Abstract
Familial hypercholesterolemia (FH) is a life-threatening genetic disease caused by mutations in the gene encoding low-density lipoprotein receptor (LDLR). As a bridge to clinical trials, we generated a “humanized” mouse model lacking LDLR and apolipoprotein B (ApoB) mRNA editing catalytic polypeptide-1 (APOBEC-1) expression and expressing a human ApoB100 transgene in order to permit more authentic simulation of in vivo interactions between the clinical transgene product, human LDLR (hLDLR), and its endogenous ligand, human ApoB100. On a chow diet, the humanized LDLR-deficient mice have substantial hypercholesterolemia and a lipoprotein phenotype more closely resembling human homozygous FH (hoFH) than in previous mouse models of FH. On injection of an adeno-associated virus serotype 8 (AAV8) vector encoding the human LDLR cDNA, significant correction of hypercholesterolemia was realized at doses as low as 1.5×1011 genome copies (GC)/kg. Given that some patients with heterozygous FH (heFH) cannot be adequately treated with current therapy, we then extended our studies to similarly “humanized” mice that were heterozygous for LDLR deficiency, and that have a lipoprotein phenotype resembling heterozygous FH. Injection of AAV8-hLDLR brought about significant reduction in total and LDL cholesterol at doses as low as 5×1011 GC/kg. Collectively, these data demonstrate the safety and efficacy of the liver-specific AAV8-hLDLR vector in the treatment of humanized mice modeling both hoFH and heFH.
Kassim and colleagues demonstrate that injection of an adeno-associated virus serotype 8 (AAV8) vector encoding the human low-density lipoprotein receptor (LDLR) cDNA results in significant correction of hypercholesterolemia in humanized mouse models of homozygous and heterozygous familial hypercholesterolemia (FH).
Introduction
Familial hypercholesterolemia (FH) is an autosomal codominant disorder caused by mutations affecting the function or expression of the gene encoding low-density lipoprotein receptor (LDLR) (Brown and Goldstein, 1986; Goldstein and Brown, 1989). Patients who inherit one mutant allele have moderate elevations in plasma LDL and develop premature coronary artery disease (CAD). The prevalence of homozygous and heterozygous FH is approximately 1 in 1 million and 1 in 500 people worldwide, respectively. Patients with two abnormal LDL receptor alleles (homozygotes or compound heterozygotes) have severe hypercholesterolemia and CAD that often presents in childhood.
Patients with homozygous FH (hoFH) are minimally responsive to conventional LDL-lowering pharmacological therapies such as HMG-CoA reductase inhibitors, which work by increasing receptor-mediated LDL uptake. The current standard of care in hoFH is LDL apheresis, a physical method of purging the plasma of LDL-C, which can transiently reduce LDL-C by more than 50% (Apstein et al., 1978). However, there is reaccumulation of LDL-C in plasma, and therefore apheresis must be repeated every 1 to 2 weeks (Leonard et al., 1981). The liver is the primary organ responsible for regulation of cholesterol homeostasis, in vivo, and the receptor for LDL plays an important role in this regulation. Hepatocytes are the cells primarily responsible for catabolizing LDL and the only cells capable of excreting cholesterol. Under normal conditions, 70% of all LDL particles are cleared by the liver and hepatic LDLR activity is the most critical regulator of plasma LDL cholesterol levels. The success of orthotopic liver transplantation in the treatment of FH provides compelling support for the hypothesis that expression of hepatic LDL receptor activity is sufficient for metabolic correction in vivo (Grossman et al., 1994, 1995). Indeed, somatic gene transfer of the LDLR cDNA to liver is effective in reducing LDL-C levels in preclinical models of FH (Kozarsky et al., 1994; Lebherz et al., 2004; Kassim et al., 2010).
The mouse is a valuable model for preclinical studies and the most commonly used mouse model of FH has been the Ldlr–/– mouse. However, deletion of LDLR in the mouse is associated with only mild hypercholesterolemia when mice are fed a chow diet (Sanan et al., 1998; Daugherty, 2002), and only on feeding of a high-fat diet do the mice develop severe hypercholesterolemia and atherosclerosis. One reason for the phenotypic difference in LDLR deficiency between mice and humans relates to the metabolism of apolipoprotein B (ApoB) (Rader and FitzGerald, 1998). Whereas humans express ApoB mRNA editing catalytic polypeptide-1 (Apobec1) in the intestine, leading to editing of the ApoB mRNA and production of a truncated form of the ApoB protein called ApoB48 by the intestine, the human liver does not express APOBEC-1 and therefore produces only the full-length version of ApoB, called ApoB100. In contrast, mice express APOBEC-1 in the liver, resulting in substantial hepatic production of ApoB48. Whereas ApoB100-containing lipoproteins require the LDLR for clearance, lipoproteins containing ApoB48 can be cleared from the plasma independent of the LDL receptor, as a result of binding of ApoE to other receptors. Thus, Ldlr–/– mice do not represent the pathophysiology of hoFH and may not be the optimal preclinical model for testing new strategies for the treatment of hoFH.
