Abstract
Context
A clinical trial was conducted to evaluate the safety and efficacy of neuroprotective therapy using granulocyte colony-stimulating factor (G-CSF) for patients with worsening symptoms of compression myelopathy. During this trial, we found that neuropathic pain associated with thoracic myelopathy was dramatically reduced after G-CSF administration in two cases.
Findings
A 32-year-old man with compression of the spinal cord at levels T7–T10 complained of spastic gait associated with spontaneous severe pain from his back to his chest. G-CSF 10 µg/kg/day was administered for 5 consecutive days; his pain was reduced 1 day after the initial G-CSF administration. One month after administration, he underwent spinal fusion surgery for decompression of the spinal cord. Six months after G-CSF administration, he showed recovery from myelopathy and no recurrence of pain. A 68-year-old man with spastic gait and bilateral thigh pain caused by ossified ligamentum flavum at T11–T12 was treated with G-CSF 10 µg/kg/day for 5 days; his pain was reduced 1 day after initial administration. One month later, he underwent a T10–T12 laminectomy. Three months after G-CSF administration, his thigh pain began to attenuate. At 6 months after administration, he showed recovery from myelopathy, and his pain was still improved compared with that before administration.
Conclusion
G-CSF may have a therapeutic effect on spinal neuropathic pain.
Keywords: Myelopathy, Spinal cord compression, Neuroprotective therapy, Granulocyte colony-stimulating factor, Thoracic myelopathy, Neuropathic pain, Spasticity, Clinical trial
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that promotes survival, proliferation, and differentiation of cells in the neutrophil lineage.1 Recent studies have indicated that G-CSF also has non-hematopoietic functions and can potentially be used for the treatment of neuronal injury, including stroke and neurodegenerative diseases.2 We previously demonstrated that G-CSF promoted the restoration of damaged spinal cord tissue and the recovery of neural function in experimental spinal cord injury (SCI) in both mice and rats.3–5 On the basis of these findings, we initiated a clinical trial to evaluate the safety and efficacy of neuroprotective therapy using G-CSF for patients with worsening symptoms of compression myelopathy.6 In phases I and IIa of the clinical trial, we recruited patients 20–75 years of age, in whom Japanese Orthopaedic Association (JOA) score for cervical and thoracic myelopathy decreased 2 points or more during a recent 1-month period.6 In the first step of this trial, G-CSF 5 µg/kg/day was intravenously administered for 5 consecutive days in five patients. We then administered G-CSF 10 µg/kg/day for 5 consecutive days in 10 patients. No serious adverse events occurred during or after treatment, and all patients showed neurological improvement, although G-CSF 10 µg/kg/day resulted in better neurological recovery. Thus, we suggested that intravenous administration of G-CSF at a dosage of 10 µg/kg/day for 5 days is an appropriate protocol for G-CSF neuroprotective therapy.6
During this trial, we encountered an unexpected finding – two patients in whom neuropathic pain associated with thoracic myelopathy was dramatically reduced after G-CSF administration. Such a pain-relieving effect of G-CSF had not been included as an endpoint in this trial. However, the effect is a significant feature with implications for future clinical use of G-CSF for compression myelopathy.
Case reports
Case 1
A 32-year-old man was admitted to our hospital complaining of progressive motor weakness of his lower extremities and gait disturbance. On admission, his JOA score for thoracic myelopathy (motor function: 0–4 points, sensory function: 0–4 points, bladder function: 0–3 points, total possible score = 11 points)7 was 4 points. He also showed spontaneous severe pain developing from his back to his chest.
Four years prior to this admission, he suffered from thoracic myelopathy because of postvertebral osseous spurs that compressed his spinal cord anteriorly at T7–T10 (Figs. 1A and B). He underwent surgical treatment for T7–T10 anterior decompression with spinal fusion. Before his first surgery, he had complained of gait disturbance and spontaneous pain from his back to his chest. After the surgery, his symptoms of myelopathy and pain were relieved. Three years after the surgery, however, his symptoms began to deteriorate.
Figure 1.
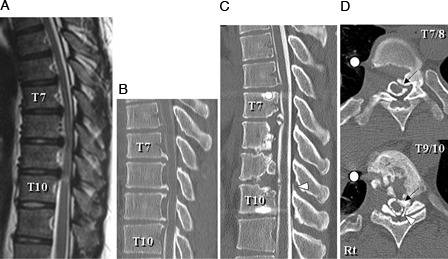
Case 1: T2-weighted midsagittal magnetic resonance image (A) and CT myelogram midsagittal reconstruction plane (B) 4 years prior to this admission showing anterior compression of the spinal cord by postvertebral osseous spurs at T7–T10. CT myelogram midsagittal reconstruction plane (C) and axial planes at T7–T8 and T9–T10 (D) on admission showing re-growth of the osseous spurs that compressed the spinal cord anteriorly at T7–T8 and T9–T10 (C, D, arrows) and a newly developed ossified ligamentum flavum (OLF) that compressed the spinal cord posteriorly at T9–T10 (C, D, arrowheads).
