Abstract
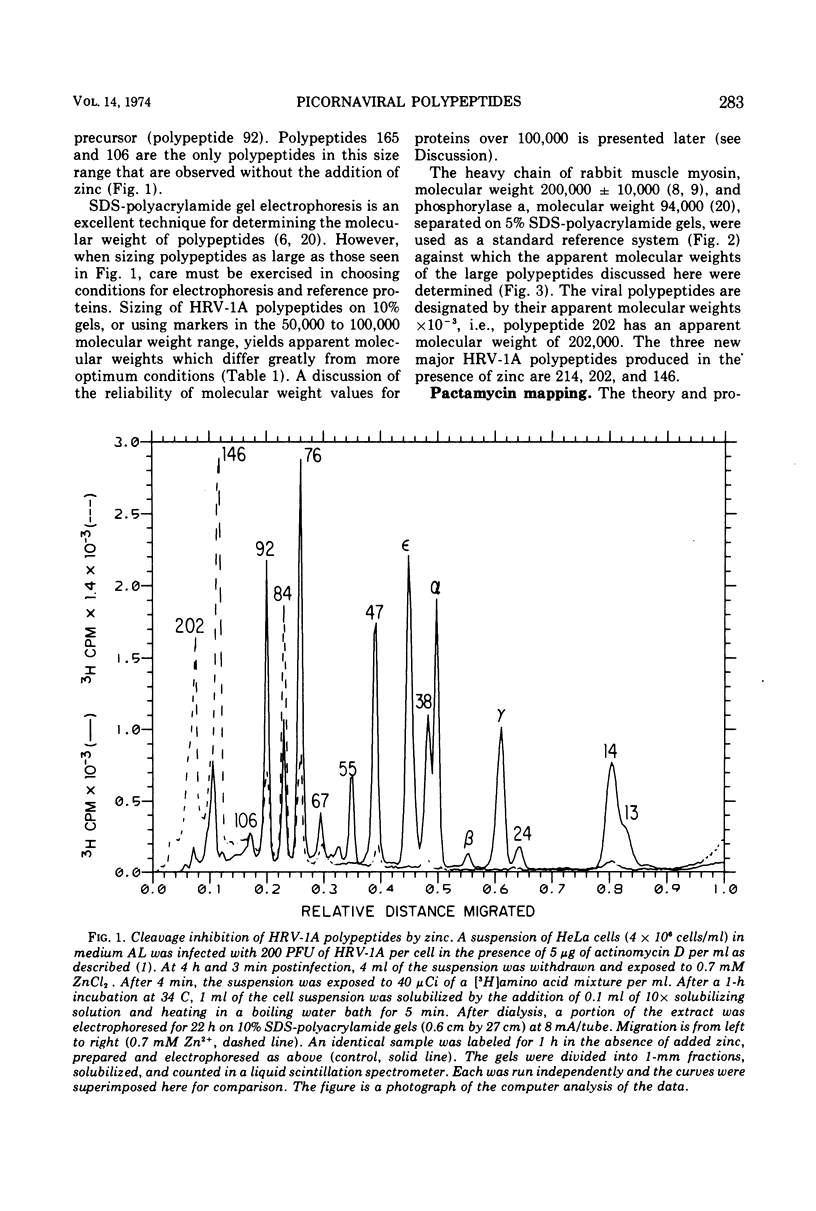
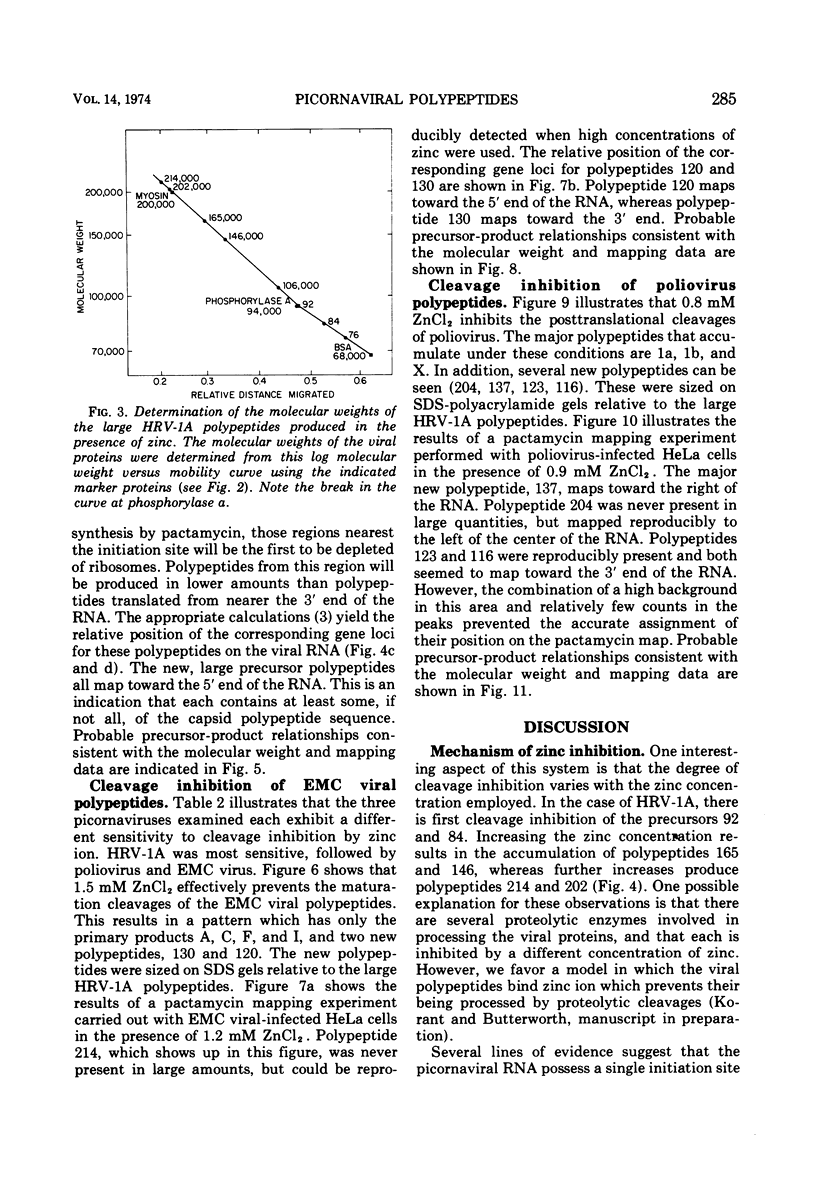
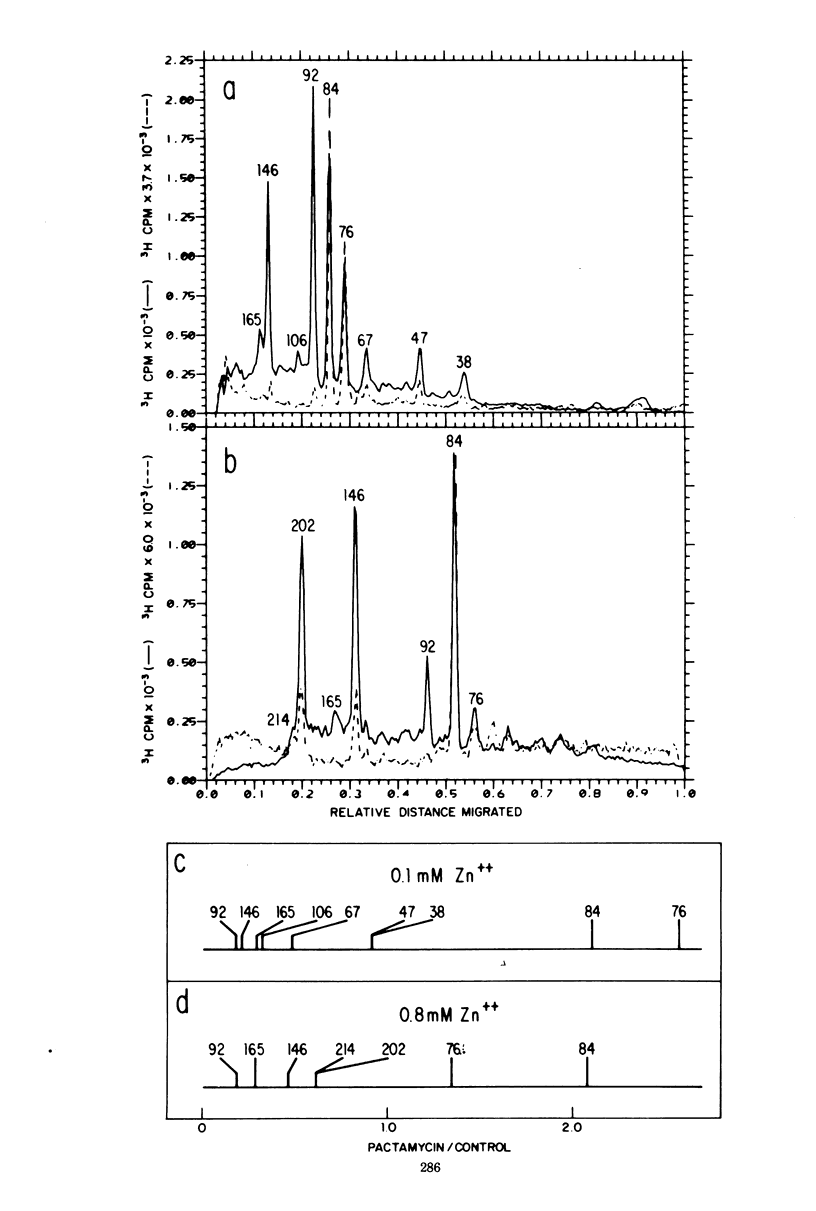
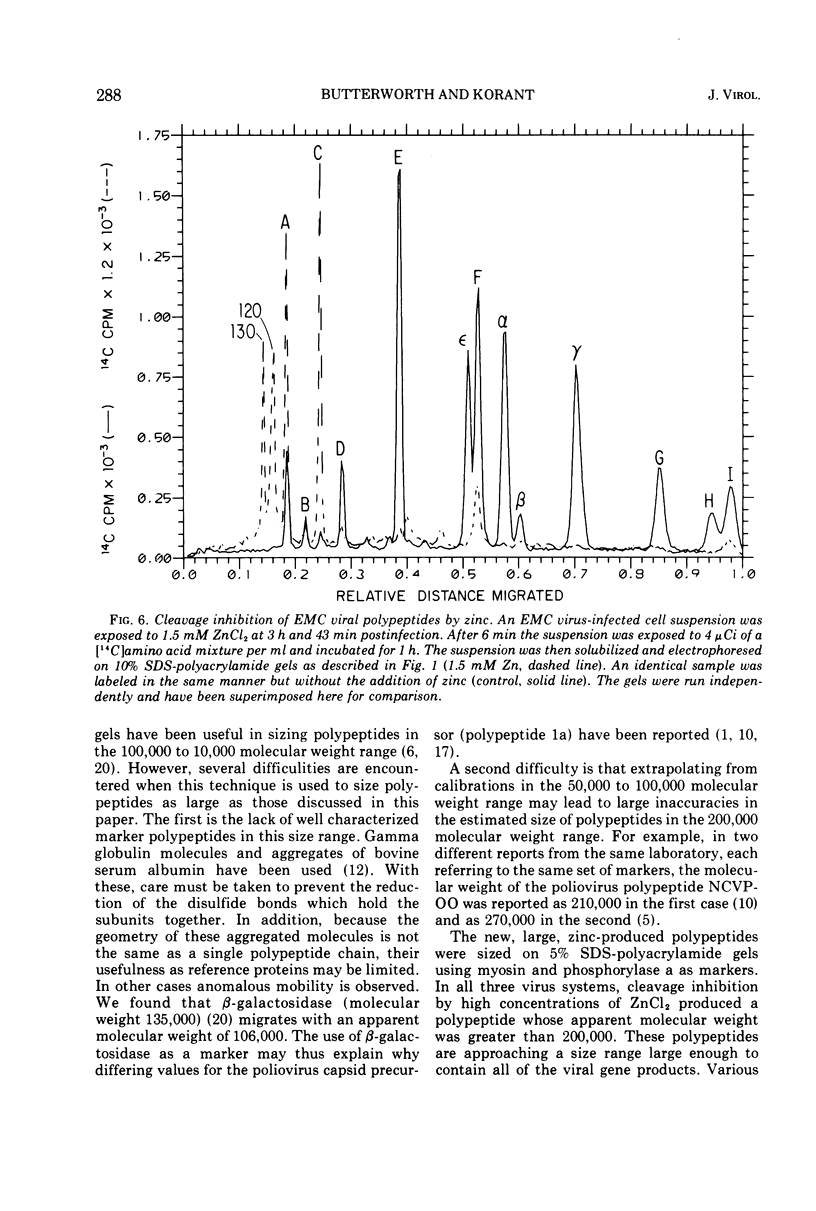
Zinc ion inhibits the posttranslational cleavages of human rhinovirus-1A, encephalomyocarditis virus, and poliovirus polypeptides. Each virus displayed a different susceptibility to zinc. However, in each case the cleavages of the capsid precursor and the cleavages analogous to the C → D → E conversion in encephalomyocarditis virus were most sensitive to zinc. Higher concentrations of zinc resulted in the buildup of even larger precursor polypeptides of a size between 106,000 and 214,000 daltons. The sizes of these polypeptides and the