Abstract
Background/objectives
Rehabilitation for individuals with spinal cord injury (SCI) is expanding to include intense, activity-based, out-patient physical therapy (PT). The study's primary purposes were to (i) examine the effectiveness of intense PT in promoting motor and sensory recovery in individuals with SCI and (ii) compare recovery for individuals who had an olfactory mucosa autograft (OMA) with individuals who did not have the OMA while both groups participated in the intense PT program.
Methods
Prospective, non-randomized, non-blinded, intervention study. Using the American Spinal Injury Association examination, motor and sensory scores for 23 (7 OMA, 6 matched control and 10 other) participants were recorded.
Results
Mean therapy dosage was 137.3 total hours. The participants’ total, upper and lower extremity motor scores improved significantly while sensory scores did not improve during the first 60 days and from initial to discharge examination. Incomplete SCI or paraplegia was associated with greater motor recovery. Five of 14 participants converted from motor-complete to motor-incomplete SCI. Individuals who had the OMA and participated in intense PT did not have greater sensory or greater magnitude or rate of motor recovery as compared with participants who had intense PT alone.
Conclusion
This study provides encouraging evidence as to the effectiveness of intense PT for individuals with SCI. Future research is needed to identify the optimal therapy dosage and specific therapeutic activities required to generate clinically meaningful recovery for individuals with SCI including those who elect to undergo a neural recovery/regenerative surgical procedure and those that elect intense therapy alone.
Keywords: Spinal cord injuries, Complete, Incomplete, Activity-based therapy, Olfactory mucosal autograft, Paraplegia, Tetraplegia, Physical therapy, Functional recovery, Motor, Sensory, Rehabilitation, Physical
Introduction
Two primary impairments after sustaining a spinal cord injury (SCI) are complete or partial loss of sensory and/or motor function below the level of injury. Rehabilitation programs have focused on strengthening the innervated muscles above the level of SCI and use of compensatory strategies to accomplish functional activities. Currently, the approach to rehabilitation is expanding to include implementation of intense, activity-based, outpatient physical therapy (PT) programs targeting recovery of function below the level of the SCI.1,2
This rehabilitation paradigm shift is supported by an accumulating body of evidence in both animal and human studies demonstrating that intense exercise enhances recovery after SCI. In rodent and feline models, exercise and locomotor training led to partial recovery of locomotion and spinal cord neurological recovery.3–6 Human studies have focused on the effects of body weight-supported treadmill training (BWSTT)7–9 and functional electric stimulation cycling.10–12 Considerable evidence demonstrates that individuals with incomplete SCI have the potential to transition from BWSTT and regain over-ground ambulation;7,8,13,14 however, even when the SCI is complete, the neural networks below the level of the lesion generate locomotor activity.7,9
The goal of activity-based therapy is to promote neuromuscular plasticity through interventions that provide activation of the neuromuscular system below the level of the spinal lesion and perform repetitive practice of the desired motor tasks.2 Activity-based, restorative therapy interventions use patterned motor activity (e.g. locomotor training and functional electrical stimulation cycling), task specific training, sensory stimulation and specific muscle activation (e.g. muscle recruitment and strengthening).12 It is our opinion that activity-based therapy programs should be task-specific2,8,15,16 and intense (high numbers of repetitions for relatively long durations)17–23 with therapy sessions occurring 3–5 times per week.17,23 Many have advocated for multi-faceted approaches to therapy for individuals with SCI.5,24–26 Individuals with SCI who participated in a multi-modal, intense exercise program improved their American Spinal Injury Association (ASIA) total motor scores (TMS) and lower extremity motor scores (LEMS).27 TMS gains correlated with total hours per week spent in intense exercise. There is likely a dose-response relationship between the amount of intense, activity-based PT and functional recovery in individuals with SCI.28
Other interventions including pharmaceutics, stem cell-based and cellular/molecular therapies are expected to have synergistic benefits with activity-based therapies that optimize spinal cord regeneration and/or recovery.17,22 Excellent reviews are available concerning stem cell-based interventions for individuals with SCI.29–31 Olfactory ensheathing cells are specialized glial cells that possess neuroregenerative properties. Transplanting olfactory ensheathing cells into the site of the SCI may allow regenerating axons to penetrate through the glial scar and establish functional connections.32–35 Other possible transplantation benefits include promotion of re-myelination, vascularization, collateral sprouting, introduction of growth factor, reduction of scar tissue, and decreased secondary damage.29,36–43 Several authors have addressed the numerous issues that must be considered prior to clinical application of olfactory tissue transplantation;32,39,41,42,44–46 however, a few neurosurgical teams have elected to proceed with transplantation in humans with SCI. A Portuguese team performed partial scar and cyst removal and transplantation of minced olfactory mucosa (olfactory mucosa autograft, OMA) without and with culture.47–49 An Australian team used cultured olfactory ensheathing cells50,51 and a Chinese team used allografts of cultured cells from fetal olfactory bulbs.52,53
This independent research group had access to individuals with SCI that had elected to undergo the OMA procedure in Portugal and return to the United States to participate in an intense, activity-based, outpatient PT program. While participating in the intense program, patients were assessed using 21 outcome measures; ASIA sensory and motor outcomes are reported here.
The primary purpose of this study was to examine the effectiveness of an intense PT intervention program in promoting motor and sensory recovery in individuals with spinal cord injury. The second purpose was to compare motor and sensory recovery for individuals with SCI who had the OMA surgical procedure and participated in the intense PT program with individuals with SCI who did not have the OMA procedure and participated in the intense PT program. The first hypothesis was that intense, activity-based PT intervention will improve ASIA motor and sensory scores in individuals with chronic SCI. The second hypothesis was that individuals who had the OMA procedure and participated in the intense, activity-based PT intervention will improve motor and sensory scores at a greater magnitude and rate when compared to individuals who participated in the intense, activity-based PT intervention and did not have the OMA procedure.
Methods
Participants
Twenty-three subjects were recruited for this prospective, non-randomized, non-blinded, intervention study. Seven individuals had elected to undergo the OMA surgery (mean time since OMA = 3.7 ± 3.9 months) and six were matched control (MC) subjects; the OMA and MC groups were matched based on level of SCI, AIS classification, gender, approximate time since injury and age (Table 1). Ten ‘other’ subjects with a wider variety of SCI levels and ASIA Impairment Scale (AIS) scores that were participating in the intense PT program were also included in this study (Table 1). Exclusion criteria included: respiratory dependency, medically unstable, restrictive musculoskeletal impairments, or moderate to severe osteoporosis. There were no significant differences between the three groups for age or time since injury.
Table 1.
