Abstract
Asthma is a chronic airway inflammatory disease characterized by eosinophilic infiltration and airway hyperresponsiveness. The over-activated Th2 and lung epithelium cells express many different cytokines, and chemokines mainly contribute to the severity of lung inflammation. Clara cell 10 kD protein (CC10) is highly expressed in airway epithelium cells and exhibits anti-inflammatory and immunomodulatory effects. Adeno-associated virus (AAV) 2/9 vector, composed of AAV2 rep and AAV9 cap genes, can efficiently and specifically target lung epithelium cells. Thus, AAV2/9 vector might carry therapeutic potential gene sequences for the treatment of asthma. This study tested whether AAV2/9 vector carrying CC10 could reduce inflammatory and asthmatic responses in OVA-induced asthmatic mouse model. The results showed that AAV2/9-CC10 vector virus significantly reduced airway hyperresponsiveness, CCL11, interleukin (IL)-4, IL-5, IL-6, IL-13, and eosinophilia in the lungs of sensitized mice. CC10 level in OVA-sensitized mice was rescued with the administration of AAV2/9-CC10 vector virus. Lung tissue remodeling, including collagen deposition and goblet cell hyperplasia, was also alleviated. However, serum levels of OVA-specific IgG1 and IgE as well as Th2 cytokine levels in OVA-stimulated splenocyte culture supernatants were at the comparable levels to the sensitized control group. The results demonstrate that AAV2/9-CC10 vector virus relieved local inflammatory and asthmatic responses in lung. Therefore, we propose that AAV2/9-CC10 vector virus guaranteed sufficient CC10 expression and had an anti-inflammatory effect in asthmatic mice. It might be applied as a novel therapeutic approach for asthma.
Wu and colleagues evaluate whether injection of adeno-associated viral vector serotype 9 (AAV2/9) carrying the Clara cell 10 kD protein (CC10) can reduce inflammatory and asthmatic responses in an OVA-induced asthmatic mouse model. AAV2/9-CC10 vector significantly reduces airway hyperresponsiveness, CCL11, interleukin (IL)-4, IL-5, IL-6, IL-13, and eosinophilia in the lungs of OVA-sensitized mice. Lung tissue remodeling is also alleviated.
Introduction
The prevalence of asthma in developed and developing countries has doubled in the past two decades, especially in children. Asthma is generally considered a chronic pulmonary inflammatory disease characterized by eosinophil infiltration, airway obstruction, and airway hyperresponsiveness (AHR) to inhaled allergens. The phenomenon indicates that inflammatory response and airway remodeling are common responses of injury to the respiratory tract (Lewitt et al., 2011; McFadden and Gilbert, 1992). The inhibition of lung inflammation may be a potential treatment for asthma. Clara cell 10-kDa protein (CC10), also known as uteroglobin or urine protein-1, was first discovered in 1968 (Beier, 1968). CC10 belongs to secretoglobin superfamily and has a variety of functions such as immunoregulation, anti-inflammation, and anti-phospholipase A2 activity (Mukherjee, 2007), indicating that CC10 may play some roles in anti-inflammatory responses. CC10-deficient mice showed more severe inflammation than wild type control (Hogan et al., 1998; Snyder et al., 2010). The serum level of CC10 decreases if injury or inflammation occurs in the lung. Previous studies suggest that CC10 may be a serum biomarker for the detection of lung injury (Dow et al., 1999; Hogan et al., 1998). In addition, gene polymorphism also influences the function of CC10 and inflammation (Yang et al., 2007). It has been proved that mucosal CC10 gene transfer can inhibit pulmonary Th2 cytokine production and eosinophilia seen in CC10-deficient mice, providing evidence for a functional role of CC10 in limiting pulmonary Th2-associated allergic responses (Hung et al., 2004). Interestingly, results from CC10-gene knockout studies of different models (Harrod et al., 1998; Hayashida et al., 2000; Johnston et al., 1997) have also shown alterations in pro-inflammatory cytokines. CC10 may play a common role in protecting the host from inflammatory insults, and its differential activity may depend on the nature and the type of inflammatory responses.
Adeno-associated virus (AAV) is a single-stranded DNA virus that belongs to the Parvoviridae family and is characterized by its nonintegrating episomal expression, nonpathogenic nature, and ability to confer stable expression (Daya and Berns, 2008). It also has the ability to infect both dividing and nondividing cells (Alexander et al., 1996). Several strains of AAVs have been isolated and characterized from humans and primates, and new serotypes are continuously discovered (Auricchio et al., 2002; Gao et al., 2004; Mori et al., 2004; Rutledge et al., 1998). Recombinant AAVs had been applied to enhance gene expression and tissue tropism. These AAVs gain the ability to infect cells that are more susceptible to other serotypes (Rabinowitz et al., 2002). AAV5 (Auricchio et al., 2002; Zabner et al., 2000), AAV6 (Halbert et al., 2001), and AAV9 (Gao et al., 2004) are the most efficient serotypes to infect lung epithelium cells. Long-term gene expression ability of AAVs in the lung tissue may enhance the efficacy for gene therapy to the lungs of asthmatic model animals. In this study, we applied a AAV2/9 viral vector carrying the CC10 gene for the treatment of an OVA-sensitized mouse model. The data showed increased CC10 gene expression level, similar to normal control, in the lungs of AAV2/9-CC10 virus-infected mice. AHR and eosinophil infiltration were also decreased in AAV2/9-CC10 virus-infected mice. Decreased Th2 cytokines, interleukin (IL)-6, and CCL11 gene expression level could be detected in the lung of AAV2/9-CC10 virus-infected mice. AAV2/9 virus induced local responses in the lung but no systemic responses. The goal of the present study was to generate an anti-inflammatory system mediated by an AAV2/9 vector virus that can be further applied for long-term management of asthma.
Materials and Methods
Generation of recombinant AAV
CC10 DNA fragment was amplified from lung cDNA with gene-specific primers, forward primer, 5′-AAGAATTCATGAAGATCGCCATCACAATC-3′; reverse primer, 5′-AACTCGAGGAATCTTAAATCTTGCTTACA-3′, by PCR. This DNA fragment was subsequently cloned into multiple cloning site of pAAV-IRES-hrGFP plasmid (Stratagene, La Jolla, CA). The AAV was generated with the tri-plasmids system (Limberis and Wilson, 2006), including pAAV/IRES/hrGFP alone or containing CC10 and humanized Renilla green fluorescent protein (hrGFP), pHelper, and pAAV2/9 (containing AAV2 rep and AAV9 cap genes). Three plasmids were co-transfected into HEK 293T cells. The titer (defined as genome copies [GC]) of AAVs in cell lysates was determined by real-time PCR analysis.