Hirano and colleagues introduced a germ line ablation into the Apobec1 gene in mice (Hirano et al., 1996). A phenotype similar to human hoFH was created when the Apobec1 ablation was bred into Ldlr–/– mice. These so-called double-knockout mice (LA-DKO), which are deficient in both Ldlr and Apobec1, develop severe hypercholesterolemia due to elevations in LDL on chow diet associated with atherosclerosis that is similar in distribution and histology to what is seen in human patients with hoFH (Powell-Braxton et al., 1998).
In a previous study, we demonstrated that a single intravenous injection of an adeno-associated virus 8 (AAV8) vector encoding mouse LDLR (mLDLR) led to a statistically significant reduction in plasma cholesterol and non- high-density lipoprotein (HDL) cholesterol levels in LA-DKO mice at doses as low as 1.5×1011 genome copies (GC)/kg (Kassim et al., 2010). Moreover, this treatment was found to trigger substantial regression and remodeling of atherosclerotic lesions. Progressing AAV8 vectors into the clinic for the treatment of hoFH requires the preclinical characterization of a vector that expresses the human LDLR (hLDLR).
As a bridge to clinical trials, we have generated a mouse model that should allow more authentic simulation of in vivo interactions between the proposed clinical transgene product, human LDLR (hLDLR), and its endogenous ligand, human ApoB100. To create this third-generation humanized FH mouse model, transgenic mice expressing the human ApoB100 transgene (Linton et al., 1993) were crossed onto the LA-DKO background to create Ldlr–/–/Apobec1–/–/human ApoB transgenic (or LA-DKO/hApoB-Tg) mice. On a chow diet these mice have higher cholesterol levels and a lipoprotein phenotype even more closely resembling hoFH than do LA-DKO animals. Here, we report results of our preclinical studies wherein we evaluated a vector, AAV8, that encodes hLDLR, in humanized LA-DKO/hApoB-Tg mice as a prelude to clinical trials.
Materials and Methods
Animals
Male C57BL/6 Ldlr–/–Apobec1–/– (DKO) mice were bred in-house and have been described elsewhere (Powell-Braxton et al., 1998). For expression and efficacy studies, mice were given unrestricted access to water and were fed a standard chow diet. The vector was injected via intravenous tail vein injection with specified genome copies (GC) per mouse. Animals were killed at the indicated times after gene transfer. For all studies, blood was obtained at least once before and at designated time points after gene transfer. LA-DKO/hApoB-Tg mice were generated by crossing Ldlr–/–Apobec1–/– with human ApoB transgenic mice. The most productive breeding strategy was to cross LA-DKO/hApoB-Tg males with heterozygous females (i.e., Ldlr+/–/Apobec1–/–/human ApoB transgenic) because LA-DKO/hApoB-Tg females are infertile. These animals were bred in-house and maintained on a chow diet. Male mice were used for all of the studies described in this paper. There was no statistically significant difference between male and female LA-DKO mice with respect to AAV8.TBG.hLDLR gene transfer as measured by genome copies in liver and drop in serum cholesterol (see Supplementary Fig. S1A and B; Supplementary Data are available online at www.liebertonline.com/hum). All study protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania (Pittsburgh, PA).
Vectors
AAV8 expressing human LDLR was generated as previously described (Lebherz et al., 2004). AAV vector was generated by triple transfection of the AAV inverted terminal repeat (ITR)-flanked construct with the packaging plasmid pAAV2/8 and the helper plasmid pdF6 as previously described (Wang et al., 2010). AAV vector particles were purified using iodixanol gradients (Lock et al., 2010; Wang et al., 2010).
Analytical methods
Plasma total cholesterol levels were measured in individual mice at each time point by enzymatic assay, using a Cobas Fara II (Roche Diagnostic Systems, Indianapolis, IN) and reagents from Sigma-Aldrich (St. Louis, MO). Plasma samples from groups of five mice were pooled at each time point and subjected to fast protein liquid chromatography (FPLC) gel filtration (Pharmacia LKB Biotechnology/GE Healthcare, Piscataway, NJ) on two Superose 6 columns (GE Healthcare). Cholesterol concentrations in the fractions were determined by enzymatic assay (Wako Pure Chemical Industries, Osaka, Japan).