Demographic and SCI characteristics for the 23 participants including the OMA, MC and Other participants in the intense PT program. Mean ± standard deviation and range are presented for age and time since injury
| Total (n = 23) | OMA (n = 7) | Matched control (n = 6) | Other (n = 10) | |
|---|---|---|---|---|
| Gender | ||||
| Female | 3 | 0 | 0 | 3 |
| Male | 20 | 7 | 6 | 7 |
| Age (years) | 30.1 ± 9.8 (15.9–48.1) | 30.3 + 9.1 (19.5–43.3) | 28.4 ± 10.5 (15.9–45.3) | 30.9 ± 10.8 (17.6–48.1) |
| no significant difference between groups F(2,22) = 0.12; P = 0.89 | ||||
| Time since injury (years) | 5.1 ± 6.4 (0.3–27.8) | 2.9 ± 1.9 (1.0–5.6) | 5.0 ± 6.2 (0.5–13.2) | 6.8 ± 8.4 (0.3–27.8) |
| no significant difference between groups F(2,22) = 0.73; P = 0.5 | ||||
| ASIA single neurologic level | C4 – T11 cauda equina | C4 – T4 | C4 – T5 | C6 – T11 cauda equina |
| AIS grade | 14 AIS A | 5 AIS A | 4 AIS A | 5 AIS A |
| 2 AIS B | 1 AIS B | 1 AIS B | 3 AIS C | |
| 5 AIS C | 1 AIS C | 1 AIS C | 1 AIS D | |
| 1 AIS D | 1 cauda equina | |||
| 1 cauda equina | ||||
| Tetraplegia | 13 tetraplegia | 6 tetraplegia | 5 tetraplegia | 2 tetraplegia |
| Paraplegia | 10 paraplegia | 1 paraplegia | 1 paraplegia | 8 paraplegia |
Procedures/interventions
All study participants read and signed the Wayne State University Human Investigation Committee and Oakland University Internal Review Board approved consents/assents and Health Insurance Portability and Privacy Act forms. The participants’ medical records and self-reports were used to obtain all demographic information. All subjects participated in an intense, outpatient PT program which utilized a multi-faceted, activity-based approach primarily aimed at recovery below the level of injury. Each subject's therapy program was individualized and updated according to the primary physical therapist's monthly examination and evaluation results and defined/revised short- and long-term goals. The intense PT program was comprised of therapeutic activities and exercises as determined by the primary physical therapist to address the patient's goals which included 1 hour of (i) pre-gait (i.e. weight-bearing in multiple positions, posture and balance training, crawling, and standing pre-gait activities) and/or gait training (i.e. BWSTT and over-ground gait training), (ii) intense therapeutic exercise (i.e. repetitive neuromuscular facilitation, mat mobility, strengthening and endurance exercises, whole body vibration, biofeedback, virtual gaming, and/or musculoskeletal interventions), and (iii) functional electric stimulation cycling or static/dynamic standing frame (glider) activities.
Outcome measures
The ASIA motor and sensory examinations were performed by SCI-experienced physical therapists at initial and discharge examination and every 30 (motor) or 60 (sensory) days while subjects were participating in the program. Using the ASIA International Standards for Neurological Classification of SCI (ISNCSCI),54 motor function of the 10 key muscle groups were recorded using a six-point ordinal scale for both sides of the body resulting in an ASIA TMS (maximum score = 100). Sensation was recorded for the 28 key sensory points using a three-point ordinal scale for both sides of the body (maximum possible scores = 112) for light touch (LT) and pin prick (PP). The ASIA motor and sensory examinations were performed by two physical therapists at initial examination (IE), every 30 (motor) or 60 (sensory) days, and upon discharge. An assistant immediately recorded the scores to minimize data entry error. Both examiners thoroughly read the ASIA ISNCSCI manual54 and were trained to perform the motor and sensory examination procedures by one of the authors (C.A.L.) in two 1.5 hour sessions. In order to establish uniform testing procedures, both examiners performed motor and sensory examinations on four volunteers (two with complete and two with incomplete SCI); then discussed the examination procedures and scoring agreements/disagreements with each other, the primary author and two ASIA ISNCSCI-trained examiners (P.V. and E. N.). The ASIA ISNCSCI has been reported to be a reliable, responsive, and valid tool to evaluate adults with SCI over time.55–58 Inter-rater reliability values for TMS, LT, and PP have been reported to be 0.98, 0.96, and 0.96, respectively, and the corresponding values for intra-rater reliability were 0.99, 0.98, and 0.99, respectively.59 Particularly after training, high inter-rater reliability values (inter-class correlation coefficients (ICC) – TMS (0.98), LT (0.96), and PP (0.89)) were reported.60
Statistical methods
In order to standardize the interpretation of the ASIA motor and sensory data or the classification procedures, recently available software61 based on the ISNCSCI54 was used to determine the ASIA sensory and motor scores, single neurological levels, AIS classification, and zone of partial preservation (ZPP). Friedman and Wilcoxin signed ranks tests were performed comparing scores obtained from IE to test three (ASIA motor scores) and IE to test two (ASIA sensory scores) (first 60 days in therapy) as well as from IE to discharge (DC) examination (regardless of therapy duration). Total LT and PP scores were partitioned into UE, trunk and LE sub-scores (maximum possible scores = 32, 44, and 36 points, respectively). Sample sizes were necessarily reduced if the IE motor or sensory sub-scores equaled the maximum possible score.
Since the subjects’ motor and sensory scores were significantly different at baseline (IE) (P ≤ 0.005, motor and sensory), an analysis of covariance (ANCOVA) was used in which the baseline score for the dependent variable was used as a covariate. Pair-wise group differences (OMA, MC, and Other) were examined controlling for multiplicity using the Sidek method.
In order to determine participants’ characteristics associated with motor recovery, a restricted maximum likelihood for parameter estimation and mixed-effects modeling approach was used.62 The mixed-effects modeling method can manage missing data, variable change across participants and explicitly examine heterogeneity at baseline and over time. The model was built sequentially assuming a linear motor score change trajectory (the individual y-intercept represented the baseline motor score and the slope represented the within subject rate of change). First, a level one model (unconditional model, random intercept) was used to examine the variability of the IE TMS. Unconditional models allow partitioning of the TMS variance into its within- and between-individual components. Intraclass correlation coefficients were computed by dividing the random effects of between- and within-subject variation by the total variation. Next, an unconditional model (random intercept/random coefficient) was used to examine the variance of the IE TMS and change in TMS over time. In the third step, a level two conditional (random intercept/random slope) model was used to introduce covariates or predictor variables hypothesized to affect the individual growth parameters. In other words, covariates were used to explain the mean motor scores at baseline and linear change rate across individuals over time.
Results
Therapy dosage
Intense, outpatient, PT dosage was targeted to be three-hour sessions, 3–5 times per week for a minimum duration of three months. For the 23 participants, actual mean therapy hours per week were 7.1, mean duration in the intense PT program was 4.6 months, mean total hours in the program was 137.3 and mean attendance rate was 89.2% (Table 2). Reasons for cancellations and/or therapy termination included transportation, medical, personal, and/or financial/insurance issues. Therapy dosage and attendance and cancellation rates partitioned for the three groups (OMA, MC, and Other) are presented in Table 2. There were no significant differences between the three groups for mean therapy duration, total therapy hours, attendance, or cancellation rates. There were significant differences between the three groups for mean hours per week spent in intense PT; pair-wise comparisons (PWC) revealed a significant difference between the OMA and Other groups and no differences between the OMA and MC or MC and Other groups. It should be noted that a pattern of participation in the intense therapy program was observed with seven subjects (1 OMA, 2 MC, and 4 Other) clearly participating in the program for longer durations (>4 months) and less hours per week (<7.6 hours per week) (LDLH) and eight subjects (5 OMA, 2 MC, and 1 Other) clearly participating in the program for relatively shorter durations (≤4 months) and greater hours per week (≥7.6 hours per week) (SDMH). Subjects in the LDLH group lived in the local area (in-state) while those in the SDMH group were from out-of-state and temporarily lived on the medical campus. Eight subjects (Mixed) could not be categorized in either group since they participated in therapy either for longer durations, more hours per week or shorter durations, less hours per week. Owing to this emerging therapy dosage pattern, this new characteristic (LDLH, SDMH, and Mixed) was examined as related to the stated hypotheses. However, it should be emphasized that total hours participating in the intense therapy program was determined to be the primary therapy dosage variable of interest. For the 23 participants, total hours of therapy were 137.3 ± 83.9 (range 52.0–337.8) with no significant difference between the three groups (OMA, MC, and Other) (F(2,20) = 1.3; P = 0.3).
Table 2.
