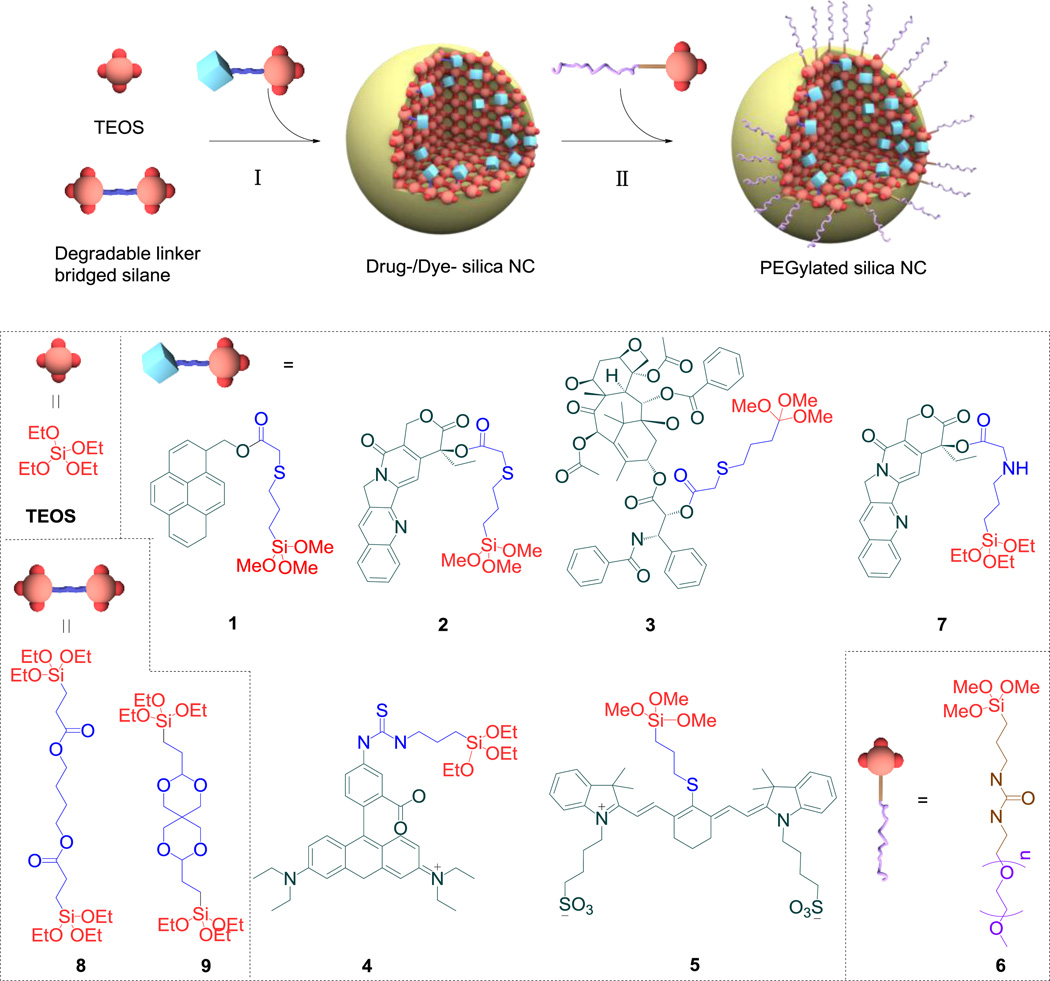
Abstract
Drug-containing nanoparticles (NPs) with monodisperse, controlled particle sizes are highly desirable for drug delivery. Accumulating evidence suggests that NPs with sizes less than 50 nm demonstrate superior performance in vitro and in vivo. However, it is difficult to fabricate monodisperse, drug-containing NPs with discrete and incremental difference in sizes required for studying and characterizing existing relationships among particle size, biologic processing, and therapeutic functionality. Here, we report a scalable process of fabricating drug-silica conjugated nanoparticles, termed drug-silica nanoconjugates (drug-NCs), which possess monodisperse size distributions and desirable particle sizes as small as 20 nm. We found that 20-nm NCs are superior to their 50-nm and 200-nm NC analogues by 2–5 and 10–20 folds, respectively, with regard to tumor accumulation and penetration, and cellular internalization. These fundamental findings underscore the importance and necessity of further miniaturizing nanomedicine size for optimized drug delivery applications.
Keywords: silica nanoparticle, nanoconjugates, chemotherapeutics, nanomedicine, drug delivery, cancer therapy, tumor penetration, cell uptake, nanoparticle biodistribution
Nanomedicines in the form of polymer-drug conjugates, micelles, nanoparticles (NPs) and vesicles have been extensively studied in the past 2–3 decades for drug and gene delivery applications.1–9 Although promising, the clinical translation of nanomedicines has proven very difficult.3 Numerous studies have been designed and performed using nanomedicine as a new modality for improved cancer treatment. However, very few nanomedicines have ever been clinically evaluated and even less been approved for clinical cancer treatment.10, 11 Although no generalized pathway exists for the clinical translation of nanomedicine, there is a consensus that a clinically applicable nanomedicine should at least possess controlled physicochemical and pharmacological properties. Specifically, such nanomedicine should have controlled size with low size dispersity, high drug loading, high loading efficiency, controlled drug-release kinetics, and sufficient stability and capability of staying non-aggregated in biological media. It should also be easily manufactured at a large-scale, from grams up to kilograms scale, and lyophilized to form solid formulation. Very few nanomedicine systems can meet all these formulation requirements that are critical to their clinical translation.
There has been growing interest in using nanomedicines for targeted or personalized cancer therapy.1, 2 Accumulating evidences show that the size of nanomedicine plays a vital role in controlling systemic and lymphatic biodistribution, tumor targeting and penetration, and cellular internalization of drug delivery vehicles.12–23 NPs with size controlled within 20–60 nm have been particularly interesting and actively pursued because some recent studies showed that NPs within this size range have distinct biodistribution, tumor penetration and cellular trafficking properties that are critical to the in vivo use of nanomedicine. For instance, Tseng and his team reported that 30-nm NPs were able to drain into the local auxiliary lymph nodes with high efficiency after footpad administration of NPs while the 100-nm NPs were nearly undetectable in these tissues.24 Chan and his co-workers reported that 20-nm and 60-nm gold NPs, as model drug delivery systems, permeate tumor tissues much more rapidly than 100-nm particles in vivo.25 Similar results were also reported by Pun and coworkers.26 Jing et al. reported that 40~50-nm NPs outperformed NPs in different size ranges for altering signaling processes that regulate various cellular functions.15
Nanomedicines are typically prepared through bottom-up approaches, such as self-assembly of amphiphilic copolymers for the preparation of micelles or vesicles, and nanoprecipitation of hydrophobic polymers for the preparation of NPs. The micellation, vesiclization and nanoprecipitation methods certainly allow for facile preparation of nanomedicines at a large scale. However, the drawbacks of these formulation methods are also obvious; the resulting micelles, vesicles or NPs often have broad particle size distributions, and variable, sometimes uncontrolled drug loading and release profiles. It is also extremely difficult to prepare NPs with narrow or mono-dispersity in size controlled within 100 nm using these conventional technologies. There were even less reports of the in vitro and in vivo properties of NPs with discrete size less than 50 nm.13, 15, 24, 25, 27 Here, we report the synthesis of drug-silica conjugated NPs (Scheme 1), termed drug-silica nanoconjugates (drug-NCs) and denoted as drug(dye)X (X = particle size in nm), which can be formulated at nearly any size ranging between 20 nm and 200 nm with mono-disperse size distribution (less than 10% coefficient of variation (CV), the ratio of the standard deviation σ to the mean μ of particle size), 10–20% drug loading and controlled drug release profiles. These drug-NCs can be easily prepared on gram scale but still with perfectly controlled size and mono-disperse size distribution. They showed size-dependent cell uptake, biodistribution and tumor penetration capability. By addressing several formulation/development issues (e.g., salt-stability, scalability and lyophilizability, etc.), we developed a potentially clinically applicable drug(dye) delivery nanomedicine platform that can be precisely controlled formulated at any size between 20 and 200 nm on large scale.
Scheme 1.
Drug/dye-silica nanoconjugate. Schematic illustration of the synthesis of drug/dye-silica nanoconjugates (NCs). The Stöber method (St) or the reverse microemulsion method (Trx) (I) was used to prepare drug/dye-silica NCs followed by in situ surface PEGylation (II). For NCs formed via the St method, TEOS was used in a solution of NH4OH/MeOH in the presence of 1, 4 or 5 or in a solution of NaF/MeOH in the presence of 2, 3 or 7. For NCs prepared via the Trx method, 8 or 9 was used in conjunction with 2 or 3 to synthesize the corresponding NCs.