The functional assay of CC10 produced from virus-infected NIH 3T3 cells in vitro
MLE-12 cells (1×106 cells/well), a mouse epithelial cell line, express pro-inflammatory cytokine after tumor necrosis factor-α (TNF-α) stimulation. The concentration of CC10-containing medium from AAV2/9-CC10 virus-infected NIH 3T3 cells was quantified by SDS-PAGE, and the band density of CC10 was compared with 14-kDa rDp2 protein and the concentration was determined. The CC10-containing medium (equivalent to 50 μg) was added to the MLE-12 cell culture system and cultured for 2 hr before TNF-α (50 ng/ml) stimulation. Supernatants were collected at 24 hr after TNF-α stimulation for IL-6 enzyme-linked immunosorbent assay (ELISA) detection.
Animals and sensitization protocol
Female BALB/c mice (8 to 10 weeks old) were sensitized by intraperitoneal injection of 50 μg OVA (Sigma, St. Louis, MO) or normal saline mixed with aluminum hydroxide (Pierce Biotechnology Inc., Rockford, IL) on days 1, 2, 3, and 14. The mice were challenged by intratracheal injection of 50 μg OVA or normal saline on days 14, 17, and 20. AHR was determined on day 21, and mice were sacrificed on day 22. Although this protocol was modified to have three instead of five OVA challenges, as in the previously described protocol (Shen et al., 2011; Wu et al., 2006), comparable asthmatic parameters were observed. Based on that, the highest reporter gene expression was detected on day 3 in the lung tissues following AAV2/9 viral vector administration (data not shown), the mice of virus vector-treated groups received 5×1011 GC viruses in 50 μl of saline on day 11 (3 days before the first OVA aerosol challenge). The mice were cared for and handled according to the guidelines of the Animal Care Committee of Chang Gung University and the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Measurement of airway hyperresponsiveness
AHR was measured 24 hr after the last challenge. Mice inhaled methacholine (Mch) (Sigma) at increasing concentrations (10, 20, 30, and 40 mg/ml) to induce airway contraction. After inhaling Mch for 3 min, mice were placed into the chamber to measure enhanced pause (Penh) for 3 min. The responses were analyzed by BioSystem XA software (Buxco Electronics, Troy, NY).
Bronchoalveolar lavage fluid collection, lung histology, and immunohistochemistry
On day 22, mouse lungs in different groups were lavaged with 1 ml of saline for three times, and the cells were collected, cytospun (Thermo Shandon Inc., Pittsburgh, PA), and stained with Liu staining solution. The numbers of differential cell counts were performed in 200 cells based on the staining characteristics of morphology. The lungs of two mice in each group were not lavaged and were saved for further histological analyses. The lung tissues were fixed with 4% formaldehyde for 18 hr and dehydrated with stepwise incubation with different ratios of ethanol solution (70%, 90%, and 100%, 30 min each time), and incubated with xylene for 20 min. Finally, dehydrated tissues were embedded with paraffin. Then, the paraffin sections (10 μm thick) were subjected to hematoxylin and eosin (H&E), Trichrome, and Periodic acid-Schiff (PAS) staining, as described in a previous study (Lappalainen et al., 2005). The hrGFP expression in each fixed lung section was determined by immunohistochemistry (IHC) with rabbit anti-hrGFP antibody (Stratagene) as previously described (Yang et al., 2009). The percentages of eosinophil infiltration (defined as inflammatory index), goblet cell hyperplasia, and collagen deposition were analyzed by MetaMorph microscopy automation and image analysis software (Molecular Devices, Sunnyvale, CA). Three sections of each mouse lung were analyzed and positive signals in five selected areas that were 100 μm2 in size in each section were counted. The data were calculated using the mean values of sensitized control mice as 100%.
Determination of OVA-specific IgG1 and IgE antibody
Serum OVA-specific IgG1 and IgE levels were determined using ELISA. Briefly, the OVA (10 μg/ml)-coated plates (Corning) were incubated with diluted serum samples. Then, the biotinylated rat anti-mouse IgG1 or IgE monoclonal antibodies (BD PharMingen, San Jose, CA) and streptavidin-conjugated horseradish peroxidase (HRP) (Sigma) were sequentially added. The plates were incubated with TMB substrate (R&D Systems, Minneapolis, MN) and the absorbance was determined with ELISA reader at 450 nm. The concentration of the IgG1 standard was defined as arbitrary unit.
Cytokine levels and RNA expression
The concentration of IL-4, IL-6, IL-10, and IL-13 were determined with Duoset ELISA kits (R&D Systems). IL-5 was analyzed by BD Pharmingen ELISA set (BD PharMingen). The assays were performed according to the manufacturers' instructions. The real-time PCR primers of IL-4, IL-5, IL-6, IL-13, β-actin (Overbergh et al., 1999), and gob5 (Wu et al., 2008) were applied for detection of RNA gene expression and results were compared to the housekeeping gene, β-actin.
Statistical analysis
Data are presented as mean±SEM. Data were analyzed by Student t test. A two-tailed p value less than 0.05 was considered statistically significant.