Interferon-γ enzyme-linked immunospot assay
Splenocytes from treated mice were harvested as described. After isolation and washing steps, splenocytes were overlaid onto a Ficoll-Paque (Amersham Biosciences/GE Healthcare) gradient layer and centrifuged for 20 min at 1000×g at room temperature to remove red blood cells. The lymphocyte band was then recovered and further washed two times in phosphate-buffered saline (PBS)–1% fetal bovine serum (FBS). To determine the number of cells secreting interferon (IFN)-γ in response to antigenic stimulation, an IFN-γ enzyme-linked immunospot (ELISPOT) assay was performed according to the manufacturer's instructions (BD Biosciences, San Jose, CA). Briefly, 96-well plates were coated with capture antibody overnight at 4°C and blocked for 2 hr at 25°C with RPMI 1640, 10% FBS, 1% penicillin–streptomycin–l-glutamine. Splenocytes were plated at 2.5×105 cells per well in T cell assay medium supplemented with immunodominant nuclear-targeted LacZ (nLacZ) peptide (ICPMYARV, 1 μg/ml; Mimotopes, Clayton, Victoria, Australia), or human LDLR peptide libraries (Mimotopes). Phorbol myristate acetate and ionomycin (PMA/I; Sigma-Aldrich) were used to stimulate a separate population of lymphocytes (plated at 2×104/well) as a nonspecific, positive control. Cells plated in medium alone (no peptide stimulation) served as a negative control. Plates were incubated for 18 hr at 37°C, 5% CO2. After incubation plates were washed vigorously in deionized water, PBS–0.05% Tween 20 (Sigma-Aldrich), and incubated for 2 hr at room temperature with biotinylated anti-mouse IFN-γ detection antibody (2 g/ml). After three washes with PBS–0.05% Tween 20, the plates were incubated with enzyme conjugate (streptavidin–horseradish peroxidase [HRP], 5 μg/ml) for 1 hr at room temperature. After a series of washes with PBS–0.05% Tween 20 and PBS, spots were developed with the 3-amino-9-ethylcarbazole (AEC) substrate set (BD Biosciences). Color development was stopped after 8 min by washing with distilled water. Plates were dried overnight at room temperature and read with an AID ELISPOT reader system (Cell Technology, Columbia, MD). Responses greater than 50 spot-forming units (SFUs) or at least 3-fold increase relative to total background were considered significant.
Immunoblotting and histochemical analysis
Levels of hepatic LDL receptor protein were determined by immunoblotting analysis as previously described (Kassim et al., 2010), using a polyclonal antiserum to rat LDL receptor (a gift from G.C. Ness, University of South Florida, Tampa, FL). Briefly, liver lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 3% gelatin and then incubated with a 1:375 dilution of rabbit anti-rat LDL receptor serum. LDL receptor-immunoreactive protein was detected with an alkaline phosphatase-conjugated second antibody.
Hematoxylin and eosin (H&E)-stained sections from formalin-fixed paraffin-embedded liver samples were examined for portal and lobular inflammation. To elucidate whether or not gene therapy led to higher lipid accumulation in the liver of LAHB mice, we examined the accumulation of lipids by the oil red O stain method. Hepatic steatosis was assessed in samples collected in O.C.T. compound by oil red O staining. Briefly, liver cryosections were fixed for 10 min in 60% isopropanol and stained with 0.3% oil red O in 60% isopropanol for 30 min and subsequently washed with 60% isopropanol. Sections were counterstained with Gill's hematoxylin, washed with acetic acid solution (4%), and mounted with aqueous solution. Once stained, sections were quantified by histomorphometry. For both H&E and oil red O analyses, a minimum of four independent fields were quantified.
Statistical analyses
Experimental groups were compared with the baseline group by Dunnett test. Repeated-measures analysis of variance was used to compare cholesterol levels among different groups of mice over time after gene transfer. Statistical significance for all comparisons was assigned at p<0.05. Graphs represent mean±SD values.