RESULTS AND DISUSSION
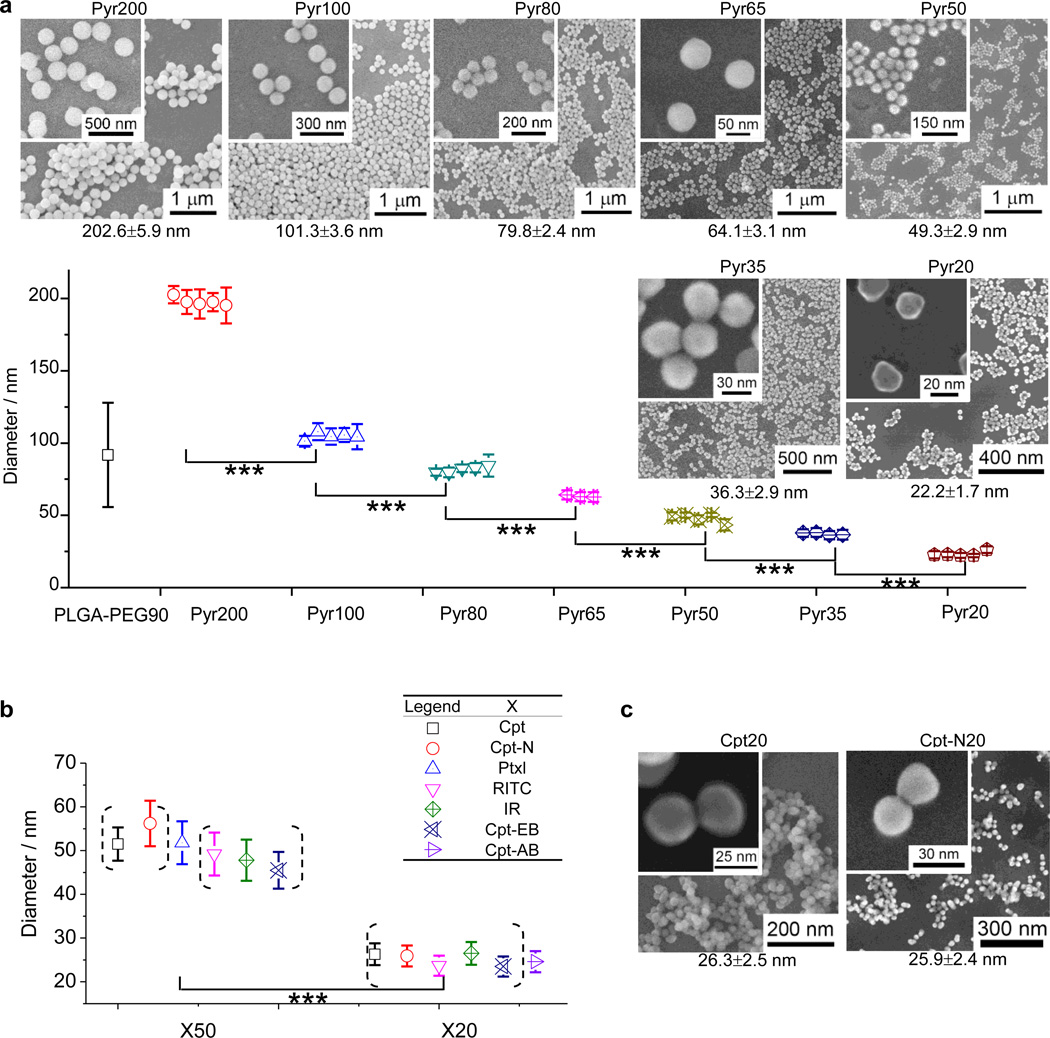
Silica NPs can be easily prepared on large scale with discrete, monodisperse particle sizes through the condensation reaction of tetraethyl orthosilicate (TEOS) or tetramethyl orthosilicate (TMOS). For example, monodisperse silica spheres with controlled sizes (50 nm - 2 µm) can be prepared in a reaction mixture of water, alcoholic solvent, ammonia, and alkyl silicate ester by controlling alcoholic solvents, different alkyl silicate esters, as well as the concentration of each component.28 Silane coupling agents containing a trialkoxysilane group can be readily incorporated into silica NPs during such condensation reaction.29 We reasoned that trialkoxysilane-containing drugs (dyes) through a degradable ester linker should be able to be condensed with TEOS or TMOS to allow the drug (dye) molecules to be incorporated into the resulting silica NPs, which can be released through the cleavage of the ester linker. To demonstrate this concept, we started with 1, a trimethyl orthosilicate that contains pyrenemethanol (Pyr-OH) as the model drug. By carefully controlling reaction conditions, we were able to prepare Pyr-NCs with discrete sizes 15 nm apart between 20 nm and 80 nm. As shown in Figure 1a and Table 1 (entry 1–5), NCs with sizes of 22.2 ± 1.7 nm (Pyr20), 36.3 ± 2.9 nm (Pyr35), 49.3 ± 2.9 nm (Pyr50), 64.1 ± 3.1 nm (Pyr65) and 80 nm (Pyr80) can be readily prepared in multigram quantities. To test the reproducibility of these conditions to prepare NCs with the corresponding size, we repeated each experiment 3 to 5 times and found that the NCs with the desired size could be precisely produced each time. For instance, the five experiment for making Pyr20 under the same condition resulted in particles with size of 22.2 ± 1.7 nm, 22.7 ± 2.4 nm, 22.9 ± 1.9 nm, 22.2 ± 1.1 nm and 26.2 ± 2.4 nm (Figure 1a). The CV values of these five experiments are 7.7, 10.6, 8.3, 5.0 and 9.2, respectively, with an average of CV value of 8.1%. The low CV values (< 10%) of Pyr20 NC indicate these particles are technically monodisperse by industry standard.30 The conditions of making pyrene-containing NCs of 35, 50, 65 and 80 nm showed similar control over NC size, monodispersity and reproducibility (Figure 1a). The hydrodynamic sizes of these Pyr-NCs were also measured by dynamic light scattering (DLS) (Supplementary Table S3), which are larger than the hard core sizes measured by scanning electron microscopy (SEM). All the PDI values measured by DLS are below or around 0.1 indicating again the high monodispersity of these Pyr-NCs. To be consistent, all NC (NP) sizes and size distributions reported in the following part of this paper, except for those in Figure 4b Table 2 and Table S3, were determined based on the SEM data of the particles, by averaging the particle size of a representative SEM image containing at least 100 particles.
Figure 1.
Precise size control of drug/dye-silica nanoconjugates (NCs). (a) Preparation of pyrene-silica nanoconjugates (Pyr-NCs) with discrete size ranging from 20 to 200 nm. Three to five separate batches of Pyr-NCs for each size were prepared to demonstrate the consistency in size control and batch-to-batch reproducibility. The diameters of NCs were determined by measuring the particle size in a representative SEM image containing at least 100 particles (mean ± standard deviation). The difference of NC diameters between each size group is extremely statistical significant with 99.9% confidence (Student’s t-test (two-tailed), ***p < 0.001). The SEM images of NCs at each size (20, 35, 50, 65, 80, 100 and 200 nm) were displayed; the higher resolution images of NCs of the corresponding size were shown in the inset. (b) Preparation of different drug/dye-silica NCs with sizes of 50 nm and 20 nm. (c) The SEM images of Cpt20 and Cpt-N20 as the examples to show the excellent size control and monodispersity of NCs with sizes of 20 nm.
Table 1.
Preparation of drug-/dye-silica nanoconjugates[a].
| Entry | Name of NC | Drug/ Dye |
Formulation[b] | Method[c] | D[d] (nm) | SD[e] (nm) |
CV%[f] | I.E.[g] (%) |
LD[h] (wt%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pyr20 | Pyr | TEOS/1 (29.4/1) | St-A | 26.6 | 2.7 | 10.2 | N/A | N/A |
| 2 | Pyr35 | Pyr | TEOS/1 (29.4/1) | St-B | 36.3 | 2.9 | 8.0 | N/A | N/A |
| 3 | Pyr50 | Pyr | TEOS/1 (29.4/1) | St-C | 43.4 | 3.9 | 9.0 | N/A | N/A |
| 4 | Pyr65 | Pyr | TEOS/1 (29.4/1) | St-D | 64.1 | 3.1 | 4.8 | N/A | N/A |
| 5 | Pyr80 | Pyr | TEOS/1 (29.4/1) | St-E | 84.4 | 7.6 | 9.0 | N/A | N/A |
| 6 | Pyr100 | Pyr | TEOS/1 (29.4/1) | St-F | 104.4 | 8.8 | 8.4 | N/A | N/A |
| 7 | Pyr200 | Pyr | TEOS/1 (29.4/1) | St-G | 195.3 | 12.8 | 6.6 | N/A | N/A |
| 8 | PLGA-PEG90[i] | N/A | PLGA-PEG | NPP | 91.8 | 36.0 | 39.2 | N/A | N/A |
| 9 | Cpt20 | Cpt | TEOS/2/6 (2.2/1/0.14) | St-A | 26.3 | 2.5 | 9.5 | 81.2 | 24.0 |
| 10 | Cpt50 | Cpt | TEOS/2/6 (2.2/1/0.14) | St-C | 51.5 | 3.8 | 7.4 | 82.9 | 24.0 |
| 11 | Cpt100 | Cpt | TEOS/2/6 (2.5/1/0.14) | St-F | 96.1 | 8.8 | 9.2 | 86.5 | 16.9 |
| 12 | Cpt200 | Cpt | TEOS/2/6 (2.2/1/0.14) | St-G | 222.7 | 16.5 | 7.4 | 80.7 | 24.0 |
| 13 | Ptxl50 | Ptxl | TEOS/3/6 (8.1/1/0.40) | St-C | 51.8 | 4.9 | 9.5 | 80.7 | 13.4 |
| 14 | RITC20 | RITC | TEOS/4/6 (58.8/1/3) | St-A | 23.7 | 2.3 | 9.7 | N/A | N/A |
| 15 | RITC50 | RITC | TEOS/4/6 (58.8/1/3) | St-C | 49.2 | 4.9 | 10.0 | N/A | N/A |
| 16 | RITC200 | RITC | TEOS/4/6 (58.8/1/3) | St-G | 188.9 | 14.4 | 7.6 | N/A | N/A |
| 17 | IR20 | IR | TEOS/5/6 (58.8/1/3) | St-A | 26.5 | 2.6 | 9.8 | N/A | N/A |
| 18 | IR50 | IR | TEOS/5/6 (58.8/1/3) | St-C | 47.8 | 4.7 | 9.8 | N/A | N/A |
| 19 | IR200 | IR | TEOS/5/6 (58.8/1/3) | St-G | 206.9 | 16.2 | 7.8 | N/A | N/A |
| 20 | Cpt-N20 | Cpt | TEOS/7/6 (3.8/1/0.20) | St-A | 25.9 | 2.4 | 9.3 | 79.3 | 15.9 |
| 21 | Cpt-N50 | Cpt | TEOS/7/6 (3.8/1/0.20) | St-C | 56.2 | 5.2 | 9.3 | 83.2 | 16.6 |
| 22 | Cpt50*[j] | Cpt | TEOS/3/6 (88.2/1/9.0) | St-C | 46.2 | 4.6 | 10.0 | 84.8 | 1.0 |
| 23 | Cpt-EB20 | Cpt | 8/2/6 (6.1/1/0.3) | Trx-A | 23.5 | 2.3 | 9.8 | 87.5 | 13.8 |
| 24 | Cpt-EB50 | Cpt | 8/2/6 (6.0/1/0.3) | Trx-B | 45.5 | 4.2 | 9.2 | 93.3 | 14.6 |
| 25 | Cpt-AB20 | Cpt | TEOS/9/2/6 (3.0/3.0/1/0.3) | Trx-C | 24.6 | 2.4 | 9.8 | 90.6 | 14.2 |
| 26 | Ptxl-EB20 | Ptxl | 8/3/6 (9.0/1/0.40) | Trx-A | 22.7 | 2.2 | 9.7 | 77.4 | 8.8 |
Formulation of monodisperse, size-specific drug/dye-silica NCs. NCs were denoted as drug(dye)X (X = the size of particles in nm). Abbreviation of drug and dye: Pyr =1- pyrenemethanol, Cpt = camptothecin, Ptxl = paclitaxel, RITC = rhodamine B isothiocyanate, IR = IR783;
Substrates used for the silica NC formulation presented in mass ratio;
NCs with perfect size control were formulated either using the St-X (X = A~G) conditions (Supplementary Table S1) or the Trx-X (X= A~C) conditions (Supplementary Table S2); NPP = nanoprecipitation.