Mean ± SD (range) therapy dosage (hours per week, duration and total hours in the intense, activity-based PT program), attendance and cancellation rates for the 23 participants and the OMA, MC and Other participants
| Total (n = 23) | OMA (n = 7) | Matched control (n = 6) | Other (n = 10) | |
|---|---|---|---|---|
| Hours per week | 7.1 ± 1.7 (5.1–11.3) | 8.5 ± 2.1 (5.3–11.3) | 6.6 ± 1.3 (4.5–8.4) | 6.4 ± 1.2 (4.1–8.5) |
| significant difference between groups = F(2,20) = 4.0; P = 0.03 | ||||
| PWC – OMA and Other (P = 0.04) | ||||
| no significant difference – OMA and MC or MC and Other | ||||
| Therapy duration (months) | 4.6 ± 2.8 (2.5–11.6) | 2.9 ± 1.2 (2.5–4.5) | 5.5 ± 3.5 (2.5–11.6) | 5.4 ± 2.6 (2.5–9.3) |
| no significant difference between groups = F(2,20) = 3.0; P = 0.07 | ||||
| Total hours in therapy | 137.3 ± 83.9 (52.0–337.8) | 95.5 ± 46.3 (53.0–175.0) | 155.8 ± 93.6 (69.0–337.8) | 155.6 ± 94.7 (52.0–332.8) |
| no significant difference between groups = F(2,20) = 1.3; P = 0.3 | ||||
| Attendance rate (%) | 89.2 ± 6.4 (74.2–96.6) | 90.5 ± 5.7 (80.0–96.3) | 88.8 ± 8.3 (74.2–96.6) | 88.5 ± 6.1 (75.7–95.0) |
| no significant difference between groups = F(2,20) = 0.2; P = 0.8 | ||||
| Cancellation rate (%) | 10.8 ± 6.3 (3.4–25.8) | 9.5 ± 5.7 (3.7–20.0) | 11.2 ± 8.3 (3.4–25.8) | 11.4 ± 6.0 (5.0–24.3) |
| no significant difference between groups = F(2,20) = 0.2; P = 0.8 | ||||
Motor recovery
Individual subject's ASIA TMS over the course of therapy for all subjects (n = 23) are presented in Fig. 1. The entire group of subjects improved significantly in their TMS from IE to test three (first 60 days) (χ2 = 14.8; P = 0.001) and from IE to DC examination (regardless of therapy duration) (Z = −3.8; P ≤ 0.005). Subjects significantly improved in their upper extremity motor score (UEMS) from IE to test three (χ2 = 13.4; P = 0.001) and from IE to DC examination (Z = −3.1; P = 0.002) (n = 12). The entire group significantly improved in their LEMS from IE to test three (χ2 = 8.1; P = 0.02) and from IE to DC examination (Z = −3.0; P = 0.003). In order to examine the magnitude of motor recovery, mean change in TMS, UEMS, and LEMS from IE to test three and IE to DC examinaton for all 23 subjects and the OMA, MC, and Other subjects was determined (Table 3). From IE to DC exam, TMS improved, on an average, 5.5 points with a mean UEMS change of 5.9 points and a mean LEMS change of 3.2 points.
Figure 1.
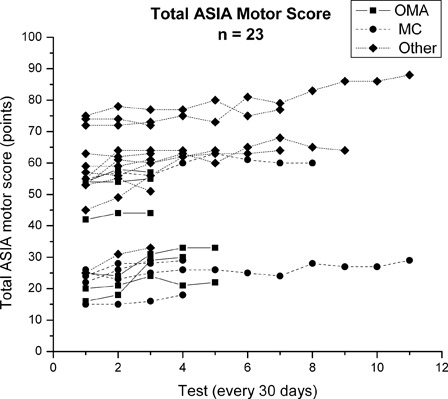
ASIA TMSs (points) for all 23 subjects (OMA (n = 7), MC (n = 6) and Other (n = 10) sub-groups) (maximum score = 100). Each line represents an individual subject's TMS over the course of their participation in the intense, activity-based PT program. Tests were conducted at approximately 30 day intervals.
Table 3.
Mean ± SD change in ASIA motor scores (points) from IE to test three and IE to discharge (DC) examination for all (n = 23) participants and the OMA, MC and Other participants in the intense PT program. PWC between the three groups were examined controlling for multiplicity using the Sidek method
| Total | OMA | Matched control | Other group | Comparisons (ANCOVA) | |
|---|---|---|---|---|---|
| Change in TMSs | |||||
| n = 23 | n = 7 | n = 6 | n = 10 | ||
| IE to test 3 | 3.6 ± 4.0 | 5.3 ± 4.2 | 2.5 ± 2.6 | 3.1 ± 4.5 | F(2,19) = 3.7; P = 0.05 |
| no significant difference PWC | |||||
| IE to DC | 5.5 ± 5.5 | 5.4 ± 4.8 | 4.3 ± 1.8 | 6.2 ± 7.5 | F(2,19) = 1.5; P = 0.24 |
| Change in UEMS (excluded subjects with initial score = 50) | |||||
| n = 12 | n = 5 | n = 5 | n = 2 | ||
| IE to test 3 | 5.2 ± 5.7 | 6.0 ± 3.2 | 4.6 ± 8.6 | 4.5 ± 3.5 | F(2,8) = 0.1; P = 0.89 |
| IE to DC | 5.9 ± 5.3 | 5.8 ± 3.9 | 6.2 ± 7.8 | 5.5 ± 2.1 | F(2,8) = 0.03; P = 0.97 |
| Change in LEMS | |||||
| n = 23 | n = 7 | n = 6 | n = 10 | ||
| IE to test 3 | 1.7 ± 2.6 | 1.0 ± 1.4 | 1.4 ± 1.7 | 2.4 ± 3.4 | F(2,18) = 2.8; P = 0.09 |
| IE to DC | 3.2 ± 4.8 | 1.3 ± 1.4 | 2.2 ± 2.3 | 5.3 ± 6.5 | F(2,18) = 2.4; P = 0.12 |
ASIA TMS over the course of therapy for each individual participant in the OMA, MC, and Other groups are presented in Fig. 1. There were no significant differences between the OMA, MC, and Other groups in magnitude of TMS, UEMS, or LEMS change from IE to test three or from IE to DC examination (Table 3). One exception was change in TMS from IE to test three; however, PWC did not confirm the group differences.
Visual inspection of the TMS data presented in Fig. 1 revealed considerable between-subject variability at the IE and in motor score changes over time. Further exploration of the IE motor scores using the mixed-effects modeling approach revealed that, as a group (n = 23), the mean IE TMS (y-intercept) was 47.4 points and significant between-subject variability (Z-ratio = 11.8; P ≤ 0.0005) was present (level one-unconditional model without covariates) (Table 4). Since IE motor score contained significant variability, it was used for prediction in subsequent unconditional/conditional models. The ICC estimates revealed that 96.3% of the IE TMS variation was between-participants and 3.7% was within-participants. Results of the second unconditional model (level one – random intercept/random coefficient) (Table 4) revealed that subjects’ TMS significantly changed over the duration (months) in the intense PT program (Z-ratio = 5.06; P ≤ 0.0005) with an average growth rate of 1.28 points per month. Since change in TMS contained significant variability, it was used for prediction in subsequent conditional models. ICC estimates revealed that 99% of the variation was between-subjects and 1.0% was within-subjects.
Table 4.
Mixed-effects modeling approach results: (i) unconditional model without covariates (only the IE TMS was allowed to vary) and (ii) unconditional model without covariates (random intercept/random coefficient) (the IE TMS (y-intercept) and TMS over therapy duration (slope) were allowed to vary). ICC are also presented
| Effect | Coefficient | Variance component | SE | Z-ratio | Probability | ICC | |
|---|---|---|---|---|---|---|---|
| Unconditional model without covariates | |||||||
| Fixed | IE TMS y-intercept (mean) | 47.42 | 4.02 | 11.80 | 0.0005 | ||
| Random | IE TMS | 368.43 | 109.46 | 0.963 | |||
| Level 1 error | 14.29 | 1.95 | 0.037 | ||||
| Unconditional model without covariates (random intercept/random coefficient) | |||||||
| Fixed | IE TMS y-intercept (mean) | 43.21 | 4.12 | 10.48 | 0.0005 | ||
| Change in TMS slope over time (month) | 1.28 | 0.25 | 5.06 | 0.0005 | |||
| Random | IE TMS | 387.00 | 115.43 | 0.987 | |||
| Change in TMS | 1.04 | 0.45 | 0.003 | ||||
| Level 1 error | 3.96 | 0.61 | 0.01 | ||||
Covariates/predictors were introduced into the level two conditional analyses (random intercept/random slope model with covariates) to attempt to explain between-subject variation in IE TMS (y-intercept) and change in TMS over time (slope) (Table 5). Having an incomplete (AIS B, C, or D) injury or paraplegia were the characteristics associated with the variability in IE TMS. Age, time since injury or being in the OMA, MC, or Other group did not explain the variation in IE TMS. Characteristics associated with change in TMS over time in therapy (slope) (motor recovery) included having an incomplete injury or paraplegia. Characteristics not associated with motor recovery included having had the OMA procedure, age, time since injury, total therapy hours, therapy hours per week or months in therapy, or being categorized in one of the three therapy dosage categories (LDLH, SDMH, or Mixed groups) (Table 5).