Results
CC10 expressed from AAV2/9-CC10–infected NIH 3T3 cells reduced IL-6 production in TNF-α–stimulated MLE-12 cells
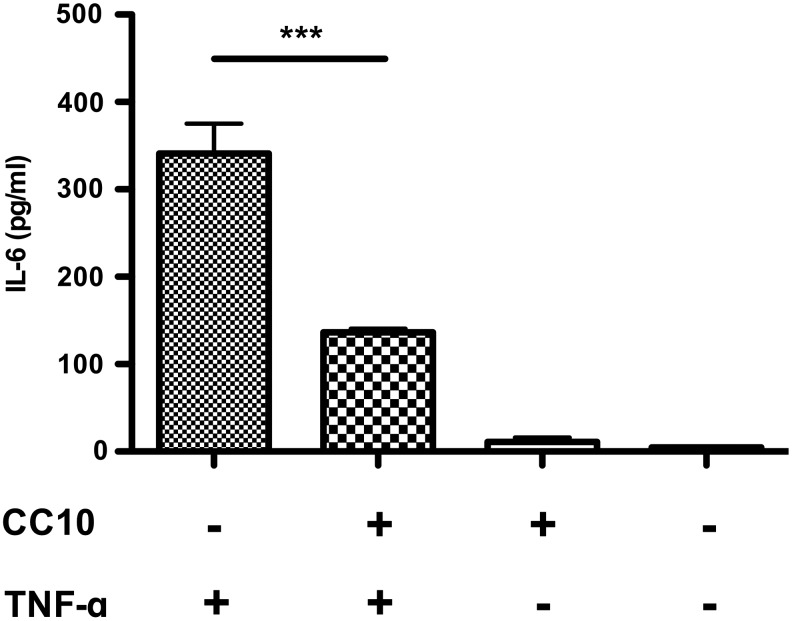
We tested the biological function of CC10 expressed from AAV2/9-CC10 virus before using it for the treatment of asthmatic mice. First, NIH 3T3 cells were infected with AAV2/9-CC10 vector virus for 3 days. The supernatant was subjected to test the inhibitory capacity of IL-6 production from TNF-α–stimulated MLE-12 cells. Fig. 1 shows that IL-6 production was significantly reduced in CC10-containing medium–treated cells compared with that from no AAV2/9-CC10 vector virus-infected cells (p<0.0001). We further applied AAV2/9-CC10 vector virus to test whether CC10 reduces asthmatic responses in OVA-sensitized mouse model.
FIG. 1.
The supernatants from AAV2/9-CC10 vector transduced NIH 3T3 cells suppressed IL-6 production in TNF-α–treated MLE-12 cells. NIH 3T3 cells were infected with AAV2/9-CC10 vector virus and the supernatants were collected at 2 days after virus infection. The concentration of CC10 in medium was quantified by SDS-PAGE. MLE-12 cells (1×106 cells/well) were pretreated with or without AAV-CC10 vector virus-infected supernatant (containing estimated 50 μg CC10) for 2 hours, and then stimulated with 50 ng/ml TNF-α for an additional 24 hours. Supernatants were collected for IL-6 detection. Data are represented as mean±SEM (***p<0.001, n=4 per group). AAV, adeno-associated virus; CC10, Clara cell 10 kD protein; IL, interleukin; TNF-α, tumor necrosis factor; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
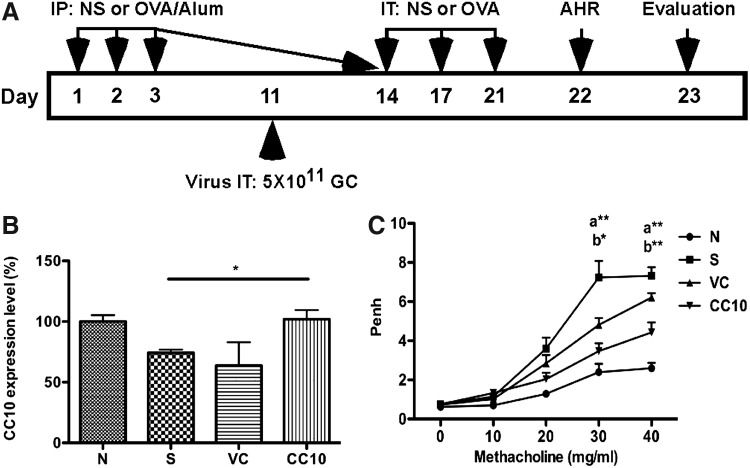
AAV2/9-CC10 vector virus rescued CC10 expression and significantly alleviated AHR in OVA-sensitized mice
After sensitization and challenge with OVA, and treatment with AAV2/9 vector viruses (Fig. 2A), we detected gene expression level of CC10 in lung after AAV2/9-CC10 vector virus administration (CC10) by real-time PCR (Fig. 2B). The results showed that the level of CC10 gene expression was reduced in OVA-sensitized control (S; p<0.05) and AAV2/9 virus control (VC; p=0.14), at comparable levels, compared with normal control (N). With AAV2/9-CC10 vector virus infection, CC10 gene expression level was rescued to the level similar to normal control mice. The CC10 gene expression level in AAV2/9-CC10 vector virus-treated mice was higher than that in AAV2/9 control mice, although not significantly (p=0.14).
FIG. 2.
AAV2/9-CC10 vector virus enhanced CC10 level and suppressed airway hyperresponsiveness in OVA-sensitized mice. (A) Brief protocol for OVA sensitization and AAV2/9 vector virus treatment. Mice were sensitized with OVA and treated with AAV2/9 vector virus as described in Materials and Methods. IP, intraperitoneal; IT, intratracheal injection. (B) The level of CC10 in lung tissue was determined by real-time PCR. Data are normalized to normal control mice (n=4 per group). (C) Airway hyperresponsiveness was measured by whole body plethysmography. Data are normalized to normal control mice and represented as enhanced pause (Penh) values (mean±SEM; n=6 per group). N, normal control; S, sensitized control; VC, AAV 2/9 control vector virus; CC10, AAV2/9-CC10 vector virus. *p<0.05; a**, p<0.01 vs. S; b*, p<0.05 vs. VC; b**, p<0.01 vs. VC.
After sensitization and OVA challenge, AHR was measured by Mch challenge using noninvasive whole body plethysmography. The Penh value of AAV2/9-CC10 vector virus-treated mice was significant lower than that of sensitized control (p<0.01) and virus control group (p<0.01) when mice were treated with 40 mg/ml Mch (Fig. 2C). A similar result was also observed when mice were treated with 30 mg/ml Mch. Taken together, AAV2/9-CC10 vector virus administration rescued CC10 level in lung and alleviated AHR in OVA-sensitized mice.