NC sizes were characterizes by SEM. Average diameter (D) and standard deviation (SD) were calculated by measuring 100 NCs in a representative SEM image;
CV%=SD/D;
I.E. = Incorporation efficiency;
LD = Drug loading;
poly(lactide-co-glycolide)-b-methoxy-PEG (PLGA13k-mPEG5k) nanoparticle prepared by nanoprecipitation and used as a negative control;
Gram-scale preparation of Cpt-NC with 50 nm particle size.
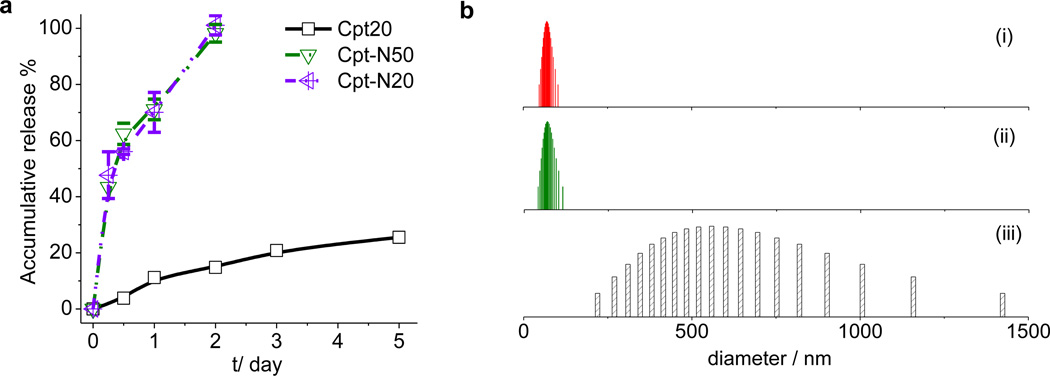
Figure 4.
Tunable drug release profiles and solid-form formulation of drug-silica nanoconjugates. (a) Release kinetics of Cpt-NCs with different linkers and sizes in 50% human serum at 37 °C. (b) NC size distributions measured by dynamic light scattering (DLS) before lyophilization (i), after lyophilization in the presence of dextrose (5%) and reconstituted with water (ii), and after lyophilization in the absence of dextrose and reconstituted with water.
Table 2.
Lyophilization of Silica NC.
| Entry | Lyoprotectant | m(Lyo)/m(NP)[a] | Do/nm[b] | D/nm[c] | D/Do[d] | Aggr. (Y/N)[e] |
|---|---|---|---|---|---|---|
| 1 | None | N/A | 102.0 | 233.5 | 2.29 | Y |
| 2 | Sodium chloride | 10 | 102.0 | 2295.1 | 22.50 | Y |
| 3 | BSA | 1 | 102.0 | 139.1 | 1.36 | N |
| 4 | BSA | 5 | 102.0 | 120.6 | 1.18 | N |
| 5 | BSA | 10 | 102.0 | 142.0 | 1.39 | N |
| 7 | Dextrose | 1 | 102.0 | 108.1 | 1.06 | N |
| 8 | Dextrose | 5 | 102.0 | 101.5 | 1.00 | N |
| 9 | Dextrose | 10 | 102.0 | 99.5 | 0.98 | N |
| 10 | None | N/A | 69.8 | 558.8 | 8.01 | Y |
| 11 | Sodium chloride | 10 | 69.8 | 2910.7 | 41.70 | Y |
| 12 | BSA | 1 | 69.8 | 103.6 | 1.48 | N |
| 13 | BSA | 5 | 69.8 | 91.0 | 1.30 | N |
| 14 | BSA | 10 | 69.8 | 97.8 | 1.40 | N |
| 15 | Dextrose | 1 | 69.8 | 84.3 | 1.21 | N |
| 16 | Dextrose | 5 | 69.8 | 68.9 | 0.99 | N |
| 17 | Dextrose | 10 | 69.8 | 71.2 | 1.02 | N |
Mass ratio of lyoprotectant (Lyo) to NP.
Do = NP size determined by DLS before lyophilization.
D = NP size determined by DLS after lyophilization in the presence of the corresponding lyoprotectant and reconstitution with water.
The ratio of NP size after and before lyophilization.
Observation of NP aggregation (Aggr.) after lyophilization and reconstitution with water (Y = aggregated; N = No aggregation).
NCs with particle size 100 nm or larger should be much easier to prepare compared to smaller particles. As expected, both 100-nm and 200-nm Pyr-NCs (Pyr100 (101.3 ± 3.6 nm) and Pyr200 (202. 6 ± 5.9 nm)) were prepared with monodisperse size distribution (CV < 10%) and high reproducibility (Figure 1a and entries 6–7 in Table 1). Statistical significances (p < 0.01) were found for all NCs with adjacent sizes between 20 and 200 nm. We compared the silica NCs with polymeric NPs prepared through nanoprecipitation (NPP) of amphiphilic copolymers and demonstrated the difference between these two methods for particle size control. Silica NCs can be easily prepared with monodisperse size distributions (CV < 10%). However, polymeric NPs prepared through NPP methods have polydisperse size distribution. For instance, poly(lactide-co-glycolide)-b-methoxy-PEG (PLGA-PEG) di-block copolymer with a 13-kDa PLGA block and 5-kDa PEG, was precipitated to form PLGA-PEG NPs. The resulting NPs showed a much broader size distribution as compared to silica NCs (CV = 39.2%, entry 8, Table 1; Supplementary Figure S1).
We next attempted to incorporate therapeutics and dyes to silica NCs using the same reactions under similar conditions. Camptothecin (Cpt), a cytotoxic chemotherapeutic agent which inhibits the DNA enzyme topoisomerase I, was converted the corresponding silane derivative 2 (Scheme 1) with Cpt connected a trialkoxysilane group via a hydrolyzable thioether ester linker, and then incorporated into the silica NCs under similar conditions used for preparing Pyr-NCs with similar sizes. As expected, remarkable control over particle size was observed for the reaction, which resulted in monodisperse Cpt-NCs in all corresponding size ranges (entries 9–12, Table 1). For the Cpt-NCs with expected size of 20, 50, 100 and 200 nm, the obtained NC sizes were 26.3 ± 2.5 nm, 51.5 ± 3.8 nm, 96.1 ± 8.8 nm and 222.7 ± 16.5 nm (Figure 1b; Supplementary Figure S2). We also attempted to incorporate other therapeutic agents (e.g., paclitaxel (Ptxl)) or fluorescent dyes (e.g., rhodamine B (RITC) and IR783) using similar approach with corresponding silane reagents (3–5) to prepare 20-nm and 50-nm NCs (Figure 1b). As expected, all obtained NCs containing Ptxl, RITC or IR783 were monodisperse (CV < 10%) and had the expected particles size (entries 13–19, Table 1). In order to increase the systemic circulation half-life and reduce aggregation of NCs in blood,31 the surface of NCs was modified with PEG via the use of 1-(2-(2-methoxyethoxy)ethyl)-3-(3-(trimethoxysilyl)propyl)urea (mPEG5k-sil) 6 (Scheme 1). The resulting pegylated NCs displayed remarkable stability in both PBS (1×) and cell medium containing 10% fetal bovine serum (FBS) (Supplementary Figure S3); the NC size remained unchanged for hours.
After we prepared drug(dye)-NCs with precisely controlled size, we next studied the size effect of these new drug delivery systems on their in vivo biodistribution, tumor tissue penetration and cellular internalization. All silica NCs involved in the following in vitro and in vivo studies have identical surface properties, spherical shape and chemical structures and compositions; particle size was the only parameter changed in these studies. Pegylated silica NCs with discrete sizes of 20, 50 and 200 nm containing rhodamine B (RITC) (termed RITC20, RITC50 and RITC200, respectively) were prepared at a 4:6 ratio of 10 (entries 14–16, Table 1; Supplementary Figure S4). To facilitate in vivo/ex vivo analysis of fluorescent NCs with reduced autofluorescence, we prepared pegylated NCs containing IR783, a near inferred (NIR) dye at a 5:6 ratio of 10; the resulting NIR active NCs with discrete sizes of 20, 50 and 200 nm were denoted as IR20, IR50 and IR200, respectively (entries 17–19, Table 1; Supplementary Figure S5).