Reconstruction images from a computed tomography (CT) myelogram showed that the grafted bone at the T7–T8, T8–T9, and T9–T10 intervertebral disc levels was absorbed, and spine fusion was not obtained (Fig. 1C). The CT images showed regrowth of osseous spurs that compressed his spinal cord anteriorly at T7–T8 and T9–T10 (Figs. 1C and D, arrows) and newly developed ossified ligamentum flavum (OLF) that compressed his spinal cord posteriorly at T9–T10 (Figs. 1C and D, arrowheads).
From the day of admission, he underwent administration of G-CSF (10 µg/kg/day) for 5 consecutive days. One day after the initial G-CSF administration, he felt relief of his back and chest pain. Visual analog scale (VAS) score of his pain was 80 mm before G-CSF administration, and it decreased to 50 mm 1 day after the initial G-CSF administration. At 1 week after the initial administration, his VAS score became 0 mm, and his pain was diminished. He also felt improved muscle strength of his legs, and his JOA score was increased to 6 points at 1 month after the administration.
According to the protocol for G-CSF neuroprotective therapy for worsening symptoms of compression myelopathy, we followed the patients without surgical treatment for 1 month after G-CSF administration.6 At 1 month after the administration, he underwent surgery for decompression of the spinal cord using a posterior approach and T4–T12 posterior instrumented fusion. At 6 months after the administration, his recovery from myelopathy was maintained (JOA score = 6 points) with no recurrence of pain.
Case 2
A 68-year-old man was admitted to our hospital with a complaint of motor weakness of his lower extremities and gait disturbance. On admission, JOA score was 4 points. In addition to the symptoms of myelopathy, he complained of spontaneous severe bilateral pain at the level of his thigh.
From 10 years earlier, his gait had become progressively unstable. Beginning 2 months previously, his gait disturbance progressed rapidly, and he could not walk without canes on admission. He had also felt severe bilateral thigh pain for the previous 2 months.
Sagittal magnetic resonance and reconstruction CT images showed that his spinal cord was severely compressed posteriorly by an OLF at T10–T11 (Figs. 2A–C).
Figure 2.
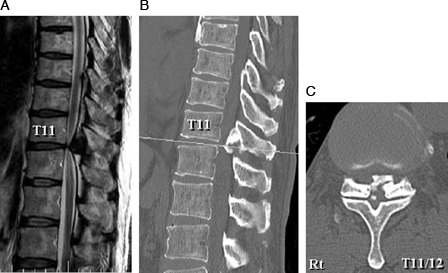
Case 2: T2-weighted midsagittal magnetic resonance image (A) and CT midsagittal reconstruction plane (B) and CT axial plane at T11–T12 (C) showing posterior compression of the spinal cord by an OLF at T11–T12.
From the day of admission, he underwent administration of G-CSF (10 µg/kg/day) for 5 consecutive days. One day after the initial G-CSF administration, he felt relief of his pain at his bilateral thigh. His VAS score for pain was 90 mm before the G-CSF administration, and it decreased to 40 mm 1 day after the initial G-CSF administration. His myelopathy also improved, and his JOA score became 6.5 points 1 month after G-CSF administration.
At 1 month after the administration, he underwent surgery for T10–T12 laminectomy. At 3 months after the administration, his pain recurred and the VAS score increased to 60 mm. After that, however, further aggravation of his pain did not occur, and the VAS score was 60 mm 6 months after the administration. The recovery from myelopathy was also maintained, and the JOA score was 6.5 points 6 months after G-CSF administration.
Discussion
Neuropathic pain has been defined as a type of pain arising from the direct consequence of a lesion affecting the somatosensory system such as in the brain, spinal cord, or peripheral nerves.8,9 Among numerous diseases of the spinal cord, neuropathic pain following SCI has been studied most commonly. Previous studies have classified neuropathic pain from spinal cord lesions into two types: at-level pain and below-level pain.1 At-level pain is characterized as pain located within two or three spinal segments below the neurological level of the spinal cord lesion. In contrast, below-level pain presents diffusely caudal to the level of the spinal cord lesion.