relative position of their gene loci on the viral RNA were determined. These data were used to place these polypeptides in the over-all scheme of viral protein processing.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Garfinkle B. D., Tershak D. R. Effect of temperature on the cleavage of polypeptides during growth of LSc poliovirus. J Mol Biol. 1971 Aug 14;59(3):537–541. doi: 10.1016/0022-2836(71)90318-4. [DOI] [PubMed] [Google Scholar]
- Gazith J., Himmelfarb S., Harrington W. F. Studies on the subunit structure of myosin. J Biol Chem. 1970 Jan 10;245(1):15–22. [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Synthesis and cleavage of enterovirus polypeptides in mammalian cells. J Virol. 1970 Mar;5(3):358–367. doi: 10.1128/jvi.5.3.358-367.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Kauer J. C., Butterworth B. E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974 Apr 12;248(449):588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Rueckert R. R. Picornaviral gene order: comparison of a rhinovirus with a cardiovirus. J Virol. 1973 Feb;11(2):341–344. doi: 10.1128/jvi.11.2.341-344.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumiantzeff M., Summers D. F., Maizel J. V., Jr In vitro protein synthetic activity of membrane-bound poliovirus polyribosomes. Virology. 1971 May;44(2):249–258. doi: 10.1016/0042-6822(71)90257-1. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