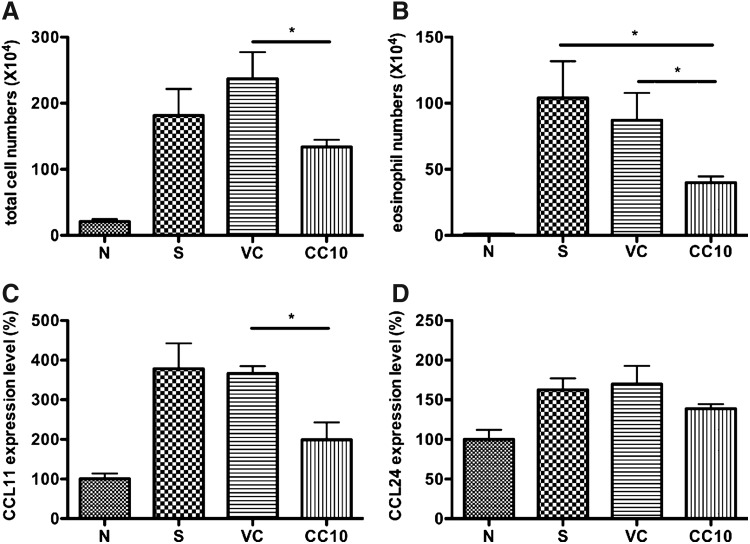
Significant reduction of eosinophilia in bronchoalveolar lavage fluid and CCL11 level in lung of AAV2/9-CC10 vector virus-treated mice
Eosinophils are multifunctional leukocytes implicated in the pathogenesis of diverse inflammatory responses, such as parasitic helminth infections and allergic diseases (Rothenberg, 1998; Weller, 1994). These inflammatory mediators of eosinophils may induce damage to airway endothelial cells and extracellular domain and leading to the recruitment of more eosinophils and Th2 cells to the airways (Kay and Klion, 2004). The results showed that the total cell number in bronchoalveolar lavage fluid (BALF) of AAV2/9-CC10 vector virus-treated mice was significantly reduced compared with AAV2/9 vector virus control group (p<0.05) (Fig. 3A). A significant reduction of eosinophils infiltration was detected in AAV2/9-CC10 vector virus-treated mice compared with that of AAV2/9 vector virus and sensitized control mice (both p<0.05) (Fig. 3B).
FIG. 3.
AAV2/9-CC10 vector virus reduced eosinophil number in BALF and CCL11 in lung from OVA-sensitized mice. (A) Total cell counts and (B) the number of eosinophils in bronchoalveolar lavage fluid (BALF) were assessed by Liu staining (n=6 per group). The level of (C) CCL11 and (D) CCL24 in lung tissue was determined by real-time PCR (n=4 per group). Data are normalized to normal control and expressed as mean±SEM. N, normal control; S, sensitized control; VC, AAV 2/9 control vector virus; CC10, AAV2/9-CC10 vector virus. *p<0.05.
CCL11 (eotaxin 1) and CCL24 (eotaxin 2) are major chemokines for the recruitment of eosinophils into the lung. Eosinophil infiltration was significantly reduced in the lungs of AAV2/9-CC10 vector virus-treated mice compared to control mice. We further determined gene expression levels of CCL11 and CCL24 in lungs by real-time PCR (Fig. 3C, 3D). The result showed that CCL11 gene expression level was reduced in AAV2/9-CC10 vector virus-treated mice compared with AAV2/9 vector virus control mice (p<0.05). We also determined CCL24, the relatively minor mediator for the recruitment of eosinophil, gene expression level among each group. Only slightly lower CCL24 gene expression level in AAV2/9-CC10 vector virus-treated mice was detected than in sensitized control and AAV2/9 virus control mice. Thus, AAV2/9-CC10 vector virus could reduce eosinophil infiltration and the CCL11 expression level in the lungs of OVA-sensitized mice.
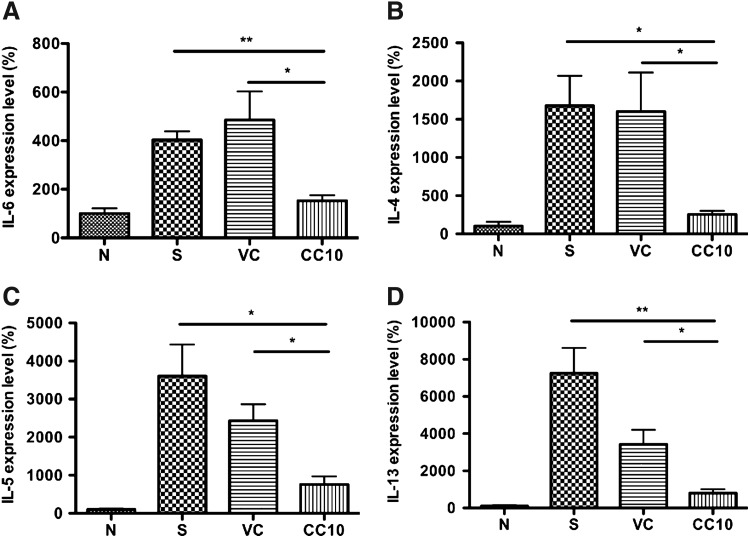
Significant reduction of IL-6 and Th2 cytokine gene expression in the lungs of AAV2/9-CC10 vector virus-treated mice
Since CC10 exhibits anti-inflammatory function, we further analyzed gene expression level of IL-6, a proinflammatory cytokine, in AAV2/9-CC10 vector virus-treated mice with real-time PCR (Fig. 4A). The results showed the reduced IL-6 gene expression level in AAV2/9-CC10 vector virus-treated mice compared with sensitized control (p<0.01) and AAV2/9 vector virus control mice (p<0.05). It has been demonstrated that CC10 may influence responses of Th2 cells but not naïve cells (Hung et al., 2004). So, we further analyzed gene expression level of Th2 cytokines in lung tissues of OVA-sensitized mice (Fig. 4B-D). Significant reduction of Th2 cytokine genes, IL-4, IL-5, and IL-13, in the lungs of AAV2/9-CC10 vector virus-treated mice was observed compared to sensitized control and virus control mice (p<0.05). The data indicate that AAV2/9-CC10 vector virus is able to reduce gene expression levels of Th2 cytokines and proinflammatory cytokine in the airways of OVA-sensitized mice.
FIG. 4.
AAV2/9-CC10 vector virus reduced IL-6 and Th2 cytokines level in lung of OVA-sensitized mice. The level of proinflammatory cytokine, (A) IL-6, and Th2 cytokines, (B) IL-4, (C) IL-5, and (D) IL-13, in lung tissue was determined by real-time PCR (n=4 per group). Data are normalized to normal control and expressed as mean±SEM. N, normal control; S, sensitized control; VC, AAV 2/9 control vector virus; CC10, AAV2/9-CC10 vector virus. *p<0.05; **p<0.01.