Table 5.
Level two conditional analyses (random intercept/random slope model with covariates)
| Characteristic | IE TMS | TMS change IE to DC exam |
|---|---|---|
| Complete or incomplete SCI | z = 2.41, P = 0.016 | z = 2.90, P = 0.004 |
| Tetraplegia or paraplegia | z = 5.38, P ≤ 0.005 | z = 4.97, P ≤ 0.005 |
| OMA, MC, and Other | z = −0.08, P = 0.94 | z = −0.68, P = 0.50 |
| Age (years) | z = 0.5, P = 0.63 | z = 1.65, P = 0.10 |
| Time since injury (years) | z = 0.01, P = 0.99 | z = −0.25, P = 0.81 |
| Total hours in intense therapy | z = 0.78, P = 0.43 | |
| Therapy dosage (hours per week) | z = −1.03, P = 0.30 | |
| Therapy duration (months) | z = −0.55, P = 0.59 | |
| LDLH, SDMH, and Mixed | z = −0.26, P = 0.79 |
Characteristics associated (italics) or not associated with IE TMS and change in TMS from initial (IE) to discharge (DC) examination for the 23 participants in the intense PT program are presented. Results included categorization into the three therapy dosage groups – LDLH, SDMH per week and mixed (either SDMH and LDLH per week).
Five subjects who had motor-complete (4 AIS A; 1 AIS B) SCI at IE converted to motor-incomplete (AIS C) SCI at DC examination (Table 6). Time since injury for the five subjects ranged from 0.5 to 2.7 years and two subjects had the OMA procedure, two subjects were MC subjects, and one subject was in the Other group. For each subject, sensory, motor and overall neurological level (NL), sensory and motor zone of partial preservation (ZPP), UE and LE TMS, and total LT and PP scores at IE and DC examination (if appropriate) are presented. In addition, changes in S4-5 LT/PP, voluntary anal contraction and/or anal sensation; specific key muscle strength changes and newly present key muscles present at the DC examination are presented. For the nine subjects (S) who had motor-complete (9 AIS A) SCI at IE and did not convert to motor-incomplete, their IE SNL/motor ZPP were: S1 C6/T1, S2 C7/C8, S7 C5/T1, S9 T10/L2, S16 T4/L3, S18 T5/L2, S19 C6/C8, S20 T11/L3, S21 T8/L1, S22 C6/C7, and S23 C6/C7.
Table 6.
Specific ASIA motor and sensory changes (italics) from initial to discharge examination for subjects that converted from a motor-complete (AIS A/B) to motor-incomplete (AIS C) spinal cord injury
| Subject number | Group | Time since injury (years) | Initial examination |
AIS IE/DC | Discharge examination |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| NL (sensory/motor/SNL overall) | ZPP (sensory/motor) | TMS (UE/LE) (points) | Total LT/PP (points) | NL (sensory/motor/SNL overall) | TMS (UE/LE) (points) | Total LT/PP (points) | ||||
| 3 | OMA | 2.7 | C4/C5/C4 | T7/T7 | 16/0 | 33/15 | A/C | C4/C6/C4 | 26/2 | 42/14 |
| 8 | Other | 2.7 | T5/T5/T5 | T12/L3 | 50/3 | 61/46 | A/C | T5/T5/T5 | 50/4 | 81/46 |
| 14 | MC | 1.1 | T5/T5/T5 | S3/L4 | 50/5 | 68/54 | A/C | T5/T5/T5 | 50/10 | 75/55 |
| 15 | MC | 0.5 | C7/C7/C7 | NA | 27/0 | 112/98 | B/C | T4/C7/C7 | 36/3 | 112/102 |
| 17 | OMA | 2.0 | C8/T1/C8 | T4/L3 | 50/1 | 39/32 | A/C | C8/T1/C8 | 50/5 | 60/32 |
| S4-5/anal sensory changes - IE to DC | Specific motor (muscle strength) changes – IE to DC | Newly present key muscles at DC | ||||||||
| 3 | LT S4-5 present | B elbow flexors 3–5 | L finger abductor grade 1 | |||||||
| B wrist extensors 3–4 | B hip flexors grade 1 | |||||||||
| B elbow extensor 1–2 | ||||||||||
| L finger flexors 1–2 | ||||||||||
| 8 | LT S4-5 and anal sensation present | L knee extension 1–2 | ||||||||
| 14 | LT/PP S4-5, anal voluntary contraction and anal sensation present | R hip flexor 1–2 | L hip flexor grade 2 | |||||||
| L ankle DF and long toe extensor grade 1 | ||||||||||
| 15 | Not applicable | Wrist extensors R 4–5, L 3–5 | L hip flexor grade 2 | |||||||
| B elbow extensors 3–5 | R hip flexor grade 1 | |||||||||
| B finger flexors 1–2 | ||||||||||
| 17 | LT S4-5 and anal sensation present | L hip flexor 1–2 | R hip flexor grade 2 | |||||||
| L knee extensor grade 1 | ||||||||||
Abbreviations: B, bilateral; L, left; R, right.
Sensory recovery
Twenty-one of the 23 participants had complete sensory data sets. Individual subject's total LT and PP scores (maximum score = 112) over the course of therapy are presented in Figs. 2A and B. From IE to test two (first 60 days of therapy), the 21 subjects significantly improved in their total LT scores (Z = −2.0; P = 0.04), but did not improve their total PP scores (Table 7). From IE to DC exam, the 21 subjects did not significantly improve in either total LT or total PP scores. Similarly, there were no significant changes in UE, trunk or LE LT or PP scores from IE to test two or over the course of therapy (IE to DC exam) (Table 7).
Figure 2.
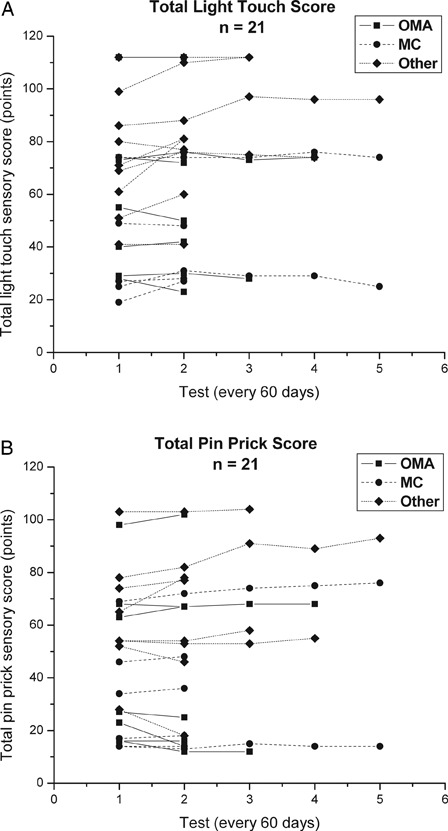
ASIA total LT (A) and PP (B) scores (points) for 21 subjects (OMA (n = 7), MC (n = 5) and Other (n = 9) sub-groups) (maximum score = 112). Each line represents an individual subject's sensory scores over the course of their participation in the intense, activity-based PT program. Tests were conducted at approximately 60 day intervals.
Table 7.