Results
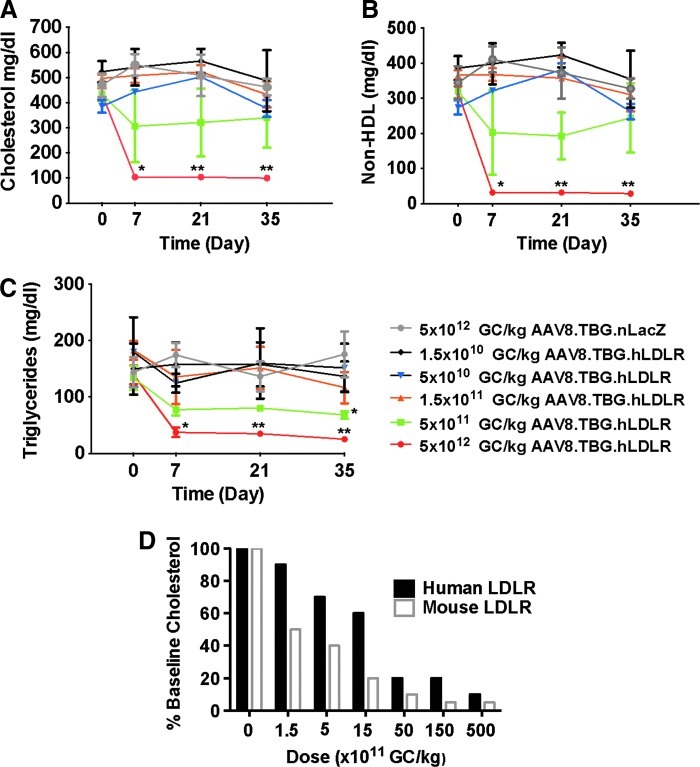
Initial studies with an AAV8 vector encoding the human LDLR (hLDLR) cDNA under the control of a liver-specific thyroxine-binding globulin (TBG) promoter were performed in male LA-DKO mice (lacking the human ApoB gene) injected with a range of doses from 1.5×1010 to 5×1012 GC/kg, and lipid correction was monitored over a period of 35 days. Although AAV8.TBG.hLDLR was effective in reducing total cholesterol (Fig. 1A), non-HDL cholesterol (Fig. 1B), and triglycerides (Fig. 1C), the lowest dose (5×1012 GC/mouse) that led to a statistically significant reduction of cholesterol (i.e., referred to as the minimally effective dose) was considerably higher than the minimal dose required for correction when using AAV8 expressing the mouse version of LDLR (1.5×1011 GC/kg; Fig. 1D and Kassim et al., 2010). The homology between human and mouse LDLR is 78% at the amino acid sequence level; within the ligand-binding domain there is 74% homology between human and mouse LDLR protein. One explanation for the apparent diminished activity of the human LDLR vector in LA-DKO mice was diminished binding of the human LDLR to mouse ApoB. We were able to address this hypothesis through the use of a third-generation humanized FH mouse model, the LA-DKO/hApoB-Tg mouse.
FIG. 1.
Evaluation of AAV8 encoding human low-density lipoprotein receptor (LDLR) in LA-DKO mice (double knockout mice that are deficient in both Ldlr and Apobec1). (A) Plasma cholesterol levels, (B) non-HDL cholesterol, and (C) triglyceride levels were examined in LA-DKO mice after treatment with AAV8.hLDLR (n=10 animals per dose group). Each point represents the mean±SD. *p<0.05, **p<0.01. (D) Presented is the percent reduction in cholesterol as compared with baseline as a function of vector dose (GC/kg). AAV8.TBG.mLDLR is based on our previously published data (Kassim et al., 2010).
The LA-DKO/hApoB-Tg line was generated by crossing Ldlr–/–Apobec1–/– mice (i.e., LA-DKO) with animals containing hApoB as a transgene, all into a C57BL/6 background. This version of the humanized mouse line is called the LA-DKO/hApoB-Tg mouse. A version of this humanized FH mouse, which is heterozygous for the Ldlr mutation, but homozygous for both the Apobec mutation and hApoB-encoding transgene, was created to simulate patients with heterozygous FH. Supplementary Table S1 summarizes the lipid and lipoprotein profiles for these new mouse strains.
As compared with the parental strain, which is deleted for Apobec1 and expresses the hApoB gene but has both wild-type LDLR alleles, LA-DKO/hApoB-Tg mice exhibit a profound elevation in total cholesterol, which is almost exclusively due to an increase in non-HDL cholesterol. Serum triglycerides were elevated approximately 2-fold over wild type. The humanized LDLR heterozygous line showed intermediate elevations in lipid/lipoprotein profiles consistent with what is observed in heterozygous patients with FH. These metabolic parameters were essentially indistinguishable between male and female animals across all genotypes.
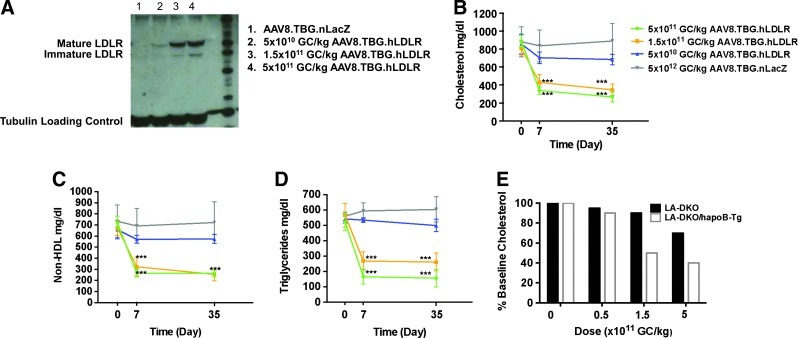
LA-DKO/hApoB-Tg mice were injected with doses of AAV8.TBG.hLDLR ranging from 5×1010 to 5×1011 GC/kg. As we anticipated, the human LDLR construct is much more effective in the LA-DKO/hApoB-Tg mouse as compared with the LA-DKO mouse (which expresses only mouse ApoB). Detectable LDLR protein expression was found at doses as low as 5×1010 GC/kg (Fig. 2A). Statistically significant reductions in cholesterol and non-HDL cholesterol correction were realized at doses as low as 1.5×1011 GC/kg (Fig. 2B and C). Specifically, treatment with 1.5×1011 GC/kg caused total cholesterol levels to drop from 808±94 to 353±76 mg/dl by day 35 after treatment (Fig. 2B). Likewise, non-HDL cholesterol levels dropped from 675±87 to 255±75 mg/dl by day 35 after injection with 1.5×1011 GC/kg (Fig. 2C). These levels of correction are comparable to those observed in studies using AAV8 to express the murine LDLR in LA-DKO mice (Fig. 2E) wherein the minimal therapeutic dose was found to be 1.5×1011 GC/kg. In addition, treatment with AAV8.TBG.hLDLR significantly reduced triglyceride levels in LA-DKO/hApoB-Tg mice (Fig. 2D). This had not previously been observed in our studies using LA-DKO mice and the AAV8.TBG.mLDLR vector (Kassim et al., 2010).