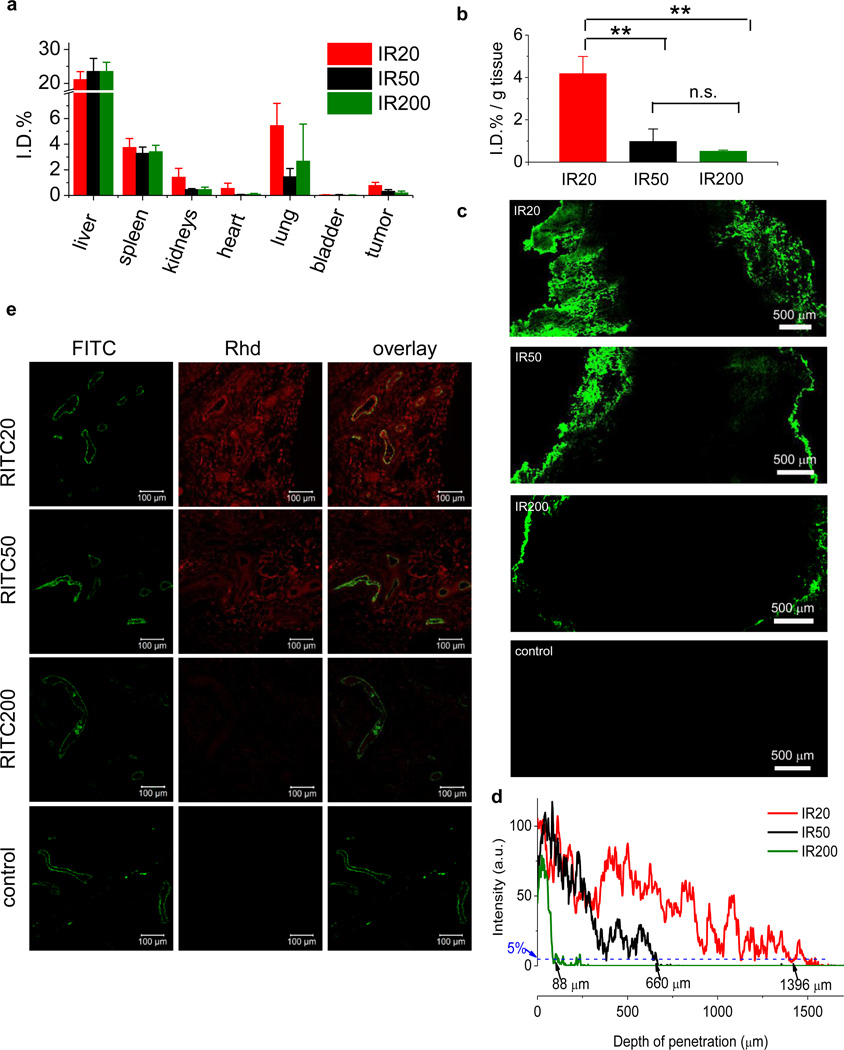
In the biodistribution study of IR20, IR50 or IR200 in vivo using C57BL/6 mice bearing subcutaneously implanted Lewis lung carcinoma (LLC), tail vein intravenous (i.v.) administration of the NCs followed by tissue harvesting 24-hour later showed that majority of NCs were accumulated in liver and spleen, few were in the respiratory and urinary systems (Figure 2a). The fluorescence of IR was found to have excellent tissue transmission; IR concentration can be quantitatively assessed in tissues with thickness 2 mm or less (Supplementary Figure S6). Importantly, NCs with smaller sizes distributed and accumulated in the tumor tissue more efficiently than NCs of larger sizes (Figure 2b). The injected doses of NCs normalized for tumor tissue weight (I.D.%/g) were 4.18 ± 0.81, 0.98 ± 0.59 and 0.52 ± 0.05 for IR20, IR50 and IR200, respectively. A decrease in particle size by 2.5-fold from 50 nm to 20 nm resulted in an increase of NC concentration by 330% in tumor tissue (from 0.98 to 4.18, **p < 0.01). In comparison, a decrease in particle size by 4-fold from 200 nm to 50 nm resulted in an increase of NC concentration in tumor tissue by only 88% (from 0.52 to 0.98). NC size showed significant influence on the systemic and tissue biodistribution, and this effect seems to be more profound for NCs below 50 nm in size. These results underscore the importance of studying nanomedicines with sizes less than 50 nm.
Figure 2.
Size effect on biodistribution and tumor penetration. (a), (b) C57BL/6 mice bearing Lewis lung carcinoma (LLC) (size: ~5.0 mm × 6.0 mm; n=3) were injected intravenously with IR20, IR50 and IR200. Mice were euthanized and the tissues were collected, excised to 2-mm or less in thickness, and analyzed (λemission = 800 nm) on an Odyssey infrared imaging system. The fidelity of utilizing Odyssey infrared imaging system for quantitative IR analysis in biological tissues was verified in a series of control studies (Supplementary Figure S6). All the organ distribution are presented as percentage of injected dose (I.D.%, a). Tumor accumulation data are presented as percentage of injected dose per gram of tumor tissue (I.D.% / g tissue, b). Student’s t-test (two-tailed) was performed for statistical analysis: n.s., not significant; highly statistical significant with 99% confidence, **p < 0.01. c), d) LLC tumors (size: ~7.0 mm × 8.0 mm, n = 3) were ex vivo cultured with IR20, IR50 or IR200 in cell culture medium for 48 h. The tumors without any treatment served as the control. The tumor sections of treatment groups (intersections, 20 µm in thickness) were collected by cryostat, mounted on glass slides and analyzed on a fluorescence microscope at (λexcitation = 780 nm). A tiling image was taken with fixed exposure time to show the NC penetration in tumor sections. Scale bar = 500 µm. The fluorescence profile in tumor section was analyzed by Image J (c) to show the depth of NC penetration in tumor tissues. To quantify the penetration, we defined the tumor tissue penetration depth as the distance from the periphery of the tumor to the site where the fluorescence intensity decreases by 95% as compared to the fluorescent intensity at tumor periphery. The penetration depths of IR20, IR50 and IR200 were found to be 1396 µm, 660 µm and 88 µm, respectively. e) C57BL/6 mice bearing LLC tumors (size: ~5.0 mm × 6.0 mm, n = 3) were injected intravenously with RITC20, RITC50 or RITC200. Mice were euthanized and dissected 24 hours post injection. Tumor sections (intersections, 5 µm in thickness) were collected in paraffin and mounted on glass slides. Fluorescence images were taken on a Zeiss LSM 700 confocal microscope. Representative two-color composite images showing the perivascular distribution of RITC-NCs (red, Rhd channel) relative to the blood vessels (green, FITC channel) in tissue sections of LLC tumors were showed and overlaid. Scale bar = 100 µm.
As the silica NCs used in our study do not have targeting ligand, the accumulation of these NCs should follow the enhanced permeation and retention (EPR) effect,32 a widely recognized passive targeting mechanism, for their accumulation and retention in the tumor tissues. While the NCs extravasate the leaky vasculatures into the tumor tissues, the capability for the NCs to diffuse away from the capillary blood vessels and vasculature should have significant effect on the retention of NCs. We went on and studied the size dependency of silica NCs diffusion/penetration in tumor tissues. We performed the tumor penetration study by incubating LLC tumors (grown in C57BL/6 mice with ~200 mg) for 48 hours in culture medium containing equal concentration of IR20, IR50 or IR200. The tumor sections (20 µm in thickness) were then analyzed by NIR fluorescence microscope. As shown in Figure 2c the size dependency of tumor penetration was obvious with IR20 penetrating tumor tissue with the greatest depth from the periphery of the tumors, followed by IR50 with intermediate penetration depth and IR200 with limited tumor penetration. To quantify the penetration, we defined the tumor tissue penetration depth as the distance from the periphery of the tumor to the site where the fluorescence intensity decreases by 95% as compared to the tumor periphery fluorescent intensity. The penetration depths of IR20, IR50 and IR200 were found to be 1,396 µm, 660 µm and 88 µm, respectively (Figure 2d). The penetration depth of IR20 is twice and sixteen times of that of IR50 and IR200, respectively. To verify the size-dependency of tumor penetration in vivo, we intravenously administered RITC20, RITC50 and RITC200 to LLC-bearing C57BL/6 mice via tail vein. Tumors were collected 24 hours post-injection, fixed and sectioned. After the blood vessel was stained with human Von Willebrand Factor antibody (green, FITC channel in Figure 2e), the tumor tissues were then analyzed using confocal microscope to study the distribution of NCs in tumor tissues relative to the blood vessels. This study showed the effect of biodistribution and diffusion collectively. RITC20 and RITC50 significantly outperformed RITC200, and diffused away from and situated distally to the blood vessel. Comparing the representative regions of interest, the fluorescence intensity of RITC20 is 4- and 22-times greater than RITC50 and RITC200, respectively. This observation of size-dependent in vivo penetration is consistent with the observations from the tumor penetration studies using ex vivo model (Figure 2c) and the size-dependent biodistribution studies (Figure 2b).