Airway tissue remodeling was reduced in the lungs of AAV2/9-CC10 vector virus-treated mice
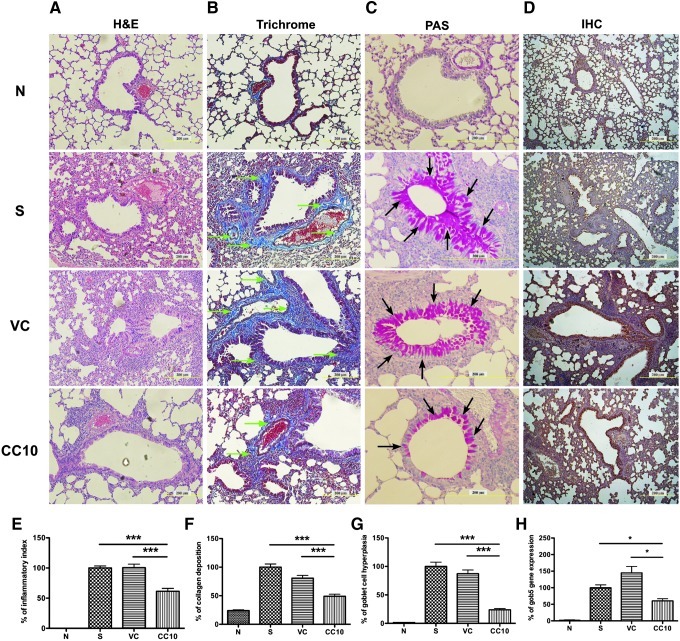
Lung samples were obtained for further tissue section staining on day 22 after sacrifice. Formaldehyde-fixed, paraffin-embedded lung samples were sliced and H&E, trichrome, and PAS stained (Fig. 5A-C). More eosinophils had infiltrated into the lungs of sensitized and virus control mice compared to the normal control mice. The bottom panel of Fig. 5A shows that fewer eosinophils had infiltrated into the lungs of AAV2/9-CC10 virus-treated mice, compared with those of sensitized and virus control mice. In addition, less collagen deposition (Fig. 5B) and goblet cell hyperplasia (Fig. 5C) were observed in the lungs of AAV2/9-CC10 vector virus-treated and normal control mice, compared to the sensitized and virus control mice. Quantitative results indicated significant reduction in eosinophils infiltrating into the lungs (presented as inflammatory index) of AAV2/9-CC10 vector virus-treated mice, compared with sensitized and virus control mice (Fig. 5E). Additionally, significant reduction of collagen deposition (Fig. 5B and 5F) and goblet cell hyperplasia (Fig. 5C and 5G) was observed in the lungs of AAV2/9-CC10 vector virus-treated and normal control mice. RNA expression levels of gob5 (Fig. 5H) and Muc-5ac (data not shown) were also reduced in the lungs of AAV2/9-CC10 vector virus-treated mice compared to those of sensitized control and virus control mice. These results demonstrated that AAV2/9-CC10 vector viruses reduced eosinophilia, collagen deposition, and goblet cells hyperplasia in the lungs of OVA-sensitized mice. These sliced lung samples were subsequently subjected to IHC staining specific for hrGFP to confirm AAV2/9 vector virus-infected cells (Fig. 5D). The data revealed that lung epithelium cells were the major targets for AAV2/9 vector virus administration.
FIG. 5.
Histopathology of lung tissues in mice treated with AAV2/9-CC10 vector viruses. Formaldehyde-fixed, paraffin-embedded lung samples were sliced and subjected to (A) hematoxylin and eosin (H&E) staining, (B) trichrome staining, (C) periodic acid-Schiff (PAS) staining, and (D) immunohistochemistry (IHC) staining specific for humanized Renilla green fluorescent protein (hrGFP). The quantitative results of the different staining were analyzed by MetaMorph and presented as percentage (mean±SEM), including (E) eosinophil infiltration (defined as inflammatory index), (F) collagen deposition (indicated with green arrows), and (G) goblet cell hyperplasia (indicated with black arrows). (H) RNA expression level of gob5 was also determined by real-time PCR. Data are normalized to sensitized controls and expressed as mean±SEM. *p<0.05 and ***p<0.001, with student t test, compared to the sensitized and viral control mice. Scale bar=200 μm. N, normal control; S, sensitized control; VC, AAV 2/9 control vector virus; CC10, AAV2/9-CC10 vector virus.
Serum levels of OVA-specific IgG1 and IgE were not affected after AAV vector virus infection
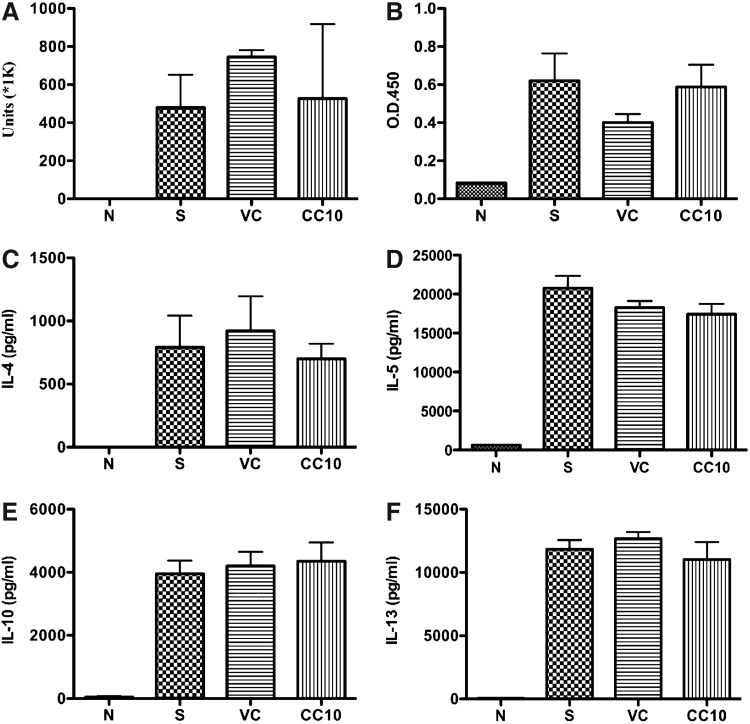
Local responses, including AHR, eosinophil infiltration, CCL11, Th2 cytokine and IL-6 gene expression, were reduced in AAV2/9-CC10 vector virus-treated mice. We further analyzed whether AAV2/9-CC10 vector virus administration affect the peripheral immune responses, OVA-specific antibodies in serum, and Th2 responses in OVA-stimulated splenocytes. The data showed no significant difference in serum level of OVA-specific IgG1 and IgE among each OVA-sensitized group (Fig. 6A, 6B). The Th2 cytokine levels in OVA-stimulated splenocytes were detected (Fig. 6C–6F). Comparable IL-4, IL-5, IL-10, and IL-13 levels were detected among the OVA-sensitized groups. Therefore, the data suggested that AAV2/9 vector virus administration could induce local instead of systemic immune responses in OVA-sensitized mice.