Mean ± SD change in ASIA sensory LT and PP scores (points) from IE to test two (first 60 days) and from IE to DC examination for 21 participants in the intense, activity-based PT program. Total, UE, trunk and LE change in LT and PP scores are presented. Sample sizes for the UE change in sensory scores are reduced due to exclusion of subjects with an IE score = 32
| Mean change in LT scores | Total | UE (n = 10) | Trunk | LE |
|---|---|---|---|---|
| IE to test 2 | 3.1 ± 6.1* | 0.7 ± 1.8 | 1.8 ± 4.1 | 1.1 ± 4.3 |
| IE to DC exam | 3.0 ± 6.4 | 0.5 ± 1.4 | 1.8 ± 4.1 | 1.2 ± 4.6 |
| Mean change in PP scores | ||||
| Total | UE (n = 13) | Trunk | LE | |
| IE to test 2 | 0.1 ± 4.9 | 0.3 ± 1.7 | 0.3 ± 3.6 | 0.2 ± 1.9 |
| IE to DC exam | 1.2 ± 6.0 | 0.9 ± 3.1 | 0.8 ± 3.8 | 0.7 ± 2.9 |
*Z = −2.0; P = 0.04.
There were no significant differences between the OMA (n = 7), MC (n = 5), and Other (n = 9) subjects in magnitude of change in total, trunk or LE LT and PP scores or UE LT scores from IE to test two or from IE to DC examination (Table 8). UE PP scores were significantly different between the three groups from IE to test two and IE to DC examination with PWC revealing a significant difference between the OMA and Other groups and no significant differences between the OMA and MC or MC and Other groups (Table 8).
Table 8.
Mean ± SD change in ASIA sensory total, UE, trunk and LE LT and PP scores (points) from IE to test two (first 60 days) and IE to DC examination for the OMA, MC and Other participants in the intense PT program. Sample sizes for the UE change in sensory scores are reduced due to exclusion of subjects with an IE score = 32
| OMA n = 7 | Matched control n = 5 | Other n = 9 | Group comparisons (ANCOVA, PWC, Sidek method) | |
|---|---|---|---|---|
| Change in total LT scores | ||||
| IE to test 2 | 0.1 ± 4.8 | 4.4 ± 3.6 | 4.6 ± 7.6 | F(2,17) = 1.7; P = 0.2 |
| IE to DC | −0.1 ± 4.8 | 2.8 ± 3.6 | 5.4 ± 8.0 | F(2,17) = 1.4; P = 0.3 |
| Change in total PP scores | ||||
| IE to test 2 | −3.0 ± 4.9 | 0.6 ± 2.1 | 2.2 ± 5.1 | F(2,17) = 0.7; P = 0.5 |
| IE to DC | −3.0 ± 4.9 | 1.2 ± 1.6 | 4.6 ± 6.5 | F(2,17) = 1.1; P = 0.4 |
| Change in UE LT scores | ||||
| n = 6 | n = 4 | |||
| IE to test 2 | −0.3 ± 1.5 | 2.3 ± 1.2 | F(1,6) = 5.3; P = 0.06 | |
| IE to DC | 0.2 ± 0.8 | 0.7 ± 2.5 | F(1,6) = 0.2; P = 0.7 | |
| Change in UE PP scores | ||||
| n = 6 | n = 4 | n = 3 | ||
| IE to test 2 | −1.2 ± 1.0 | 1.0 ± 0.8 | 1.7 ± 2.1 | F(2,9) = 6.1; P = 0.02 |
| OMA and Other (P = 0.04) | ||||
| OMA/MC or MC/Other not significant | ||||
| IE to DC | −1.2 ± 1.0 | 1.0 ± 0.8 | 4.0 ± 5.2 | F(2,9) = 4.4; P = 0.05 |
| OMA and Other (P = 0.05) | ||||
| OMA/MC or MC/Other not sig | ||||
| Change in trunk LT scores | ||||
| IE to test 2 | 1.4 ± 5.6 | 2.5 ± 2.1 | 1.9 ± 3.8 | F(2,15) = 0.6; P = 0.6 |
| IE to DC | 0.9 ± 5.6 | 2.5 ± 2.1 | 2.4 ± 3.5 | F(2,15) = 0.6; P = 0.56 |
| Change in trunk PP scores | ||||
| IE to test 2 | −1.1 ± 3.2 | −0.6 ± 3.1 | 1.3 ± 4.1 | F(2,17) = 0.2; P = 0.82 |
| IE to DC | −1.1 ± 3.2 | 1.0 ± 2.8 | 2.2 ± 4.4 | F(2,17) = 0.25; P = 0.78 |
| Change in LE LT scores | ||||
| IE to test 2 | −1.0 ± 1.6 | 1.5 ± 1.9 | 2.8 ± 5.9 | F(2,15) = 1.7; P = 0.2 |
| IE to DC | −1.1 ± 1.5 | 1.3 ± 2.5 | 3.3 ± 6.4 | F(2,15) = 1.6; P = 0.2 |
| Change in LE PP scores | ||||
| IE to test 2 | 0.6 ± 1.5 | −0.8 ± 1.8 | 0.6 ± 2.3 | F(2,17) = 0.9; P = 0.42 |
| IE to DC | 0.6 ± 1.5 | −0.6 ± 1.9 | 1.6 ± 4.0 | F(2,17) = 1.0; P = 0.38 |
Discussion
Effectiveness of intense, activity-based physical therapy
Intense, activity-based PT was effective in promoting motor recovery in individuals with SCI. Participation in the intense PT program improved TMS at a mean rate of 1.3 points per month. On average, TMS, UEMS, and LEMS improved 5.5, 5.9, and 3.2 points, respectively, from initial to discharge examination. Similarly, individuals with chronic SCI who participated in a six month, intense exercise program demonstrated a mean change of 4.8 points in TMS and 3.3 points in LEMS.27 Gains of this magnitude in individuals that were, on average, five years post-SCI are noteworthy since expected motor recovery in individuals greater than 18 months post-SCI are limited to 1–2 points per year.63,64
In contrast, from initial to discharge examination, intense PT was not effective in promoting sensory recovery in individuals with SCI. Interventions directly targeting sensory recovery such as sensory integration or retraining were not a primary focus of the intense PT program; however, sensory input from repetitive movement, weight bearing, therapeutic activities and manual facilitation was expected to improve sensation. Proprioception or sensory modalities other than the superficial sensations of LT and PP may have changed with intense therapy but were not monitored. It is unclear why the participants improved in their LT sensory scores within the first 60 days of therapy; whereas, LT and PP sensory scores did not significantly change over the entire course of therapy. Nine of the 21 subjects had sustained a thoracic injury. It is our observation that the sensory level can vary especially in the thoracic region due to the subjectivity of the thoracic dermatome locations. Saddiki-Trak et al.65 described the existence of a band of altered, often abnormal, sensation at the boundary between the sentient and insentient skin on the anterior trunk of individuals with thoracic complete SCI. This zone can extend for as much as 12 cm inferior to the insentient skin and has an average threshold (relative to the grams of force needed for detection) that was 58% greater than controls. This may or may not explain the variability in sensation over time.
Further exploration of the motor data revealed that participants with an incomplete (AIS B, C or D) SCI or paraplegia demonstrated greater motor recovery. Similarly, Harness et al.27 reported that individuals who were motor-incomplete had significantly greater gains in motor recovery over a six-month intense exercise period. In general, individuals with incomplete as compared with complete tetraplegia have greater UE motor recovery and highly variable LE recovery.64,66,67 Individuals with thoracic/lumbar and incomplete SCI had greater gains in mobility and self-care as compared with those with cervical and complete SCI.68 Even though intense PT participants with incomplete SCI or paraplegia had greater motor recovery; some participants with complete SCI demonstrated motor recovery as well.