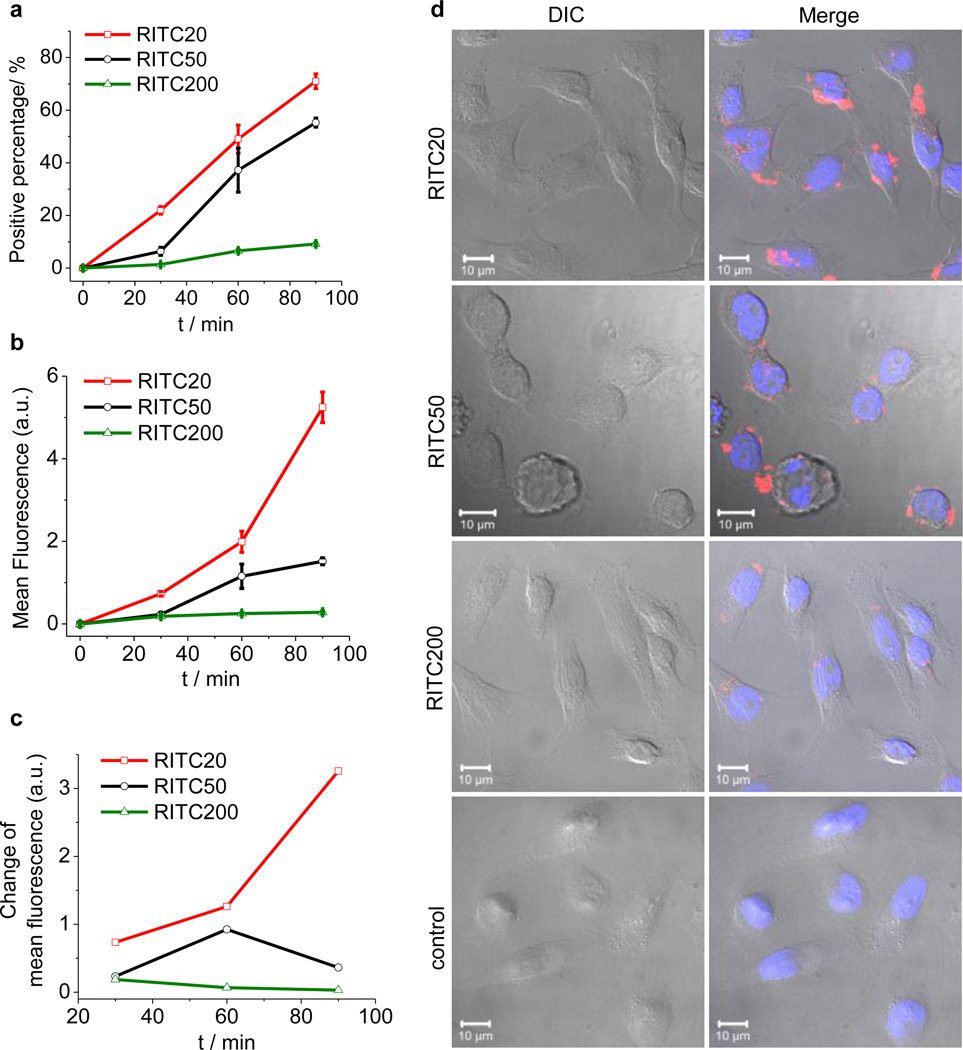
While the NCs diffuse into tumor tissue, whether the NCs stay in interstitial extracellular matrix or are internalized and reside inside the cells should impact the penetration depth in tumor tissue as well as the capability of retention. We thus compared the size-dependent uptake of these NCs in HeLa cells. Cellular internalization of RITC20, RITC50 or RITC200 into the HeLa cells for 30, 60, or 90 minute incubation was analyzed by fluorescence-assisted flow cytometry (FACS) to assess the kinetics of NC internalization (Figure 3a). We found that smaller NCs were internalized into HeLa cells faster and more efficiently than NCs with larger size, in terms of both percentage of the fluorescent cells and total accumulated mean fluorescence intensity. The number of fluorescent cells accounts for 1.4%, 6.6% and 9.2% of the total treated cells for 30-, 60- and 90-minute incubation with RITC200. These numbers were 6.6%, 37.2% and 55.2% for RITC50, and 21.9%, 49.1% and 71.0% for RITC20, respectively (Figure 3a). The fluorescence intensities of cells for 30-, 60- and 90-minute incubation with RITC200 were 0.18, 0.25 and 0.28, in arbitrary units of FACS. These numbers were 0.23, 1.15 and 1.52 for RITC50 and 0.73, 1.99 and 5.25 for RITC20, respectively (Figure 3b). The 20-nm NC was therefore internalized 18.7 times and 3.5 times more than 200-nm and 50-nm NC for a total of 90-minute incubation. Interestingly, comparing the fluorescence intensity change of the three 30-minute blocks (0–30 min, 30–60 min and 60–90 min), we found the rate of internalization of 20-nm NC (RITC20) in HeLa cells were accelerating in the first 90 minutes while the accumulation of 200-nm NC (RITC200) were evidently de-accelerating (Figure 3c). For RITC200, 90-minute incubation versus 30-minute incubation resulted in an increase of the number of the fluorescent cells by 660% (9.2% vs. 1.4%), but the total accumulated fluorescence intensity was only increased by 56% (0.28 vs. 0.18), suggesting that not all internalized RITC200 can be effectively retained inside of the cells and exocytosis might occur simultaneously.33 In contrast, 90-minute incubation versus 30-minute incubation of RITC20 resulted in an increase of the number of fluorescence cells by 340% (71% vs. 21%) and the total accumulated fluorescence intensity by 720% (5.25 vs. 0.73), clearly indicating that 20-nm particle can be effectively internalized and retained in the cells, and the internalization/retention process become more favorable for the duration of study. The size-dependent cell-uptake and retention was also verified by confocal microscopy study (Figure 3d), which demonstrated that NCs with smaller sizes were internalized and retained inside the cells more efficiently than the NCs with larger sizes.
Figure 3.
NC size effect on cellular internalization. (a), (b), (c) Internalization of RITC-NCs into HeLa cells over 90 min incubation at 37 °C evaluated by the percentage of cells containing internalized NCs (a), mean fluorescence of treated cells (b) and change of mean fluorescence every 30 minutes (c). (d) Confocal laser scanning microscopy images of HeLa cells after 1 h incubation at 37 °C with RITC20, RITC50 and RITC200 (red). The nuclei of cells were stained by DAPI (blue). Top panel: differential interference contrast (DIC); bottom panel: overlay of DIC, DAPI and Rhd channels. Scale bar = 10 µm.
The 20-nm silica NC outperforms the 50-nm NC and the 200-nm NC by ~2–5 times and ~10–20 times, respectively, in terms of biodistribution, tumor tissue penetration, and internalization to cancer cells. Collectively, 20-nm NCs breach the three physiological barriers (systemic, tissue, and cellular) that are critical to drug delivery significantly better than larger particles. We are currently exploring whether the NCs containing targeting ligand will follow the same size dependency as what we observed in this study with the use of non-targeting NCs. Because particles less than 10 nm may be subject to significant renal clearance and rapid fenestration into other tissues (e.g., lymphatic system), which is undesirable for sustained circulation that is critical to NC passive targeting and tumor tissue accumulation via EPR effect, NC around 20-nm may be close to the optimal size for drug delivery application.
Silica NCs have other promising properties that are noteworthy. First, this nano-fabrication process as shown in Scheme 1 allows the incorporation of drug (dye) molecules in high yields (up to 24%) (Table 1) that are comparable to or higher than the FDA-approved drug delivery systems, such as Doxil (~10%).34 Drug burst release is a long-standing formulation challenge of nanocarriers with drug encapsulated in polymeric NPs or adsorbed in mesoporous silica NPs, which causes undesirable dose dumping, significant side effects, and reduced long-term therapeutic efficacy. Since the drug release kinetics of drug-NCs is determined by the hydrolysis of the thioether ester bond linker, the release kinetics of drug from NCs are more controllable with essentially no burst release (Figure 4a). In human serum, Cpt20 with the hydrophobic thioether ester linker between Cpt and the silica particles showed sustained drug release with 14.8% of CPT being released in 48 hours (Figure 4a); the IC50 value of Cpt20 in HeLa cells was found to be 220 nM. When the linker was changed to a hydrophilic amine ester as in Cpt-N20 (entry 20, Table 1; Figure 1c), which was prepared by using 7 as the corresponding drug-containing silane reagent (Scheme 1), the Cpt release kinetics can be dramatically accelerated with Cpt being 100% released within 48 hours, resulting in a much lower IC50 value (9.0 nM, Supplementary Table S4). This could be due to the fact that hydrophilic amine ester is more assessable by water and esterase which can accelerate the cleavage of the ester bond. By controlling the feed ratio of 2/7 during Cpt-NC fabrication to the ratios of these two different linkers, the Cpt release half-life can be precisely adjusted ranging from 24 hours to about two weeks.
Besides controlled particle size, drug loading and release kinetics, other issues critical to the clinical translation of NP drug delivery system, such as scalability, lyophilizability, and toxicity, should also be addressed. These issues may also present the bottleneck to the clinical translation of a nanomedicine. We found the silane chemistry could be easily used for the large-scale preparation of drug-containing NCs. We tested the preparation of one gram of 50-nm Cpt-NC in one pot, and successfully obtained NCs with the expected size (46.4 ± 4.6 nm) in quantitative yield within one day (entry 22, Table 1; Supplementary Figure S2). The NP fabrication process that allows preparation of very small drug delivery NPs with remarkable control over size and monodispersity and with excellent scalability is unprecedented and offers clear advantages over many other nanomedicine preparation methods.
Aiming to formulate solid silica NCs without aggregation, we tested the lyophilization of silica NCs in the presence of various lyoprotectants (Table 2). We found dextrose was overall the best lyoprotectant for silica NC. Silica NCs lyophilized in 1 mL 5% dextrose solution (known as D5W, routinely used for drug administration in clinic) resulted in solid formulation of silica NCs with essentially no change of particle sizes after lyophilization and re-constitution in water (Figure 4b).