FIG. 2.
Evaluation of AAV8.TBG.hLDLR in LA-DKO/hApoB-Tg mice. (A) Representative Western blot examining human LDLR (hLDLR) protein expression in liver lysates on day 35 after vector administration. (B) Plasma cholesterol, (C) non-HDL cholesterol, and (D) triglyceride levels were examined in LA-DKO/hApoB-Tg mice after treatment with AAV8.TBG.hLDLR (n=10 animals per dose group). Each point represents the mean±SD. ***p<0.001. (E) Evaluation of AAV8.TBG.hLDLR in LA-DKO and LA-DKO/hApoB-Tg mice. Presented is the average reduction in serum cholesterol as a function of vector dose (GC/kg).
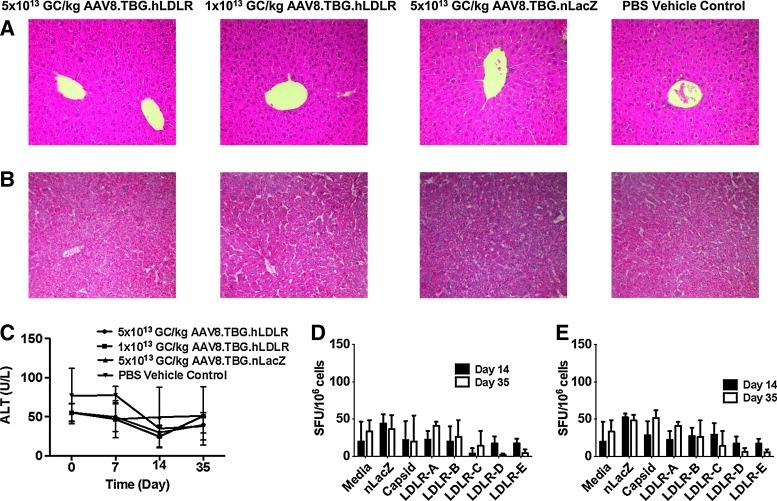
Additional evaluations included liver pathology as assessed by measurement of serum transaminases, histopathology, and T cell responses to the capsid and transgene product. LA-DKO/hApoB-Tg mice injected with the highest dose of AAV8.TBG.hLDLR or AAV8.TBG.LacZ that can be delivered (5×1013 GC/kg) were used for these analyses. No significant liver histopathology was detected in animals, based on hematoxylin and eosin staining of livers (Fig. 3A) and liver transaminase measurements (Fig. 3C). Likewise, no abnormal lipid accumulation was observed in the livers of vector-treated mice as seen by oil red O staining (Fig. 3B). Lymphocytes were harvested from the spleen (Fig. 3D) or liver (Fig. 3E) of vector-treated animals to evaluate for transgene- and capsid-specific T cell responses via IFN-γ ELISPOT. No transgene- or capsid-specific T cell responses were elicited in mice injected with the highest dose of AAV8.TBG.hLDLR vector.
FIG. 3.
Safety of AAV8.TBG.hLDLR in LA-DKO/hApoB-Tg mice. (A) Hematoxylin–eosin (H&E) and (B) oil red O staining of samples from mice 35 days after vector administration. (C) Serum alanine aminotransferase (ALT) levels were monitored over a 35-day period. Lymphocytes were harvested from the (C) liver and (D) spleen of mice treated with 1×1012 AAV8.TBG.hLDLR or 1×1012 AAV8.TBG.nLacZ and stimulated with either the immunodominant nLacZ epitope or a human LDLR peptide library to determine the transgene-specific response. Cells were stimulated with the capsid peptide library of AAV8 to determine the capsid response. Data represent means±SD for four mice per group.