Conversion from complete to incomplete injury is limited in those with chronic SCI; AIS conversions from A to C and C to D have been reported to be 1.1% and 21.5%, respectively, from year one to five post-SCI.69 Ninety percent of individuals with complete tetraplegia at one month post-SCI remained complete at three years post-injury and only 0.05% recovered some LE function.63 Comparatively, in the current study, 5 of 14 participants (35%) with motor-complete SCI (four participants AIS A; one participant AIS B) at IE converted to motor-incomplete (AIS C) at the DC examination and one participant converted from AIS C to D. The four participants classified as AIS A who converted to motor-incomplete had extensive IE motor ZPP (motor ZPP extended 11–14 segments below the SNL) as compared with the nine participants with motor-complete SCI who did not convert to motor-incomplete SCI (motor ZPP extended 1–10 segments below the SNL). Therefore, a majority of the motor score changes were due to strengthening muscles that were already present; presumably due to muscle fiber hypertrophy, type IIx/IIb to IIa fiber-type conversion and/or increased capillary density.70,71 However, motor score increases were also due to newly gained function of key muscles below the ZPP (Table 6). Four of the five individuals who converted to motor-incomplete SCI had motor recovery below their ZPP and partial recovery of LE motor function (Table 6). In principle, recovery within the ZZP could be due to central and/or peripheral nervous system plasticity while recovery below the ZPP would possibly require at least some central nervous system repair.64 It should be noted that, at the time of discharge, the LE muscles received ASIA grades of one or two; therefore, muscle strength was considered non-functional.64 It is unknown if further participation in the intense therapy program would have led to the attainment of functional muscle strength (greater than ASIA grade three). Since conversion from AIS A–C and recovery below the ZPP rarely occurs in those with complete SCI,72,73 recovery in individuals with motor-complete SCI indicates that participation in the intense PT program did promote greater-than-expected motor recovery.
Harness et al.27 reported a weak correlation (r = 0.53) between hours per week participating in an intense exercise program and change in ASIA motor scores. Conversely, in the current study, therapy dosage (total therapy hours, hours per week, and therapy duration) did not significantly correlate with motor recovery. Since this research project was carried out in a clinical setting, dosage could not be well controlled. Participation in the intense PT program varied due to factors such as motivation, medical complications, and insurance/financial issues. The amount of variability in dosage as measured by therapy hours per week or months within the program was not anticipated. Individuals that were in the program less hours per week over a longer therapy duration did not differ in motor outcomes as compared with those that were in the program for more hours per week over a shorter therapy duration. The key variable to examine; therefore, was determined to be total hours of intense, activity-based PT. On average, 137 total hours of intense PT was effective in promoting motor recovery; therefore some minimally effective dosage appears to have been reached for this cohort of participants. There may be a yet-unidentified, optimal therapy dosage that generates motor recovery. Currently, there is no consensus as to the optimal therapy dosage for activity-based, outpatient PT. Dosages used in past studies or recommended dosages included 7.3 hours per week over six months,27 thirty-three hours per week for 1.8 years,47 nine hours per week,74 or six hours per day, six days per week over a six months duration.75,76 Future dose-response research must be performed in order to identify the optimal, effective therapy dosage required to create clinically meaningful change in motor recovery after SCI. It is important to identify the optimal therapy dosage in order to minimize over-utilization of insurance benefits and/or participants’ financial resources; however, individual differences must be considered. In addition, the number of points of improvement in total ASIA motor scores which indicate a clinically meaningful- or functional-change has not been identified. Since functional outcome measures were recorded for the 23 participants in the intense PT program, the associated functional changes will be forth-coming in future publications.
It may not be the therapy dosage,68,77,78 but rather the therapeutic activity or combination of activities,2,27 the number of exercise repetitions per session,20,21 the exercise/rest ratio and/or the timing of therapy initiation that drives recovery for individuals with SCI. The intense, out-patient PT program utilized an activity-based approach comprised of a number of therapeutic activities and exercise strategies. Whereas frequency and duration of participation in the intense PT program was recorded, the therapeutic exercises and activities were not quantified precisely. There is a need to perform future research which would precisely quantify the minutes spent performing each type of therapeutic activity relative to recovery outcomes after SCI. The SCIRehab project is in the process of precisely classifying and quantifying interventions across disciplines in six in-patient SCI rehabilitation facilities.79–82 A similar, multi-site study specifically quantifying interventions and outcomes for individuals participating in out-patient, activity-based programs including this study's site is in the planning stages.
Is olfactory mucosa autograft a factor?
Individuals with SCI who had elected to undergo the OMA procedure and then participated in the intense, activity-based, outpatient PT program did not have greater sensory recovery or greater magnitude or rate of motor recovery when compared to individuals who participated in the intense PT program and had not had the OMA. The OMA and MC groups were not significantly different in terms of gender, age, time since injury, level of injury, AIS grades or therapy dosage. Lima et al.47,49 have reported significant sensory and motor recovery following OMA plus rehabilitation. From baseline (pre-OMA) to the final examination post-OMA, after 28 ± 11 months of intense rehabilitation, ASIA UEMS, and LEMS improved, on average, 4.5 and 5.0 points, respectively.49 However, as clearly stated by the authors, ‘one of the limitations of this study was that there was no control group with rehabilitation alone to separate the effects of rehabilitation, and OMA and rehabilitation.’49 Eight of the 20 subjects had participated in a year or more of intense rehabilitation before the OMA procedure with no change in AIS grade and LEMS remained zero, which led the authors to state that ‘rehabilitation alone was not sufficient for recovery.’49 It is unclear if the content of the pre- and post-operative rehabilitation was the same. In two other phase I/IIA studies, five individuals with chronic SCI, C5-T12, motor-complete83 and six individuals with complete T4-T7 SCI51 who had undergone the OMA procedure had no significant motor or sensory recovery and none of the LE muscles that scored zero at baseline improved at the 6 or 36 month follow-up. Chhabra et al. (2009) had instructed their subjects to follow a defined and standardized physical rehabilitation program; however, the dosage was not recorded and ‘the compliance…may have been low’;83 whereas, Mackey-Sim et al.51 had not instructed their subjects to follow ‘any particular exercise regime.’ These results along with the results of the current study indicate that intense PT alone may be the key factor in promoting motor recovery for individuals with SCI. On the other hand, intense PT after the various neural recovery/neuroregenerative surgical procedures including the OMA procedure appears to be an essential component for motor recovery.36,47,83
It should be noted that there was a tendency for the matched control as compared to the OMA participants to improve their UE LT scores over the first 60 days in the intense PT program; however, this group difference was not sustained over the entire course of therapy. It is uncertain if this is a true finding or is another example of the apparent variability of sensory score outcomes when tested repeatedly over time. As stated previously, thoracic sensation may vary due to the extensive sentient-insentient band present in the anterior torso.65 Variability in cervical dermatome locations as illustrated by the various dermatome maps may also explain the variability observed in the repeated sensory testing.84 It appears important to examine the stability of sensory scores over time and establish the number of levels of sensory return that indicates true recovery of sensation in individuals with SCI. On the other hand, it does appear that there was a true difference between the OMA and Other participants as to change in UE PP scores; the Other participants improved their UE PP scores at both the 60 day and discharge examination as compared to the OMA participants. It is unclear whether the loss of UE PP sensation in the OMA group is a true loss of PP sensation or whether a reduction of, on average, 1.2 points is within the normal variance observed when performing repeated testing of PP sensation over time. Substantial gains in both LT and PP sensation were reported in subjects who had the OMA procedure plus rehabilitation. However, 1 of 7 (2006) and 1 of 20 (2009) subjects had LT and PP sensory loss at 3–18 months post-OMA presumably due to ‘some sensory axons being damaged during the surgical procedure.’47,49
The findings of the current study and past studies that included individuals having had the OMA must be considered preliminary due to the small sample sizes. Additional limitations for the current study include the inability to examine a non-therapy control group; if individuals were not receiving therapy, they did not consistently attend scheduled examinations every 30 days. There was no access to motor and sensory data immediately after the OMA surgery and long-term follow-up after discharge from the intense therapy program was not possible. Attempts were made to keep the examiners blinded as to who had had the OMA; however, two participants inadvertently verbally informed the examiners of their OMA history. Efforts were made to have the same examiner perform the repeated ASIA motor and sensory measures for each individual participant; however, due to scheduling issues, this was not possible for 4 of the 23 participants. For two of these four participants, the physical therapist who was treating the patient was also the examiner who performed the ASIA motor testing.