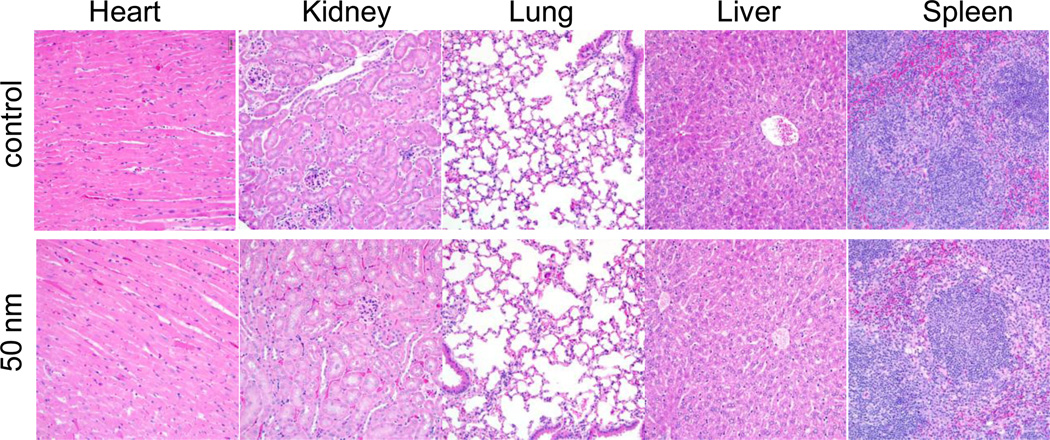
Recent studies showed that silica NPs can decompose in blood within a few days,35, 36 suggesting that this class of NPs can be eliminated by either hepatic or renal clearance,37, 38 thereby minimizing concerns for cumulative tissue damage and associated toxicity. In vitro study (MTT assay; Supplementary Table S4) showed almost no toxicity of blank silica NPs (IC50>1 mM). Acute in vivo toxicity experiments were performed after i.v. administration of 50 nm silica NPs in C57BL/6 mice at very high dose up to 250 mg/kg. There was no mortality or deterioration under general conditions observed in any of the groups. In addition, there were no treatment related clinical signs and change of body weights. Representative sections of various organs taken 24 h after injections from control mice receiving PBS and mice receiving silica NPs were stained by hematoxylin and eosin, and evaluated by an independent pathologist (Figure 5). The absence of immune or inflammatory reactions after NC administration supports their lack of toxicity. To facilitate faster degradation, we prepared bis-silane agents containing pH-sensitive ester (8) or orthoester domain (9). They can be very successfully incorporated to silica NC to make mono-disperse silica NC (entries 23–26, Table 1; supplementary Figure S7). These silica NC fabrication methods are not only independent of agents, but are also independent of linker (e.g., in the context of using 7, entries 20–21, Table 1) or addition of other silane reagents (e.g., in the context of using degradable (8) or pH-sensitive (9) silane agent, entries 23–26, Table 1). Study of the in vivo degradation and clearance of regular silica NCs and 8 or 9-containing silica NCs are underway.
Figure 5.
Histopathology of mouse tissues following an intravenous injection of silica nanoparticles via a tail vein. Representative sections of various organs taken from control mice receiving PBS and mice receiving 250 mg/kg 50 nm blank silica nanoparticles 24 h post injection were stained byhematoxylin and eosin. No organs of a mouse given silica nanoparticles showed any acute inflammations.
CONCLUSIONS
Silica NPs have been used in various drug and gene delivery applications.22, 23, 39–60 For example, silica NPs with stably bound photosensitizer were used for photodynamic therapy;57 mesoporous silica NPs were explored extensively for the encapsulation and delivery of chemotherapeutics;46, 48, 49, 51–53, 58, 60–63 silica NPs were also used in gene delivery.47, 48, 59, 64 These studies set up the cornerstone for the continuous advancement and novel design of silica NP based nanomedicine. In this paper, we streamlined a process for developing potentially clinically applicable drug-silica nanoconjugate delivery system with well-controlled physicochemical and pharmacological properties. To the best of our knowledge, this is the only report of a drug/dye delivery nanomedicine platform that can be easily prepared in gram- or larger scale in dry powder form and can be controlled formulated to any desirable size ranging from 20 to 200 nm with monodisperse particle size distribution (CV < 10%). The in vitro and in vivo studies using NCs with discrete sizes of 20, 50, and 200 nm demonstrate that smaller particle size is more efficient in bypassing the systemic, tissue, and cellular barriers, the three physiological barriers that are critical for effective drug delivery. Collectively, 20-nm silica NCs outperform 50-nm and 200-nm NCs by ~2–5 and ~10–20 times, respectively, in terms of tumor biodistribution, tumor tissue penetration, and cell internalization. Due to formulation challenges, FDA-approved drug-delivery nanomedicines and others under clinical and preclinical investigations mostly have sizes over 100 nm. Our study not only demonstrates substantial opportunities to further reduce nanomedicine size that may favorably impact their in vitro and in vivo performance, in particular, for sizes ranging from 20 to 50 nm, but also provides a platform technology to make such particles with these desired properties for clinical drug delivery applications and fundamental studies.
EXPERIMENTAL SECTION
General procedure for the preparation of Pyr-silica nanoconjugates28, 65–67
Methanol (1.0 mL), DI water (0.27 mL) and concentrated ammonia (0.24 mL) were mixed. TEOS (62.5 µL, 0.28 mmol) was then added to the solvent mixture followed by the addition of a DMSO solution (20 µL) of 1 (2 mg, 4.3 µmol). The mixture was stirred at a stirring rate of 100 rpm at RT for 12 h. The resulting Pyr-NCs were collected by centrifugation at 15k rpm and washed by ethanol (3 × 1 mL). One drop of a dilute solution of silica NCs in ethanol on a silicon wafer was allowed to dry in air and then analysed by SEM at 5 kV. The NC size (200 nm in this case) was determined by averaging at least 100 particles on a representative SEM image. Fabrication of monodisperse Pyr-NCs with other sizes can be similarly achieved by tuning the concentrations of TEOS, water and ammonia (Table 1 and Supplementary Table S1). Cpt-NCs, Ptxl-NCs, RITC-NCs and IR-NCs with monodisperse, controlled sizes were prepared under similar conditions with the corresponding silane substrate 2–7 (see Supplementary Information).
General procedure of preparing Cpt- or Ptxl-NCs via modified Stöber method45
The silica NCs of various sizes were prepared using Stöber method as described in the Method section of the paper without the addition of 1. The obtained silica NCs (4.1 mg) were re-dispersed in a mixture of EtOH/DI water (0.7 mL/0.2 mL) followed by the addition of 2 (1.7 mg) in DMSO (100 µL). After the mixture was stirred for 10 min, a NaF aqueous solution (10 mg/mL, 25 µL) was added. After 12 h of reaction, 6 (10 mg/mL, 100 µL) was added. The mixture was stirred for another 12 h. The supernatant of the mixture was analysed by HPLC to determine the unreacted 2 in order to determine the incorporation efficiency of drugs to NCs. The drug loadings were calculated based on the feed ratio of drugs and the incorporation efficiency. The NCs were collected by centrifugation at 15k rpm. The isolated NCs were washed with ethanol (3 × 1 mL) and re-dispersed in DI water or 1× PBS buffer before use. The preparation of Ptxl-NC was similar except for addition of 3 (1.0 mg).
Preparation of RITC/IR-NCs via Stöber method
The silica NCs (27.5 mg) of various sizes were prepared as described above without the addition of drug(dye)-sil reagents. After the reaction was complete, without isolating the NCs, a methanol solution of 4 (10 mg/mL, 100 µL) was added to the silica NC solution. The mixture was stirred for 12 h in dark. A methanol solution of 6 (10 mg/mL, 100 µL) was added. RITC-NCs were collected by centrifugation at 15k rpm, washed with ethanol (3 × 1 mL), and re-dispersed in DI water or 1× PBS buffer before use. IR- NCs were similarly prepared using 5 instead of 4.
Preparation of Cpt- or Ptxl- silica NCs using degradable silane 8 or 9 via a reverse micro-emulsion process
During the NC fabrication through the reverse micro-emulsion process, Triton X-100 and n-hexanol were employed as the surfactant and the co-surfactant, respectively. To prepare 20-nm Cpt-NCs containing degradable ester bond (Cpt-EB20, Table 1), cyclohexane (7.5 mL), n-hexanol (1.8 mL) and Triton X-100 (1.77 mL) were mixed and stirred for 20 min. DI water (480 µL) and 8 (80 µL) were added over the course of 20 minutes. Ammonia hydroxide (28%, 60 µL) was added to initiate the reaction. After 24 h, 2 (17.9 mg, 0.03 mmol) in dichloromethane solution (500 µL) was added. The reaction solution was stirred for another 12 h. A methanol solution of 6 (10 mg/mL, 600 µL) was added. The supernatant of the mixture was analysed by HPLC to quantify the unreacted 2 in order to determine the incorporation efficiency of drugs to NCs. The drug loading was determined based on the feed ratio of 2 versus 8 and TEOS, and the incorporation efficiency of 2 to NC. The emulsion was disrupted by the addition of 10-mL ethanol. The NC (Cpt-EB20) was collected by centrifugation at 15k rpm and washed with ethanol (3 × 1 mL). Cpt-EB50, Cpt-AB20 and Ptxl-EB20 (entries 23–26, Table 1) were prepared by following similar condition as summarized in Supplementary Table S2.
Release kinetics
The NC (Cpt20, Cpt-N20 or Cpt-N50) was dispersed in 50% reconstituted human serum (Sigma-Aldrich) (0.6 mg/mL), equally distributed to 20 vials with 1 mL NC solution per vial, and then incubated at 37°C. At selected time intervals, one selected vial of each group was taken out of the incubator. The NC solution was mixed with equal volume of methanol (1 mL) and centrifuged at 15,000 rpm for 10 min. The supernatant (1 mL) was transferred to an Eppendorf tube without disturbing the precipitates (NCs) and tuned to pH 2 with phosphoric acid (85%, 100 µL). The resulting solution was directly injected into HPLC equipped with an analytical C18 column (Luna C18, 250 × 4.6 mm, 5 μ, Phenomenex, Torrance, CA, USA). A mixture of acetonitrile and water (containing 0.1% TFA) at a volume ratio of 1:3 was used as the mobile phase. The flow rate was set at 1 mL/min. The area of the HPLC peak of the released Cpt (λabs = 370 nm) was intergraded for the quantification of Cpt as compared to a standard curve of free Cpt prepared separately. The Cpt release kinetic profiles from Cpt20, Cpt-N20 and Cpt-N50 were showed in Figure 4a.