In case 1, the patient complained of spontaneous severe pain developing from his back to his chest. We suggest that his pain is a typical at-level pain originating from the spinal cord lesions at vertebral levels T7–T10. In case 2, the patient complained of spontaneous severe bilateral thigh pain corresponding to dermatome levels L2–L3. In this patient, the spinal cord was compressed by a T11–T12 OLF. Anatomically, the spinal cord level compressed by a T11–T12 OLF is considered to be the upper portion of the epiconus, where multiple spinal cord segments (usually L2–L5) are densely located.10 Thus, we suggest that the thigh pain of this patient is also at-level pain. In the present two cases, G-CSF administration resulted not only in recovery from myelopathy, but also in reduction of neuropathic pain. In case 1, the VAS score was 80 mm before G-CSF administration, and it became 0 mm at 1 week after administration. In case 2, the pre-administration VAS score was 90 mm, and it decreased to 40 mm 1 day after G-CSF administration. In both cases, decompression surgery was performed 1 month after G-CSF administration. Thus, we suggest that the pain reduction observed in the present two cases during the 1 month after G-CSF administration was caused by the pharmacological effect of G-CSF and not by surgery. After surgery, however, the VAS score of both cases did not necessarily reflect the neuroprotective effect of G-CSF. Despite the confounding factor of surgery, the present findings suggest that G-CSF may have a therapeutic effect on neuropathic pain in patients with thoracic compression myelopathy.
To the best of our knowledge, no reports of experimental studies of G-CSF administration in an animal model of spinal neuropathic pain have been published. In our studies using animal models of compression-induced and contusive SCI, intravenously administered G-CSF resulted in functional recovery by (1) promoting the migration of bone marrow-derived cells into the damaged spinal cord, (2) directly suppressing the neural apoptosis that occurs via G-CSF receptors at the injured spinal cord, and (3) decreasing the expression of inflammatory cytokines such as IL-1β and TNF-α.3–5 Ro et al.9 administered G-CSF to animal models of peripheral neuropathic pain, and demonstrated that G-CSF increased the number of opioid-contained polymorphonuclear cells and relieved neuropathic pain. We suggest that such mechanisms may participate in the pain-relieving effect of G-CSF on spinal neuropathic pain, although further studies are required to fully clarify all of the underlying mechanisms.
Among numerous diseases of the spinal cord, neuropathic pain following SCI has been studied most extensively.8 Investigators have suggested that pregabalin, gabapentin, and tricyclic antidepressants are optimal first-line treatments for neuropathic pain associated with SCI. Furthermore, serotonin–norepinephrine reuptake inhibitors are considered to be second-line choices, and tramadol, opioids, and lamotrigine are used as third-line options. However, these researchers concluded that such oral pharmacological intervention is often inadequate, commonly resulting in a reduction of only 20–30% in pain intensity.8 To date, therefore, no effective therapies for spinal neuropathic pain have been established.
Conclusion
To the best of our knowledge, this is the first report showing the therapeutic effect of G-CSF on neuropathic pain associated with compression myelopathy. We cannot deny the possibility that the placebo effect of injection and the surgical intervention contributed to the pain relief. On the basis of the experience of the present cases, however, we intend to advance to a clinical trial to verify the feasibility of using G-CSF for relief of spinal neuropathic pain. If the efficacy and safety of G-CSF treatment for spinal neuropathic pain is confirmed and clinical use of G-CSF therapy is approved, a novel and effective approach for the treatment of this disorder will be available.
Acknowledgement
This work was supported by a Health Labour Science Research Grant of Japan.
References
- 1.Roberts AW. G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors 2005;23(1):33–41 [DOI] [PubMed] [Google Scholar]
- 2.Schneider A, Kuhn HG, Schäbitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle 2005;4(12):1753–7 [DOI] [PubMed] [Google Scholar]
- 3.Kawabe J, Koda M, Hashimoto M, Fujiyoshi T, Furuya T, Endo T, et al. Granulocyte colony-stimulating factor (G-CSF) exerts neuroprotective effects via promoting angiogenesis after spinal cord injury in rats. J Neurosurg Spine 2011;15(4):414–21 [DOI] [PubMed] [Google Scholar]
- 4.Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, et al. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res 2007;1149:223–31 [DOI] [PubMed] [Google Scholar]
- 5.Nishio Y, Koda M, Kamada T, Someya Y, Kadota R, Mannoji C, et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 2007;66(8):724–31 [DOI] [PubMed] [Google Scholar]
- 6.Sakuma T, Yamazaki M, Okawa A, Takahashi H, Kato K, Hashimoto M, et al. Neuroprotective therapy using granulocyte-colony stimulating factor for patients with worsening symptoms of compression myelopathy, part 1: a phase I and IIa clinical trial. Eur Spine J 2011;21(3):482–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki M, Mochizuki M, Ikeda Y, Sodeyama T, Okawa A, Koda M, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine 2006;31(13):1452–60 [DOI] [PubMed] [Google Scholar]
- 8.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 2008;22(6):455–75 [DOI] [PubMed] [Google Scholar]
- 9.Ro LS, Chen SR, Chao PK, Lee YL, Lu KT. The potential application of granulocyte colony stimulating factor therapy on neuropathic pain. Chang Gung Med J 2009;32(3):235–46 [PubMed] [Google Scholar]
- 10.Toribatake Y, Baba H, Kawahara N, Mizuno K, Tomita K. The epiconus syndrome presenting with radicular-type neurological features. Spinal Cord 1997;35(3):163–70 [DOI] [PubMed] [Google Scholar]