FIG. 6.
Systemic immune responses were not affected in OVA-sensitized mice treated with AAV2/9 vector viruses. Serum and splenocyte samples (n=6 per group) were collected at the end of the sensitization protocol. Splenocytes were stimulated with 100 μg/ml OVA for 6 days and supernatants were collected for Th2 cytokine determination. The levels of (A) OVA-IgG1, (B) OVA-IgE, (C) IL-4, (D) IL-5, (E) IL-10, and (F) IL-13 were measured using enzyme-linked immunosorbent assay. Serum samples were diluted (IgG1: 1:40000; IgE: 1:5). Data are represented as mean±SEM. N, normal control; S, sensitized control; VC, AAV 2/9 control vector virus; and CC10, AAV2/9-CC10 vector virus.
Discussion
In the current study, the first to use AAV2/9 vector virus carrying lung-specific gene transfer for asthma, we demonstrated that CC10 gene transfer with AAV2/9 expressing virus alleviated airway inflammation of OVA-sensitized mice. CC10 expression level in OVA-sensitized mice was rescued after AAV2/9-CC10 vector virus treatment to the similar level of normal control mice. In addition to the reduction of CCL11 in the lungs, AHR, eosinophil infiltration, and the IL-6 level were significantly reduced in AAV2/9-CC10 vector virus-treated mice. Serum levels of OVA-specific IgE, IgG1, and Th2 cytokines of OVA-stimulated splenocytes, however, were not affected by AAV2/9 vector virus treatment. These results indicated that CC10 might be a good candidate for the treatment of lung inflammation. It is also provide a strong support that AAV2/9 vector virus could serve as a potential therapeutic vector for the treatment of asthma, without inducing systemic immune responses.
CC10 plays an important role in controlling airway inflammatory reactions (Chen et al., 2001; Chiba et al., 2006; Harrod et al., 1998). To determine the physiological functions of CC10, two gene deficient mice, with different genetic background, have been studied (Stripp et al., 1996; Zhang et al., 1997). CC10-deficient mice expressed abnormal deposit of fibronectin and collagen, which caused renal failure (Zhang et al., 1997). A detailed histopathological analysis subsequently proved an abnormally high level of IgA and deposition of complement C3 in the glomeruli (Zheng et al., 1999). Similar results were also observed using transgenic mice expressing CC10-antisense mRNA (Zhang et al., 2000). The abnormal deposit of fibronectin and collagen causing renal failure could be rescued with the treatment of recombinant CC10 (Zhang et al., 1997). The CC10-deficient mice were susceptible to allergen-induced pulmonary inflammation that induced local pulmonary fibrosis (Lee et al., 2006) and to lung tumor genesis by cigarette smoke (Yang et al., 2004). The exacerbated pulmonary eosinophilic infiltration and increased levels of inflammatory cytokines IL-4, IL-5, IL-9, and IL-13 were also demonstrated in some CC10-deficient mice sensitized with OVA (Chen et al., 2001). That was the first article to mention the importance of CC10 in asthmatic responses. The function of CC10 has been proved to reduce Th2 cytokine production in activated Th2 cells but not naïve T cells (Hung et al., 2004). Compared to allergen-sensitized control mice, the decreased IL-4, IL-5, and IL-13 were detected in CC10-deficient mice that received intratracheal injection of CC10 plasmid (Chen et al., 2001) and the current AAV2/9-CC10 vector virus. Following the previously described method (Hammad et al., 2003; Lambrecht et al., 2000), the similar percentages of FITC-OVA+ dendritic cells (DCs) detected in brachial draining and mediastinal lymph nodes of mice with or without AAV2/9-CC10 vector virus administration suggest that antigen uptake or migration of DCs was not affected (data not shown). The reduction of Th2 cytokines by CC10 carried with AAV2/9 may be through the down-regulation of IL-4–dependent GATA-3 expression (Hung et al., 2004). Alternatively, CC10 may indirectly block PGD2 receptor-mediated NF-κB activation and hence suppress COX-2 gene expression (Mandal et al., 2004). Moreover, some recent evidence demonstrated that CC10 might attenuate proinflammatory molecules, such as chitinase 3-like 1 protein and osteopontin, expression in allergic airway diseases (Liu et al., 2011; Wang et al., 2010).
Some studies applied adenovirus vector which exhibits capacity of large size of therapeutic potential DNA segments for the treatment of airway diseases (Barnett et al., 2002). Particularly, adenovirus vectors carried immune modulatory genes such as IL-10, IL-12 (Hsu et al., 2010), or uteroglobin-related protein 1 (Chiba et al., 2006) to control mice allergic inflammation. Most studies showed reduced eosinophilia and levels of Th2 cytokines, including IL-4, IL-5, and IL-13, in treated mice (Chiba et al., 2006). But the expression level of CC10 (mRNA and protein) in lung tissue or BALF of adenovirus-empty and adenovirus-CC10 vector-treated OVA-sensitized mice was similar. It is known that administration of adenovirus vector induces a variety of innate immune responses and subsequently induced adaptive immune responses in the days following virus administration (Liu and Muruve, 2003; Muruve, 2004). Tissue-specific tropism is another important concern for using viral vectors; however, adenovirus and lentiviral vectors exhibit broad tissue tropism becomes a limitation to reduce the off-target effect for the use of these vectors. Moreover, strong responses from host immune system are also induced by capsid proteins from adenovirus vectors, which is another severe disadvantage (Douglas, 2007). Our data also showed no significance difference of OVA-specific IgG1 and IgE between vector virus-treated or virus-untreated group. The data indicated that AAV2/9 vector virus induced local instead of systemic immune response.