Lyophilization of silica NPs in the presence of lyoprotectants
Silica NPs were prepared at TEOS/6 ratio (wt/wt) of 19.6:1 using Stöber method as described previously (St-B and St-D) and analysed with DLS. One of the selected lyoprotectants (Table S2) was added at different lyoprotectant/NP ratio (varying from 1:1 to 10:1 wt/wt) to the NP solution. The solution was lyophilized. The solid-form silica NP/lyoprotectant was reconstituted with 2-mL DI water to prepare a NP aqueous solution at a concentration of 10 mg/mL. The reconstituted silica NP was analysed by DLS (Figure 4b). The silica NP lyophilized in the absence of lyoprotectant and reconstituted with water was used as the negative control.
Cellular internalization of RITC-NCs
The HeLa cells were used to investigate the uptake of RITC20, RITC50 and RITC200 (Table 1). HeLa cells (50,000) were seeded in a 4-well chamber slide for 24 h (37 °C, 5% CO2). Cells were washed once with opti-MEM and then incubated for 1 h (37 °C, 5% CO2) with opti-MEM (1 mL) containing 100 µg/mL corresponding RITC-NCs. The cells were then washed by PBS (1 mL) for three times, fixed with 4% paraformaldehyde and subsequently imaged on a confocal laser scanning microscope. Nuclei were stained by DAPI. Cells without the addition of RITC-NCs were imaged as the control. The cell uptake kinetics of RITC-NCs was also studied. HeLa cells (100, 000) were seeded in a 12-well plate for 24 h. RITC-NCs (100 µg/ml) were incubated with the cells in opti-MEM (1 mL) over a time course ranging from 30 min to 90 min (37 °C, 5% CO2). The cells were then washed with PBS (3 × 1 mL) and detached via trypsinization. Cells were fixed with 4% paraformaldehyde for flow cytometry analysis (10,000 cells analysed, red fluorescence, PE channel). Both the percentage of the fluorescent cells relative to the total analysed cells and the fluorescence intensity of the fluorescence-positive cells were assessed. All experiments were performed in triplicate.
Ex vivo tumor penetration study
C57BL/6 mice (female, 12–13 week old) bearing LLC tumors were sacrificed to collect the tumors when the tumors grew to ~ 7.0 × 8.0 mm. Tumors (n = 3) were ex vivo cultured with IR20, IR50 or IR200 (Table 1) at concentration of 3 mg/mL NC in cell medium for 48 h. Tumor without any treatment served as the control. Tumor sections (20 µm thickness) were collected by cryostat and mounted on glass slides. Fluorescent images were taken on a Zeiss Axiovert 200M fluorescence microscope with 780 nm laser excitation. A tiling image was taken with fixed exposure time to show the NC penetration in tumor sections. The fluorescence intensity in tumor sections was analysed by Image J. To quantify the penetration of NCs, we defined the tumor tissue penetration depth as the distance from the periphery of the tumor to the site where the fluorescence intensity decreased by 95% as compared to the fluorescent intensity at the tumor periphery.
In vivo tumor penetration study
LLC tumor-bearing C57BL/6 mice were divided randomly into groups of three (n = 3) and were treated when the mean tumor diameter was in the range of 5.0~6.0 mm. Each animal received a PBS solution of RITC20, RITC50 or RITC200 (200 µL, 50 mg/mL) through tail vein administration. The animals were euthanized 24 hours after administration. The tumors were collected, fixed by 10% formalin, and then embedded in paraffin prior for tissue sectioning and immunohistochemical staining. A tissue section with approximate thickness of 5 µm were collected from each tumor, mounted on glass slides, and allowed to air-dry. Fluorescence images were taken on a Zeiss LSM 700 confocal microscope. Tissue sections were imaged with a 10×/0.3 lens. Developing tumor neovasculature within each tumor section was identified by the expression of Von Willebrand Factor (Factor VIII-related antigen) by incubating slides with a rabbit polyclonal anti-human Factor VIII antibody (1:200) for 30 minutes at room temperature. Following primary antibody incubation, glass slides were stained with a FITC-conjugated goat polyclonal anti-rabbit antibody (1/250) for 4 hours in the dark, then coverslipped using VECTASHIELD mounting media (Burlingame, CA). FITC fluorescence representing endothelial cells was visualized using 488 nm laser excitation. Red fluorescence of rhodamine, representing silica NCs, was visualized with 555 nm laser excitation.
In vivo biodistribution study
C57BL/6 mice bearing LLC tumors (~5.0 × 6.0 mm) (n = 3) were divided into three groups, minimizing tumor size variations between groups. Mice were injected intravenously with IR20, IR50 and IR200 at a dose of 150 mg/kg. Mice were euthanized and dissected 24 hours post injection. The major organs (liver, spleen, kidney, heard, bladder, lung and tumor) were collected and fixed in 10% formalin. The fluorescent intensity of IR-NCs in each organ was measured ex vivo at 800 nm emission using Odyssey infrared mouse imaging system (LI-COR, Lincoln, NE, USA). The concentration of the IR-NCs in each organ was determined by comparing its fluorescent intensity against a standard curve of IR-NCs.
In vivo biocompatibility study
Silica NPs of 50 and 200 nm in diameter were prepared by St-C and St-G methods, respectively, using TEOS/6=19.6/1. They were administered intravenously (200 µL, 25 mg/mL) via lateral tail vein to the C57BL/6 mice (n = 3) at a dose of 250 mg silica NC/kg. The animals were sacrificed 24 hours later by carbon dioxide. Organs including heart, lung, liver, spleen, kidney, stomach, small intestine, and large intestine were fixed in 10% neutral buffered formalin for 48 hours. The fixed tissues were then processed and trimmed, embedded in paraffin, sectioned to a thickness of 5 µm, and stained with hematoxylin and eosin for microscopic examination. Characterization of all the collected target tissues for inflammatory cell infiltrate including macrophages and neutrophils were performed by systemic microscopic evaluation at 400× magnification and analysed by an independent pathologist.
Statistical Analyses
Student T-Test (two tailed) comparisons at 95% confidence interval were used for statistical analysis. The results were deemed significant at 0.01 < p ≤ 0.05, highly significant at 0.001 < p ≤ 0.01, and extremely significant at p ≤ 0.001.
Supplementary Material
ACKNOWLEDGMENT
J.C. acknowledges supports from the NIH (Director’s New Innovator Award program 1DP2OD007246-01 and 1R21CA152627). L.T. was funded at University of Illinois at Urbana-Champaign from NIH National Cancer Institute Alliance for Nanotechnology in Cancer ‘Midwest Cancer Nanotechnology Training Center’ Grant R25 CA154015A.