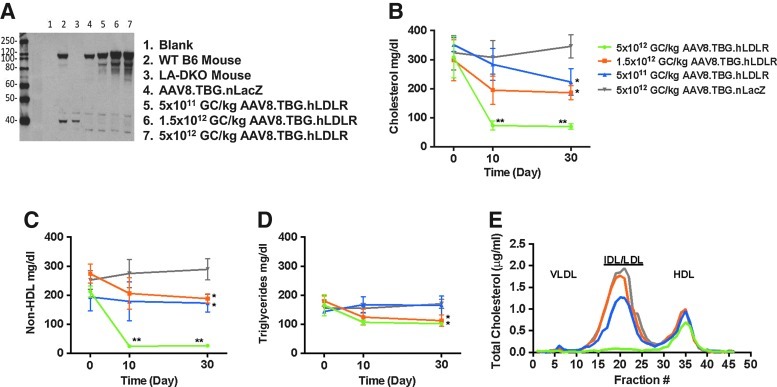
We evaluated the efficacy of the AAV8.TBG.hLDLR vector in humanized LDLR heterozygous mice as well. Animals were injected with a range of doses from 5×1011 to 5×1012 GC/kg. The baseline level of endogenous LDLR in humanized heterozygous mice is shown in Western blots (Fig. 4A, lane 4) from livers of animals that received control LacZ vector. A single intravenous injection of AAV8.TBG.hLDLR at a dose of 5×1011GC/kg led to an increase in LDLR protein in the heterozygous mice (Fig. 4A, lane 5) and a statistically significant reduction in total cholesterol from 325±95 to 200±80 mg/dl (Fig. 4B). Further decreases in serum lipids were obtained with higher doses of vector; serum cholesterol and non-HDL cholesterol were essentially normalized at a dose of 5×1012 GC/kg. The same overall trend was observed with respect to non-HDL cholesterol and triglyceride levels (Fig. 4C and D). No significant increase in serum transaminase levels was observed in animals after vector injection (data not shown). To gain insight into the effects of AAV8.TBG.LDLR expression on plasma lipoproteins, we separated lipoproteins by FPLC gel filtration (Fig. 4E). This approach demonstrated that 5×1012 GC/kg was the only dose that led to substantial reductions in the intermediate-density lipoprotein (IDL)/LDL peak compared with AAV8.TBG.nLacZ-treated heterozygous mice (Fig. 4E). There were no marked differences among the four groups in the very low-density lipoprotein (VLDL) and HDL fractions (Fig. 4E).
FIG. 4.
A higher dose of AAV8.TBG.hLDLR is required to achieve full correction in the humanized form of heterozygous FH mice (Ldlr+/–Apobec1–/–ApoB+/+). (A) Western blots examining hLDLR protein expression in liver lysates of representative mice 30 days after vector administration. (B) Plasma cholesterol, non-HDL cholesterol (C), and triglyceride (D) levels were examined in heterozygous mice after treatment with AAV8.hLDLR (n=6 animals per dose group). Each point represents the mean±SD. *p<0.05, **p<0.01. (E) Pooled mouse plasma from AAV-injected LAHB mice (n=6 per dose group) was analyzed by FPLC fractionation and the cholesterol content of each fraction was determined. IDL, intermediate-density lipoprotein; VLDL, very low-density lipoprotein.
Discussion
Detailed characterizations of FH homozygotes have demonstrated that molecular heterogeneity leads to genotype-specific variation in the level of residual LDL receptor function, which may correlate with severity and progression of the disease (Goldstein and Brown, 1982; Sprecher et al., 1984, 1985). Sprecher and colleagues characterized the residual LDL receptor activity in fibroblasts of 14 patients with FH and attempted to correlate this with several clinical indices (Sprecher et al., 1985). They demonstrated a statistically significant inverse correlation between residual LDL receptor activity and (1) pretreatment levels of cholesterol and LDL, and (2) age of onset of angina pectoris. They also noted an association between LDL receptor activity and cholesterol reduction in response to conventional pharmacological therapy. In another study, Goldstein and Brown analyzed 57 homozygous deficient patients for residual LDL receptor activity in fibroblasts and classified them as receptor negative (<2% of control, n=31) and receptor defective (>2% of control, n=26) (Goldstein and Brown, 1982). Receptor status was correlated with age of onset and severity of CAD. Manifestations of CAD developed before the age of 10 years in 10 of 31 receptor-negative patients (32%) and in only 1 of 26 receptor-defective patients (4%). Furthermore, 8 of 31 of the receptor-negative patients (25%) died of the sequelae of CAD before the age of 25 years (mean, 11 years), whereas only 1 of 26 of the receptor-defective patients (4%) died during this time interval. These studies, in addition to the extensive evidence in the literature, and our previous experience with the ex vivo gene therapy trial for hoFH (Grossman et al., 1994, 1995) suggest that even a small amount of residual LDL receptor activity will be beneficial when compared with those who are truly receptor defective.