It must be emphasized that the rate of motor recovery for the OMA and MC groups was not different over the course of intense PT; but it cannot be assumed that motor recovery is a linear process. Lima et al.47 reported a possible bi-modal pattern in recovery post-OMA with early (3–6 months) and late (greater than one year) peaks. Since the subjects participated in the intense PT program for an average of 4.6 months, it is likely that the current study captured the early recovery peak; however, any later recovery may have been missed. This again emphasizes the importance of determining the minimally effective dosage, duration, and content of an intense PT program for both individuals with SCI who elect to undergo a neural recovery/regenerative surgical procedure and those that elect intense PT alone.
Conclusion
This study provides encouraging evidence as to the effectiveness of an intense, activity-based, outpatient PT program designed for individuals with SCI. Motor recovery (TMS as measured by the ASIA ISNCSCI examination), improved at a mean rate of 1.3 points per month over the intense therapy period. In contrast, intense therapy was not effective in promoting sensory recovery. Participants with an incomplete SCI or paraplegia had greater motor recovery; however, five of 14 participants with complete SCI converted from motor-complete to motor-incomplete. Individuals with SCI who had elected to undergo the OMA procedure and then participated in the intense PT program did not have greater sensory recovery or greater magnitude or rate of motor recovery as compared to individuals who had not had the OMA. Future therapy dose-response and therapy-content research must be performed in order to identify the specific therapeutic activities and optimal, effective therapy dosage required to generate clinically meaningful recovery for individuals with SCI including those who elect to undergo a neural recovery surgical procedure and those that elect intense, outpatient, activity-based PT alone.
Acknowledgements
This project was supported by the Rehabilitation Institute of Michigan Del Harder fund. We thank Susan Harrington, PT (data collection); Leah Maddern, BS, BA, and Bridget McDermott, S.P.T. (data processing); Jeffery D. Baril, PT and Christopher J. Hinze, PT (writing assistance); Scott Millis, PhD, Med, ABPP (statistical assistance) and Edward C. Nieshoff, M.D. (Director, Outpatient Spinal Cord Injury Services; ASIA trained examiner), and Phuong Vu PT (ASIA trained examiner).
References
- 1.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther 2006;86(10):1406–25 [DOI] [PubMed] [Google Scholar]
- 2.Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18(2):183–202 [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004;127(6):1403–14 [DOI] [PubMed] [Google Scholar]
- 4.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 1998;79(3):1329–40 [DOI] [PubMed] [Google Scholar]
- 5.Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev 2008;45(2):229–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol 2004;476(2):130–45 [DOI] [PubMed] [Google Scholar]
- 7.Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull 2009;78(1):I–VI [DOI] [PubMed] [Google Scholar]
- 8.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 2006;66(4):484–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma 2002;19(10):1217–29 [DOI] [PubMed] [Google Scholar]
- 10.McDonald JW, Becker D. Spinal cord injury: promising interventions and realistic goals. Am J Phys Med Rehabil 2003;82(10 Suppl.):S38–49 [DOI] [PubMed] [Google Scholar]
- 11.Barbeau H, Nadeau S, Garneau C. Physical determinanats, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma 2006;23(3–4):571–85 [DOI] [PubMed] [Google Scholar]
- 12.Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev 2009;15(2):112–6 [DOI] [PubMed] [Google Scholar]
- 13.Wernig A. Long-term body-weight supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 2006;44(4):265–6 [DOI] [PubMed] [Google Scholar]
- 14.Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective wellbeing. Spinal Cord 2005;43(5):291–8 [DOI] [PubMed] [Google Scholar]
- 15.Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Lacquaniti F. Recovery of forward stepping in spinal cord injured patients does not transfer to untrained backward stepping. Exp Brain Res 2004;157(3):377–82 [DOI] [PubMed] [Google Scholar]
- 16.Betker AL, Desai A, Nett C, Kapadia N, Szturm T. Game-based exercises for dynamic short sitting balance rehabilitation of people with chronic spinal cord and traumatic brain injuries. Phys Ther 2007;87(10):1389–98 [DOI] [PubMed] [Google Scholar]
- 17.Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci 2008;31(8):410–8 [DOI] [PubMed] [Google Scholar]
- 18.Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair 2005;19(1):33–45 [DOI] [PubMed] [Google Scholar]
- 19.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 2005;94(4):2844–55 [DOI] [PubMed] [Google Scholar]
- 20.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther 2007;31(1):3–10 [DOI] [PubMed] [Google Scholar]
- 21.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil 2009;90(10):1692–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belegu V, Oudega M, Gary DS, McDonald JW. Restoring function after spinal cord injury: promoting spontaneous regeneration with stem cells and activity-based therapies. Neurosurg Clin N Am 2007;18(1):143–68, xi [DOI] [PubMed] [Google Scholar]
- 23.Kloosterman MG, Snoek GJ, Jannink MJ. Systematic review of the effects of exercise therapy on the upper extremity of patients with spinal-cord injury. Spinal Cord 2009;47(3):196–203 [DOI] [PubMed] [Google Scholar]
- 24.Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma 2006;23(3–4):571–85 [DOI] [PubMed] [Google Scholar]
- 25.Harkema S, Dobkin B, Edgerton V. Pattern generators in locomotion: implications for recovery of walking after spinal cord injury. Top Spinal Cord Inj Rehabil 2000;6:82–96 [Google Scholar]
- 26.Gregory CM, Bowden MG, Jayaraman A, Shah P, Behrman A, Kautz SA, et al. Resistance training and locomotor recovery after incomplete spinal cord injury: a case series. Spinal Cord 2007;45(7):522–30 [DOI] [PubMed] [Google Scholar]
- 27.Harness ET, Yozbatiran N, Cramer SC. Effects of intense exercise in chronic spinal cord injury. Spinal Cord 2008;46(11):733–7 [DOI] [PubMed] [Google Scholar]
- 28.Choquette S, Hamel M, Boissy P. Accelerometer-based wireless body area network to estimate intensity of therapy in post-acute rehabilitation. J Neuroeng Rehabil 2008;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol 2008;209(2):368–77 [DOI] [PubMed] [Google Scholar]
- 30.Keirstead HS. Stem cells for the treatment of myelin loss. Trends Neurosci 2005;28(12):677–83 [DOI] [PubMed] [Google Scholar]
- 31.Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res 2007;161:217–33 [DOI] [PubMed] [Google Scholar]
- 32.Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci 2007;8(4):312–9 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci 1998;18(24):10514–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu J, Feron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125(1):14–21 [DOI] [PubMed] [Google Scholar]
- 35.Ramon-Cueto A, Santos-Benito FF. Cell therapy to repair injured spinal cords: olfactory ensheathing glia transplantation. Restor Neurol Neurosci 2001;19(1–2):149–56 [PubMed] [Google Scholar]
- 36.Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, et al. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain 2008;131(1):264–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J Neurosci 2003;23(28):9428–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raisman G. Repair of spinal cord injury by transplantation of olfactory ensheathing cells. C R Biol 2007;330(6–7):557–60 [DOI] [PubMed] [Google Scholar]
- 39.Richter MW, Roskams AJ. Olfactory ensheathing cell transplantation following spinal cord injury: hype or hope? Exp Neurol 2008;209(2):353–67 [DOI] [PubMed] [Google Scholar]
- 40.Pastrana E, Moreno-Flores MT, Avila J, Wandosell F, Minichiello L, Diaz-Nido J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem Int 2007;50(3):491–8 [DOI] [PubMed] [Google Scholar]
- 41.Boyd JG, Skihar V, Kawaja M, Doucette R. Olfactory ensheathing cells: historical perspective and therapeutic potential. Anat Rec B New Anat 2003;271(1):49–60 [DOI] [PubMed] [Google Scholar]
