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A. Harvey B.G. Yao J., et al. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- Matthews Q.L. Fatima A. Tang Y., et al. HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS. One. 2010;5:e11815. doi: 10.1371/journal.pone.0011815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K. Tatsis N. Korioth-Schmitz B., et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 2007;81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A.Y. Azar M.R. Warren N.A., et al. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol. Pharm. 2010;7:431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- Moreno A.Y. Janda K.D. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol. Biochem. Behav. 2009;92:199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwanegbo E. Vardas E. Gao W., et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma C. Overstreet M.G. Guedon J.M., et al. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine. 2011;29:1683–1689. doi: 10.1016/j.vaccine.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra P.A. Poveda G.A. Ramsey B., et al. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101:1013–1019. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- Roberts D.M. Nanda A. Havenga M.J., et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Siegfried W. Yoshimura K., et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Yoshimura K. Trapnell B.C., et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Seregin S.S. Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert. Opin. Biol. Ther. 2009;9:1521–1531. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- Setoguchi Y. Jaffe H.A. Chu C.S., et al. Intraperitoneal in vivo gene therapy to deliver alpha 1-antitrypsin to the systemic circulation. Am. J. Respir. Cell Mol. Biol. 1994;10:369–377. doi: 10.1165/ajrcmb.10.4.8136153. [DOI] [PubMed] [Google Scholar]
- Shiver J.W. Fu T.M. Chen L., et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Sprangers M.C. Lakhai W. Koudstaal W., et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) Results from the 2009 National survey on drug use and health: Volume I. Summary of national findings. 2010. www.samhsa.gov/data/2k9/2k9Resultsweb/web/2k9results.htm. [Jul 26;2012 ]. www.samhsa.gov/data/2k9/2k9Resultsweb/web/2k9results.htm
- Sumida S.M. Truitt D.M. Kishko M.G., et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N. Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel F.W. Liu C. Simecka J.W., et al. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum. Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- Wee S. Hicks M.J. De B.P., et al. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology. 2012;37:1083–1091. doi: 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.M. Gene therapy for cystic fibrosis: challenges and future directions. J. Clin. Invest. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S. Krause A. Rivara M., et al. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Invest. 2005;115:1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Li Q. Ertl H.C., et al. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Nunes F.A. Berencsi K., et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]