Our present study develops a treatment system, delivered directly into mouse lung by intratracheal injection, with AAV2/9 vector carrying CC10, that exhibits anti-inflammatory and immunomodulatory activity, for asthma and can down-regulate inflammatory and asthmatic responses in OVA-sensitized mice. Recombinant AAV2 vectors have been applied to clinical trials for several diseases such as α1 anti-trypsin deficiency (Brantly et al., 2006; Flotte et al., 2011), cystic fibrosis (Moss et al., 2007), hemophilia (Jiang et al., 2006; Manno et al., 2003), Parkinson's disease (Christine et al., 2009; Lewitt et al., 2011; Marks et al., 2010), and rheumatoid arthritis (Mease et al., 2009, 2010). The duration of gene expression mediated by AAV2/9 vector virus was also tested in our model. The data showed that gene expression of hrGFP could be detected more than 3 months after virus infection (data not shown). Few articles used AAV2 carrying therapeutic genes for the treatment of asthma. Intratracheal injection of AAV2 with IL-4 receptor antagonist vector significantly inhibited the development of airway eosinophilia and Th2-type cytokines in BALF (Zavorotinskaya et al., 2003). A balanced Th1/Th2 cytokine profile can be achieved by delivering AAV2 T-bet vector in OVA-induced allergic mice (Wang et al., 2008). Although AAV2 has been one of the most commonly used serotypes of AAV (Bainbridge et al., 2008; Marks et al., 2008; McPhee et al., 2006; Mendell et al., 2010; Worgall et al., 2008), recombinant AAVs composed of rep gene from AAV2 and cap gene from different serotypes can be used to enhance gene expression and target specific tissue (Halbert et al., 2000). Compared to the AAV2/2 vector, higher levels and longer duration of gene expression were detected in the lung tissues when the exogenous genes were transduced by either AAV2/5 or AAV2/9 viral vector (Gao et al., 2004; Zabner et al., 2000). AAV2/9 vector has been applied for the treatment of human diseases, such as anti-hepatitis B virus infection and Parkinson's disease (Chen et al., 2009; Xue et al., 2010). AAV2/9 vector also showed more efficient gene expression and resistance to second administration than AAV2/5 vector virus for the transduction of mouse lung (Limberis and Wilson, 2006). The authors also suggested that AAV2/9 vector virus is better to prevent the activation of mucosal immunity. Our IHC data also showed that lung epithelia cells are the major targets for AAV2/9 vector virus. Rescued CC10 level was observed in lung after AAV2/9-CC10 vector virus administration.
In conclusion, a single treatment of AAV2/9-CC10 vector virus significantly reduced asthmatic responses and airway remodeling in OVA-sensitized mice. Serum OVA-specific immunoglobulin concentration and cytokines of OVA-stimulated splenocytes were not affected, suggesting the local modulation of immune responses to allergen challenge. This experimental study suggests that CC10 can be a good candidate through the application of AAV2/9 for the treatment of asthma and the use of AAV2/9 vector can be further applied to long-term gene expression for the management of asthma.
Acknowledgments
We deeply appreciate Dr. James M. Wilson at the University of Pennsylvania for kindly gift of AAV2/9 plasmid. This work was supported, in part, by grants from the National Science Council (NSC98-2320-B-182-030-MY3, NSC95-2320-B-182-043-MY3, NSC 98-2320-B-182-020-MY3, and NSC 94-2314-B-182A-092) of the Republic of China, as well as from Chang Gung Memorial Hospital (CMRPD190512, CMRPD1A0171, and CMRPG260281).
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander I.E. Russell D.W. Spence A.M., et al. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum. Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- Auricchio A. O'Connor E. Weiner D., et al. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J.W. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Barnett B.G. Crews C.J. Douglas J.T. Targeted adenoviral vectors. Biochim. Biophys. Acta. 2002;1575:1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Beier H.M. Uteroglobin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochim. Biophys. Acta. 1968;160:289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. gene ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Chen C.C. Sun C.P. Ma H.I., et al. Comparative study of anti-hepatitis B virus RNA interference by double-stranded adeno-associated virus serotypes 7, 8, and 9. Mol. Ther. 2009;17:352–359. doi: 10.1038/mt.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.C. Zhang Z. Myers A.C., et al. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J. Immunol. 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- Chiba Y. Kurotani R. Kusakabe T., et al. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2006;173:958–964. doi: 10.1164/rccm.200503-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine C.W. Starr P.A. Larson P.S., et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S. Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.T. Adenoviral vectors for gene therapy. Mol. Biotechnol. 2007;36:71–80. doi: 10.1007/s12033-007-0021-5. [DOI] [PubMed] [Google Scholar]
- Dow S.W. Elmslie R.E. Fradkin L.G., et al. Intravenous cytokine gene delivery by lipid-DNA complexes controls the growth of established lung metastases. Hum. Gene. Ther. 1999;10:2961–2972. doi: 10.1089/10430349950016375. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Trapnell B.C. Humphries M., et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R., et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Rutledge E.A. Allen J.M., et al. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Allen J.M. Miller A.D. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H. De Heer H.J. Soullie T., et al. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J. Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- Harrod K.S. Mounday A.D. Stripp B.R., et al. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am. J. Physiol. 1998;275:L924–930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- Hayashida S. Harrod K.S. Whitsett J.A. Regulation and function of CCSP during pulmonary Pseudomonas aeruginosa infection in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L452–459. doi: 10.1152/ajplung.2000.279.3.L452. [DOI] [PubMed] [Google Scholar]
- Hogan S.P. Mould A.W. Young J.M., et al. Cellular and molecular regulation of eosinophil trafficking to the lung. Immunol. Cell Biol. 1998;76:454–460. doi: 10.1046/j.1440-1711.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- Hsu C.Y. Leu S.J. Chiang B.L., et al. Cytokine gene-modulated dendritic cells protect against allergic airway inflammation by inducing IL-10(+)IFN-gamma(+)CD4(+) T cells. Gene Ther. 2010;17:1011–1021. doi: 10.1038/gt.2010.39. [DOI] [PubMed] [Google Scholar]