Footnotes
Supporting Information. Synthesis and characterization of siliane reagents. SEM images, calibration, formulation condition and toxicity of silica particles. These materials are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Duncan R. Polymer Conjugates as Anticancer Nanomedicines. Nat. Rev. Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 2.Davis ME, Chen Z, Shin DM. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 4.Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang LF. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim MS, Kwon YJ. Acid-Transforming Polypeptide Micelles for Targeted Nonviral Gene Delivery. Biomaterials. 2010;31:3404–3413. doi: 10.1016/j.biomaterials.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Bae JW, Pearson RM, Patra N, Sunoqrot S, Vukovic L, Kral P, Hong S. Dendron-Mediated Self-Assembly of Highly Pegylated Block Copolymers: A Modular Nanocarrier Platform. Chem. Commun. 2011;47:10302–10304. doi: 10.1039/c1cc14331j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina SH, Tekumalla V, Chevliakov MV, Shewach DS, Ensminger WD, El-Sayed MEH. N-Acetylgalactosamine-Functionalized Dendrimers as Hepatic Cancer Cell-Targeted Carriers. Biomaterials. 2011;32:4118–4129. doi: 10.1016/j.biomaterials.2010.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagalkot V, Farokhzad OC, Langer R, Jon S. An Aptamer-Doxorubicin Physical Conjugate as a Novel Targeted Drug-Delivery Platform. Angew. Chem., Int. Ed. 2006;45:8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 9.Ghadiali JE, Lowe SB, Stevens MM. Quantum-Dot-Based Fret Detection of Histone Acetyltransferase Activity. Angew. Chem., Int. Ed. 2011;50:3417–3420. doi: 10.1002/anie.201008263. [DOI] [PubMed] [Google Scholar]
- 10.Wagner V, Dullaart A, Bock AK, Zweck A. The Emerging Nanomedicine Landscape. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Jablonowski H, Goebel F, Arasteh K, Spittle M, Rios A, Aboulafia D, Galleshaw J, Dezube B. Randomized Comparative Trial of Pegylated Liposomal Doxorubicin Versus Bleomycin and Vincristine in the Treatment of Aids-Related Kaposi's Sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J. Clin. Oncol. 1998;16:683–691. doi: 10.1200/JCO.1998.16.2.683. [DOI] [PubMed] [Google Scholar]
- 12.Dreher MR, Liu WG, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J. Natl. Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 13.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 14.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The Effect of Particle Design on Cellular Internalization Pathways. Proc. Natl. Acad. Sci. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-Mediated Cellular Response Is Size-Dependent. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 16.Mitragotri S, Lahann J. Physical Approaches to Biomaterial Design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning Payload Delivery in Tumour Cylindroids Using Gold Nanoparticles. Nat. Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Gao X. Plasmonic Fluorescent Quantum Dots. Nat. Nanotechnol. 2009;4:571–576. doi: 10.1038/nnano.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farokhzad OC, Cheng JJ, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted Nanoparticle-Aptamer Bioconjugates for Cancer Chemotherapy in Vivo. Proc. Natl. Acad. Sci. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AM, Mohs AM, Nie S. Tuning the Optical and Electronic Properties of Colloidal Nanocrystals by Lattice Strain. Nat. Nanotechnol. 2009;4:56–63. doi: 10.1038/nnano.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. Size and Shape Effects in the Biodistribution of Intravascularly Injected Particles. J. Controlled Release. 2010;141:320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Malugin A, Ghandehari H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. ACS Nano. 2011;5:5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Wang ST, Su H, Chen KJ, Armijo AL, Lin WY, Wang YJ, Sun J, Kamei K, Czernin J, et al. A Supramolecular Approach for Preparation of Size-Controlled Nanoparticles. Angew. Chem., Int. Ed. 2009;48:4344–4348. doi: 10.1002/anie.200900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating Tumor Targeting Efficiency of Nanoparticles through Design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 26.Goodman TT, Olive PL, Pun SH. Increased Nanoparticle Penetration in Collagenase-Treated Multicellular Spheroids. Int. J. Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 27.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, et al. Accumulation of Sub-100 Nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 28.Stober W, Fink A, Bohn E. Controlled Growth of Monodisperse Silica Spheres in Micron Size Range. J. Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 29.Stein A, Melde BJ, Schroden RC. Hybrid Inorganic-Organic Mesoporous Silicates-Nanoscopic Reactors Coming of Age. Adv. Mater. 2000;12:1403–1419. [Google Scholar]
- 30.Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. Monodisperse Mfe2o4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 31.Caliceti P, Veronese FM. Pharmacokinetic and Biodistribution Properties of Poly(Ethylene Glycol)-Protein Conjugates. Adv. Drug Deliv. Rev. 2003;55:1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 32.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and the Epr Effect in Macromolecular Therapeutics: A Review. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 33.Chithrani BD, Chan WCW. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien MER, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, et al. Reduced Cardiotoxicity and Comparable Efficacy in a Phase Iii Trial of Pegylated Liposomal Doxorubicin Hcl (Caelyx™/Doxil®) Versus Conventional Doxorubicin for First-Line Treatment of Metastatic Breast Cancer. Annals. of Oncology. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 35.Finnie KS, Waller DJ, Perret FL, Krause-Heuer AM, Lin HQ, Hanna JV, Barbe CJ. Biodegradability of Sol-Gel Silica Microparticles for Drug Delivery. J. Sol-Gel Sci. and Tech. 2009;49:12–18. [Google Scholar]
- 36.Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable Luminescent Porous Silicon Nanoparticles for in Vivo Applications. Nat. Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho MJ, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, Kim HO, Sheen YY, Jeong JY. The Impact of Size on Tissue Distribution and Elimination by Single Intravenous Injection of Silica Nanoparticles. Toxicol. Lett. 2009;189:177–183. doi: 10.1016/j.toxlet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 38.He XX, Nie HL, Wang KM, Tan WH, Wu X, Zhang PF. In Vivo Study of Biodistribution and Urinary Excretion of Surface-Modified Silica Nanoparticles. Analy. Chem. 2008;80:9597–9603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 39.Li LL, Tang FQ, Liu HY, Liu TL, Hao NJ, Chen D, Teng X, He JQ. In Vivo Delivery of Silica Nanorattle Encapsulated Docetaxel for Liver Cancer Therapy with Low Toxicity and High Efficacy. ACS Nano. 2010;4:6874–6882. doi: 10.1021/nn100918a. [DOI] [PubMed] [Google Scholar]
- 40.Della Rocca J, Huxford RC, Comstock-Duggan E, Lin W. Polysilsesquioxane Nanoparticles for Targeted Platin-Based Cancer Chemotherapy by Triggered Release. Angew. Chem., Int. Ed. 2011;50:10330–10334. doi: 10.1002/anie.201104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unger K, Rupprecht H, Valentin B, Kircher W. The Use of Porous and Surface Modified Silicas as Drug Delivery and Stabilizing Agents. Drug Dev. Ind. Pharm. 1983;9:69–91. [Google Scholar]
- 42.Wang L, Wang KM, Santra S, Zhao XJ, Hilliard LR, Smith JE, Wu JR, Tan WH. Watching Silica Nanoparticles Glow in the Biological World. Anal. Chem. 2006;78:646–654. [Google Scholar]
- 43.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, et al. Multimodal Silica Nanoparticles Are Effective Cancer-Targeted Probes in a Model of Human Melanoma. J. Clin. Invest. 2011;121:2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan EY, Fu YL, Wang X, Ding Y, Qian HQ, Wang CH, Hu Y, Jiang XQ. Hollow Chitosan-Silica Nanospheres for Doxorubicin Delivery to Cancer Cells with Enhanced Antitumor Effect in Vivo. J. Mater. Chem. 2011;21:3147–3155. [Google Scholar]
- 45.Jin YH, Lohstreter S, Pierce DT, Parisien J, Wu M, Hall C, Zhao JXJ. Silica Nanoparticles with Continuously Tunable Sizes: Synthesis and Size Effects on Cellular Contrast Imaging. Chem. Mater. 2008;20:4411–4419. [Google Scholar]
- 46.Klichko Y, Liong M, Choi E, Angelos S, Nel AE, Stoddart JF, Tamanoi F, Zink JI. Mesostructured Silica for Optical Functionality, Nanomachines, and Drug Delivery. J. Am. Ceram. Soc. 2009;92:S2–S10. doi: 10.1111/j.1551-2916.2008.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia TA, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of Sirna and DNA Constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng HA, Liong M, Xia TA, Li ZX, Ji ZX, Zink JI, Nel AE. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein Sirna to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng H, Yang S, Li ZX, Xia T, Chen J, Ji ZX, Zhang HY, Wang X, Lin SJ, Huang C, et al. Aspect Ratio Determines the Quantity of Mesoporous Silica Nanoparticle Uptake by a Small Gtpase-Dependent Macropinocytosis Mechanism. ACS Nano. 2011;5:4434–4447. doi: 10.1021/nn103344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nanomedicine. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable Cds Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003;125:4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 52.Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Stimuli-Responsive Controlled-Release Delivery System Based on Mesoporous Silica Nanorods Capped with Magnetic Nanoparticles. Angew. Chem., Int. Ed. 2005;44:5038–5044. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 53.Taylor KML, Kim JS, Rieter WJ, An H, Lin WL, Lin WB. Mesoporous Silica Nanospheres as Highly Efficient Mri Contrast Agents. J. Am. Chem. Soc. 2008;130:2154–2155. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 54.Rieter WJ, Kim JS, Taylor KML, An HY, Lin WL, Tarrant T, Lin WB. Hybrid Silica Nanoparticles for Multimodal Imaging. Angew. Chem., Int. Ed. 2007;46:3680–3682. doi: 10.1002/anie.200604738. [DOI] [PubMed] [Google Scholar]
- 55.Kim JS, Rieter WJ, Taylor KML, An H, Lin WL, Lin WB. Self-Assembled Hybrid Nanoparticles for Cancer-Specific Multimodal Imaging. J. Am. Chem. Soc. 2007;129:8962–8963. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbe C, Bartlett J, Kong LG, Finnie K, Lin HQ, Larkin M, Calleja S, Bush A, Calleja G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004;16:1959–1966. [Google Scholar]
- 57.Hulchanskyy TY, Roy I, Goswami LN, Chen Y, Bergey EJ, Pandey RK, Oseroff AR, Prasad PN. Organically Modified Silica Nanoparticles with Covalently Incorporated Photosensitizer for Photodynamic Therapy of Cancer. Nano Lett. 2007;7:2835–2842. doi: 10.1021/nl0714637. [DOI] [PubMed] [Google Scholar]
- 58.Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous Silica Nanoparticles Deliver DNA and Chemicals into Plants. Nat. Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 59.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically Modified Silica Nanoparticles: A Nonviral Vector for in Vivo Gene Delivery and Expression in the Brain. Proc. Natl. Acad. Sci. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small. 2007;3:1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 61.Liong M, Angelos S, Choi E, Patel K, Stoddart JF, Zink JI. Mesostructured Multifunctional Nanoparticles for Imaging and Drug Delivery. J. Mater. Chem. 2009;19:6251–6257. [Google Scholar]
- 62.Lu J, Liong M, Li ZX, Zink JI, Tamanoi F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small. 2010;6:1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferris DP, Lu J, Gothard C, Yanes R, Thomas CR, Olsen JC, Stoddart JF, Tamanoi F, Zink JI. Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells. Small. 2011;7:1816–1826. doi: 10.1002/smll.201002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hom C, Lu J, Liong M, Luo HZ, Li ZX, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles Facilitate Delivery of Sirna to Shutdown Signaling Pathways in Mammalian Cells. Small. 2010;6:1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corma A, Diaz U, Arrrica M, Fernandez E, Ortega I. Organic-Inorganic Nanospheres with Responsive Molecular Gates for Drug Storage and Release. Angew. Chem., Int. Ed. 2009;48:6247–6250. doi: 10.1002/anie.200902208. [DOI] [PubMed] [Google Scholar]
- 66.Kim JW, Kim LU, Kim CK. Size Control of Silica Nanoparticles and Their Surface Treatment for Fabrication of Dental Nanocomposites. Biomacromolecules. 2007;8:215–222. doi: 10.1021/bm060560b. [DOI] [PubMed] [Google Scholar]
- 67.Ha SW, Camalier CE, Beck GR, Lee JK. New Method to Prepare Very Stable and Biocompatible Fluorescent Silica Nanoparticles. Chem. Comm. 2009:2881–2883. doi: 10.1039/b902195g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.