Translating gene therapy into the clinic requires preclinical evaluation of the efficacy and safety of the candidate vector in an authentic animal model that accurately recapitulates the disease etiology observed in humans. In this study, we evaluated the vector expressing the human LDLR gene in a mouse model of hoFH. Although the LA-DKO mouse studies revealed dramatic improvement in lipid levels, the minimally effective dose was significantly higher than in our previously published studies examining vectors expressing the syngeneic murine LDLR gene in LA-DKO mice. We hypothesized that this difference was due to species specificity in ligand (ApoB)–receptor (LDLR) interactions. To test this hypothesis, we introduced the human ligand for LDLR (human ApoB100) via a germ line transgene in the LA-DKO mouse. This animal (LA-DKO/hApoB-Tg) better modeled the human disease of hoFH in terms of baseline lipoprotein profile. In the LA-DKO/hApoB-Tg mouse, the minimal effective dose of AAV8.TBG.hLDLR was found to be as low as 1.5×1011 GC/kg, confirming a greater efficacy of expression of the human LDLR transgene in the setting of human ApoB100 expression. The minimal effective dose of 1.5×1011 GC/kg translates to 3×1012 GC for a 20-kg adolescent and 1×1013 GC for a 70-kg adult, which is well within the reach of current manufacturing protocols.
We also tested the AAV8.TBG.hLDLR vector in a similarly humanized mouse model of heterozygous LDLR deficiency, which has a lipoprotein phenotype resembling that of human heFH. This is, to our knowledge, the first study to examine the potential of LDLR gene therapy for heFH. We demonstrated that complete correction could be achieved at doses as low as 5×1012 GC/kg and less than complete but statistically significant reductions in serum lipids at a dose as low as 5×1011 GC/kg. Optimal reductions in the plasma concentrations of LDL-cholesterol in patients with heFH in whom baseline values on a cholesterol-lowering diet exceed 300 mg/dl, or in those patients with multiple risk factors or known coronary artery disease, are often not achieved with monotherapy and in such patients combination drug therapy is necessary (Huijgen et al., 2008). The refractory nature of many patients with heterozygous FH underscores the need for these patients to be seen in a specialty lipid disorders clinic where expertise in combination drug therapy is available and where potentially newer therapeutic approaches may be available (Vuorio et al., 2004). Within this context, gene therapy may prove to be an attractive alternative option.
Several gene therapy studies have demonstrated inhibition or regression of atherosclerosis progression. Of regression studies, the most common strategy has been to use liver-directed gene transfer of antiatherogenic apolipoproteins including ApoA-I and ApoE (Tsukamoto et al., 1997, 1999, 2000; Tangirala et al., 1999, 2001; Reis et al., 2001). We have previously shown that AAV8-mediated expression of the murine form of LDLR in LA-DKO mice markedly reduced plasma cholesterol levels and was remarkably effective in reducing preexisting fatty streaks, as measured by en face lesion analysis, and in remodeling aortic root plaques to a more stable-looking phenotype. This effect was observed despite the fact that animals were maintained on a high-fat diet throughout the study (Kassim et al., 2010). In the current study, our results suggest that the intravenous injection of a low dose of an AAV8-based vector encoding the human LDLR cDNA in humanized mice lacking LDLR and APOBEC-1 and expressing human ApoB100 is comparably effective in reducing plasma cholesterol levels. Future studies are planned to examine whether AAV8.TBG.hLDLR demonstrates a similar effect on the atherosclerotic lesions of both homozygous and heterozygous LA-DKO/hApoB-Tg mice.
Last, we demonstrate that the expression of human LDLR is sustained without evidence of substantial hepatic inflammation or toxicity. Although previous studies have demonstrated that AAV8 can result in prolonged and durable in vivo gene expression in both mice (Sarkar et al., 2004; Sands, 2011) and humans (Nathwani et al., 2011), it will be important to conduct longer term studies at a single dose to examine the durability of the AAV8-mediated expression of LDLR in LA-DKO/hApoB-Tg mice. Collectively, the results described in this paper suggest that AAV8-based gene therapy for hoFH may be feasible and support further development of this approach.