- Hung C.H. Chen L.C. Zhang Z., et al. Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J. Allergy Clin. Immunol. 2004;114:664–670. doi: 10.1016/j.jaci.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Jiang H. Pierce G.F. Ozelo M.C., et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Johnston C.J. Mango G.W. Finkelstein J.N., et al. Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am. J. Respir. Cell Mol. Biol. 1997;17:147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- Kay A.B. Klion A.D. Anti-interleukin-5 therapy for asthma and hypereosinophilic syndrome. Immunol. Allergy Clin. North Am. 2004;24:645–666. doi: 10.1016/j.iac.2004.06.007. vii. [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N. De Veerman M. Coyle A.J., et al. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen U. Whitsett J.A. Wert S.E., et al. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- Lee Y.C. Zhang Z. Mukherjee A.B. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett. 2006;580:4515–4520. doi: 10.1016/j.febslet.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Lewitt P.A. Rezai A.R. Leehey M.A., et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Limberis M.P. Wilson J.M. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Muruve D.A. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- Liu W. Li Z. Luo Q., et al. The elevated expression of osteopontin and vascular endothelial growth factor in sinonasal inverted papilloma and its relationship with clinical severity. Am. J. Rhinol. Allergy. 2011;25:313–317. doi: 10.2500/ajra.2011.25.3662. [DOI] [PubMed] [Google Scholar]
- Mandal A.K. Zhang Z. Ray R., et al. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J. Exp. Med. 2004;199:1317–1330. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Marks W.J., Jr. Ostrem J.L. Verhagen L., et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Marks W.J., Jr. Bartus R.T. Siffert J., et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- McFadden E.R., Jr. Gilbert I.A. Asthma. N. Engl. J. Med. 1992;327:1928–1937. doi: 10.1056/NEJM199212313272708. [DOI] [PubMed] [Google Scholar]
- McPhee S.W. Janson C.G. Li C., et al. Immune responses to AAV in a phase I study for Canavan disease. J. Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Mease P.J. Hobbs K. Chalmers A., et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann. Rheum. Dis. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- Mease P.J. Wei N. Fudman E.J., et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J. Rheumatol. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- Mendell J.R. Campbell K. Rodino-Klapac L., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Wang L. Takeuchi T., et al. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Moss R.B. Milla C. Colombo J., et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- Mukherjee A.K. Correlation between the phospholipids domains of the target cell membrane and the extent of Naja kaouthia PLA(2)-induced membrane damage: evidence of distinct catalytic and cytotoxic sites in PLA(2) molecules. Biochim. Biophys. Acta. 2007;1770:187–195. doi: 10.1016/j.bbagen.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Overbergh L. Valckx D. Waer M., et al. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J.E. Rolling F. Li C., et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M.E. Eosinophilia. N. Engl. J. Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- Rutledge E.A. Halbert C.L. Russell D.W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J.J. Chiang M.S. Kuo M.L., et al. Partially purified extract and viscolin from Viscum coloratum attenuate airway inflammation and eosinophil infiltration in ovalbumin-sensitized mice. J. Ethnopharmacol. 2011;135:646–653. doi: 10.1016/j.jep.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Snyder J.C. Reynolds S.D. Hollingsworth J.W., et al. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am. J. Respir. Cell Mol. Biol. 2010;42:161–171. doi: 10.1165/rcmb.2008-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp B.R. Lund J. Mango G.W., et al. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am. J. Physiol. 1996;271:L656–664. doi: 10.1152/ajplung.1996.271.4.L656. [DOI] [PubMed] [Google Scholar]
- Wang H. Long X.B. Cao P.P., et al. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am. J. Respir. Crit. Care Med. 2010;181:908–916. doi: 10.1164/rccm.200904-0597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Y. Yang M. Xu X.P., et al. Intranasal delivery of T-bet modulates the profile of helper T cell immune responses in experimental asthma. J. Investig. Allergol. Clin. Immunol. 2008;18:357–365. [PubMed] [Google Scholar]
- Weller P.F. Eosinophils: structure and functions. Curr. Opin. Immunol. 1994;6:85–90. doi: 10.1016/0952-7915(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Worgall S. Sondhi D. Hackett N.R., et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- Wu C.A. Peluso J.J. Shanley J.D., et al. Murine cytomegalovirus influences Foxj1 expression, ciliogenesis, and mucus plugging in mice with allergic airway disease. Am. J. Pathol. 2008;172:714–724. doi: 10.2353/ajpath.2008.070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.J. Chen L.C. Kuo M.L. Attenuated Salmonella typhimurium reduces ovalbumin-induced airway inflammation and T-helper type 2 responses in mice. Clin. Exp. Immunol. 2006;145:116–122. doi: 10.1111/j.1365-2249.2006.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.Q. Ma B.F. Zhao L.R., et al. AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson's disease. Gene Ther. 2010;17:83–94. doi: 10.1038/gt.2009.113. [DOI] [PubMed] [Google Scholar]
- Yang C.J. Liu Y.K. Liu C.L., et al. Inhibition of acidic mammalian chitinase by RNA interference suppresses ovalbumin-sensitized allergic asthma. Hum. Gene Ther. 2009;20:1597–1606. doi: 10.1089/hum.2008.092. [DOI] [PubMed] [Google Scholar]
- Yang R. Tan X. Thomas A.M., et al. Alanine-glutamine dipeptide (AGD) inhibits expression of inflammation-related genes in hemorrhagic shock. JPEN J. Parenter. Enteral. Nutr. 2007;31:32–36. doi: 10.1177/014860710703100132. [DOI] [PubMed] [Google Scholar]
- Yang Y. Zhang Z. Mukherjee A.B., et al. Increased susceptibility of mice lacking Clara cell 10-kDa protein to lung tumorigenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent carcinogen in cigarette smoke. J. Biol. Chem. 2004;279:29336–29340. doi: 10.1074/jbc.C400162200. [DOI] [PubMed] [Google Scholar]
- Zabner J. Seiler M. Walters R., et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavorotinskaya T. Tomkinson A. Murphy J.E. Treatment of experimental asthma by long-term gene therapy directed against IL-4 and IL-13. Mol. Ther. 2003;7:155–162. doi: 10.1016/s1525-0016(02)00050-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Kundu G.C. Yuan C.J., et al. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Kundu G.C. Zheng F., et al. Insight into the physiological function(s) of uteroglobin by gene-knockout and antisense-transgenic approaches. Ann. N. Y. Acad. Sci. 2000;923:210–233. doi: 10.1111/j.1749-6632.2000.tb05532.x. [DOI] [PubMed] [Google Scholar]
- Zheng F. Kundu G.C. Zhang Z., et al. Uteroglobin is essential in preventing immunoglobulin A nephropathy in mice. Nat. Med. 1999;5:1018–1025. doi: 10.1038/12458. [DOI] [PubMed] [Google Scholar]