- 42.Toft A, Scott DT, Barnett SC, Riddell JS. Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain 2007;130(4):970–84 [DOI] [PubMed] [Google Scholar]
- 43.Iwatsuki K, Yoshimine T, Kishima H, Aoki M, Yoshimura K, Ishihara M, et al. Transplantation of olfactory mucosa following spinal cord injury promotes recovery in rats. Neuroreport 2008;19(13):1249–52 [DOI] [PubMed] [Google Scholar]
- 44.Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx 2004;1(4):424–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleitman N. Keeping promises: translating basic research into new spinal cord injury therapies. J Spinal Cord Med 2004;27(4):311–8 [DOI] [PubMed] [Google Scholar]
- 46.Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair 2006;20(1):5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 2006;29(3):191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escada PA, Lima C, da Silva JM. The human olfactory mucosa. Eur Arch Otorhinolaryngol 2009;266(11):1675–80 [DOI] [PubMed] [Google Scholar]
- 49.Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair 2009;24(1):10–22 [DOI] [PubMed] [Google Scholar]
- 50.Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 2005;128(12):2951–60 [DOI] [PubMed] [Google Scholar]
- 51.Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 2008;131(9):2376–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, Chen L, Wang H, Xiu B, Li B, Wang R, et al. Influence of patients’ age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin Med J (Engl) 2003;116(10):1488–91 [PubMed] [Google Scholar]
- 53.Huang H, Wang H, Chen L, Gu Z, Zhang J, Zhang F, et al. Influence factors for functional improvement after olfactory ensheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2006;20(4):434–8 [PubMed] [Google Scholar]
- 54.American Spinal Injury Association International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003. Spring;26(Suppl. 1):S50–6 [DOI] [PubMed] [Google Scholar]
- 55.Furlan JC, Noonan V, Singh A, Fehlings M. Assessment of disability in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma 2010;27:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savic G, Bergstrom EM, Frankel HL, Jamous MA, Jones PW. Inter-rater reliability of motor and sensory examinations performed according to American Spinal Injury Association standards. Spinal Cord 2007;45(6):444–51 [DOI] [PubMed] [Google Scholar]
- 57.Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma 2011;28(8):1445–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furlan JC, Fehlings MG, Tator CH, Davis AM. Motor and sensory assessment of patients in clinical trials for pharmacological therapy of acute spinal cord injury: psychometric properties of the ASIA standards. J Neurotrauma 2008;25(11):1273–301 [DOI] [PubMed] [Google Scholar]
- 59.Cohen ME, Ditunno JF, Donovan WH, Maynard FM. A test of the 1992 international standards for neurological and functional classification of spinal cord injury. Spinal Cord 1998;36:554–60 [DOI] [PubMed] [Google Scholar]
- 60.Marino RJ, Jones L, Kirshblum S, Tal J, Dasgupta A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spin cord injury. J Spinal Cord Med 2008;31(2):166–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linassi G, Li Pi Shan R, Marino RJ. A web-based computer program to determine the ASIA impairment classification. Spinal Cord 2010;48(2):100–4 [DOI] [PubMed] [Google Scholar]
- 62.Chu BC, Millis S, Arango-Lasprilla JC, Hanks R, Novack T, Hart T. Measuring recovery in new learning and memory following traumatic brain injury: a mixed-effects modeling approach. J Clin Exp Neuropsychol 2007;29(6):617–25 [DOI] [PubMed] [Google Scholar]
- 63.Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil 1993;74(3):242–7 [PubMed] [Google Scholar]
- 64.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007;45(3):190–205 [DOI] [PubMed] [Google Scholar]
- 65.Saddiki-Traki F, Tremblay N, Dykes RW, Derraz S, El-Khamlichi A, Harrisson M. Differences between the tactile sensitivity on the anterior torso of normal individuals and those having suffered complete transaction of the spinal cord. Somatosen Mot Res 1999;16(4):391–401 [DOI] [PubMed] [Google Scholar]
- 66.Ditunno JF, Jr, Cohen ME, Hauck WW, Jackson AB, Sipski ML. Recovery of upper extremity strength in complete and incomplete tetraplegia: a multicenter study. Arch Phys Med Rehabil 2000;81(4):389–93 [DOI] [PubMed] [Google Scholar]
- 67.Kirshblum SC, O'Connor KC. Levels of spinal cord injury and predictors of neurologic recovery. Phys Med Rehabil Clin N Am 2000;11(1):1–27, vii [PubMed] [Google Scholar]
- 68.Graves DE, Frankiewicz RG, Carter RE. Gain in functional ability during medical rehabilitation as related to rehabilitation process indices and neurologic measures. Arch Phys Med Rehabil 1999;80(11):1464–70 [DOI] [PubMed] [Google Scholar]
- 69.Kirshblum S, Millis S, McKinley W, Tulsky D. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil 2004;85(11):1811–7 [DOI] [PubMed] [Google Scholar]
- 70.Duffell LD, Rowlerson AM, Donaldson NDN, Harridge SDR, Newham DJ. Effects of endurance and strength-directed electrical stimulation training on the performance and histological properties of paralyzed human muscle: a pilot study. Muscle Nerve 2010;42(5):756–63 [DOI] [PubMed] [Google Scholar]
- 71.Gordon T, Mao J. Muscle atrophy and procedures for training after spinal cord injury. Phys Ther 1994;74(1):50–60 [DOI] [PubMed] [Google Scholar]
- 72.Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil 1999;80(11):1391–6 [DOI] [PubMed] [Google Scholar]
- 73.Fisher CG, Noonan VK, Smith DE, Wing PC, Dvorak MF, Kwon BK. Motor recovery, functional status, and health-related quality of life in patients with complete spinal cord injuries. Spine (Phila Pa 1976) 2005;30(19):2200–7 [DOI] [PubMed] [Google Scholar]
- 74.Tefertiller C. Shepherd center spinal cord injury rehabilitation description. Atlanta 2011 [cited 7/11/2011]. Available from: http://www.shepherd.org/patient-care/spinal-cord-injury [Google Scholar]
- 75.Young W. The case for umbilical cord mononuclear cell transplants and lithium, panel discussion and breakout session. Working to Walk – United to Fight Paralysis; 2010 conference. 2010; Phoenix, Arizona [Google Scholar]
- 76.Young W. Review of bone marrow stem cell therapy of spinal cord injury. 2011 [cited 7/18/2010]. Available from: http://sci.rutgers.edu/forum/archive/index.php/t-156170.html [Google Scholar]
- 77.Heinemann AW, Hamilton B, Linacre JM, Wright BD, Granger C. Functional status and therapeutic intensity during inpatient rehabilitation. Am J Phys Med Rehabil 1995;74(4):315–26 [DOI] [PubMed] [Google Scholar]
- 78.Aronow HU. Rehabilitation effectiveness with severe brain injury: translating research into policy. J Head Trauma Rehabil 1987;2(3):24–36 [Google Scholar]
- 79.Natale A, Taylor S, LaBarbera J, Bensimon L, McDowell S, Mumma SL, et al. SCIRehab Project series: the physical therapy taxonomy. J Spinal Cord Med 2009;32(3):270–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whiteneck G, Dijkers M, Gassaway J, Lammertse DP. The SCIRehab Project: classification and quantification of spinal cord injury rehabilitation treatments. Preface. J Spinal Cord Med 2009;32(3):249–50 [PMC free article] [PubMed] [Google Scholar]
- 81.Whiteneck G, Gassaway J, Dijkers M, Backus D, Charlifue S, Chen D, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Inpatient treatment time across disciplines in spinal cord injury rehabilitation. J Spinal Cord Med 2011;34(2):133–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor-Schroeder S, LaBarbera J, McDowell S, Zanca JM, Natale A, Mumma S, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Physical therapy treatment time during inpatient spinal cord injury rehabilitation. J Spinal Cord Med 2011;34(2):149–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chhabra HS, Lima C, Sachdeva S, Mittal A, Nigam V, Chaturvedi D, et al. Autologous olfactory mucosal transplant in chronic spinal cord injury: an Indian Pilot Study. Spinal Cord 2009;47(12):887–95 [DOI] [PubMed] [Google Scholar]
- 84.Eschback KS, Herbison GJ, Ditunno JF. Sensory root level recovery in patients with Frankel A quadriplegia. Arch Phys Med Rehabili 1992;73(7):618–22 [PubMed] [Google Scholar]