Supplementary Material
Acknowledgments
Sources of funding: National Heart, Lung, and Blood Institute (P01-HL059407; J.M.W.) and National Institute of Diabetes and Digestive and Kidney Diseases (P30-DK047757; J.M.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
References
- Apstein C.S. Zilversmit D.B. Lees R.S. George P.K. Effect of intessive plasmapheresis on the plasma cholesterol concentration with familial hypercholesterolemia. Atherosclerosis. 1978;31:105–115. doi: 10.1016/0021-9150(78)90157-0. [DOI] [PubMed] [Google Scholar]
- Brown M.S. Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Daugherty A. Mouse models of atherosclerosis. Am. J. Med. Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L. Brown J.C. Familial hypercholesterolemia, lipoprotein and lipid metabolism disorders. In: Scriver C.R., editor. Metabolic Basis of Inherited Disease II. McGraw-Hill; New York: 1989. pp. 1215–1250. [Google Scholar]
- Goldstein J.L. Brown M.S. The LDL receptor defect in familial hypercholesterolemia: Implications for pathogenesis and therapy. Med. Clin. North Am. 1982;66:335–362. doi: 10.1016/s0025-7125(16)31424-9. [DOI] [PubMed] [Google Scholar]
- Grossman M. Raper S.E. Kozarsky K., et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat. Genet. 1994;6:335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- Grossman M. Rader D.J. Muller D.W., et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat. Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- Hirano K.-I. Young S.G. Farese R.V.J., et al. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J. Biol. Chem. 1996;271:9887–9890. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
- Huijgen R. Vissers M.N. Defesche J.C., et al. Familial hypercholesterolemia: Current treatment and advances in management. Expert Rev. Cardiovasc. Ther. 2008;6:567–581. doi: 10.1586/14779072.6.4.567. [DOI] [PubMed] [Google Scholar]
- Kassim S.H. Li H. Vandenberghe L.H., et al. Gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis. PLoS One. 2010;5:e13424. doi: 10.1371/journal.pone.0013424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K.F. McKinley D.R. Austin L.L., et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J. Biol. Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- Lebherz C. Gao G. Louboutin J.P., et al. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J. Gene Med. 2004;6:663–672. doi: 10.1002/jgm.554. [DOI] [PubMed] [Google Scholar]
- Leonard J.V. Clarke M. Macartney F.J. Slack J. Progression of atheroma in homozygous familial hypercholesterolaemia during regular plasma exchange. Lancet. 1981;2:811. doi: 10.1016/s0140-6736(81)90225-7. [DOI] [PubMed] [Google Scholar]
- Linton M.F. Farese R.V. Chiesa G., et al. Transgenic mice expressing high plasma-concentrations of human apolipoprotein B100 and lipoprotein(A) J. Clin. Invest. 1993;92:3029–3037. doi: 10.1172/JCI116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M. Alvira M. Vandenberghe L.H., et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C. Tuddenham E.G. Rangarajan S., et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton L. Véniant M. Latvala R.D., et al. A mouse model of human familial hypercholesterolemia: Markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat. Med. 1998;4:934–938. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- Rader D.J. Fitzgerald G.A. State of the art: Atherosclerosis in a limited edition. Nat. Med. 1998;4:899–900. doi: 10.1038/nm0898-899. [DOI] [PubMed] [Google Scholar]
- Reis E.D. Li J. Fayad Z.A., et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J. Vasc. Surg. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- Sanan D.A. Newland D.L. Tao R., et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: No accentuation by apolipoprotein(a) Proc. Natl. Acad. Sci. U.S.A. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M.S. AAV-mediated liver-directed gene therapy. Methods Mol. Biol. 2011;807:141–157. doi: 10.1007/978-1-61779-370-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R. Tetreault R. Gao G., et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- Sprecher D.L. Schaefer E.J. Kent K.M., et al. Cardiovascular features of homozygous familial hypercholesterolemia: Analysis of 16 patients. Am. J. Cardiol. 1984;54:20–30. doi: 10.1016/0002-9149(84)90298-4. [DOI] [PubMed] [Google Scholar]
- Sprecher D.L. Hoeg J.M. Schaefer E.J., et al. The association of LDL receptor activity, LDL cholesterol level, and clinical course in homozygous familial hypercholesterolemia. Metabolism. 1985;34:294–299. doi: 10.1016/0026-0495(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Tangirala R.K. Tsukamoto K. Chun S.H., et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Tangirala R.K. Pratico D. Fitzgerald G.A., et al. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J. Biol. Chem. 2001;276:261–266. doi: 10.1074/jbc.M003324200. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K. Smith P. Glick J.M. Rader D.J. Liver-directed gene transfer and prolonged expression of three major human ApoE isoforms in ApoE-deficient mice. J. Clin. Invest. 1997;100:107–114. doi: 10.1172/JCI119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K. Tangirala R. Chun S.H., et al. Rapid regression of atherosclerosis induced by liver-directed gene transfer of ApoE in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:2162–2170. doi: 10.1161/01.atv.19.9.2162. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K. Tangirala R.K. Chun S., et al. Hepatic expression of apolipoprotein E inhibits progression of atherosclerosis without reducing cholesterol levels in LDL receptor-deficient mice. Mol. Ther. 2000;1:189–194. doi: 10.1006/mthe.2000.0028. [DOI] [PubMed] [Google Scholar]
- Vuorio A.F. Kovanen P.T. Gylling H. Hypolipidemic treatment of heterozygous familial hypercholesterolemia: A lifelong challenge. Expert Rev. Cardiovasc. Ther. 2004;2:405–415. doi: 10.1586/14779072.2.3.405. [DOI] [PubMed] [Google Scholar]
- Wang L. Wang H. Bell P., et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.