Abstract
Infection of enucleated TC-7 monkey cells with rabies virus resulted in the synthesis of virus-directed RNA and the production of rabies antigens but not of infectious virus. The yield of infectious vesicular stomatitis virus from enucleated TC-7 cells, on the other hand, was almost as high as that from intact cells. Inhibition of the mitochondrial functions of enucleated cells by treatment with ethidium bromide did not influence the development of rabies antigens or the production of infectious vesicular stomatitis virus.
Full text
PDF

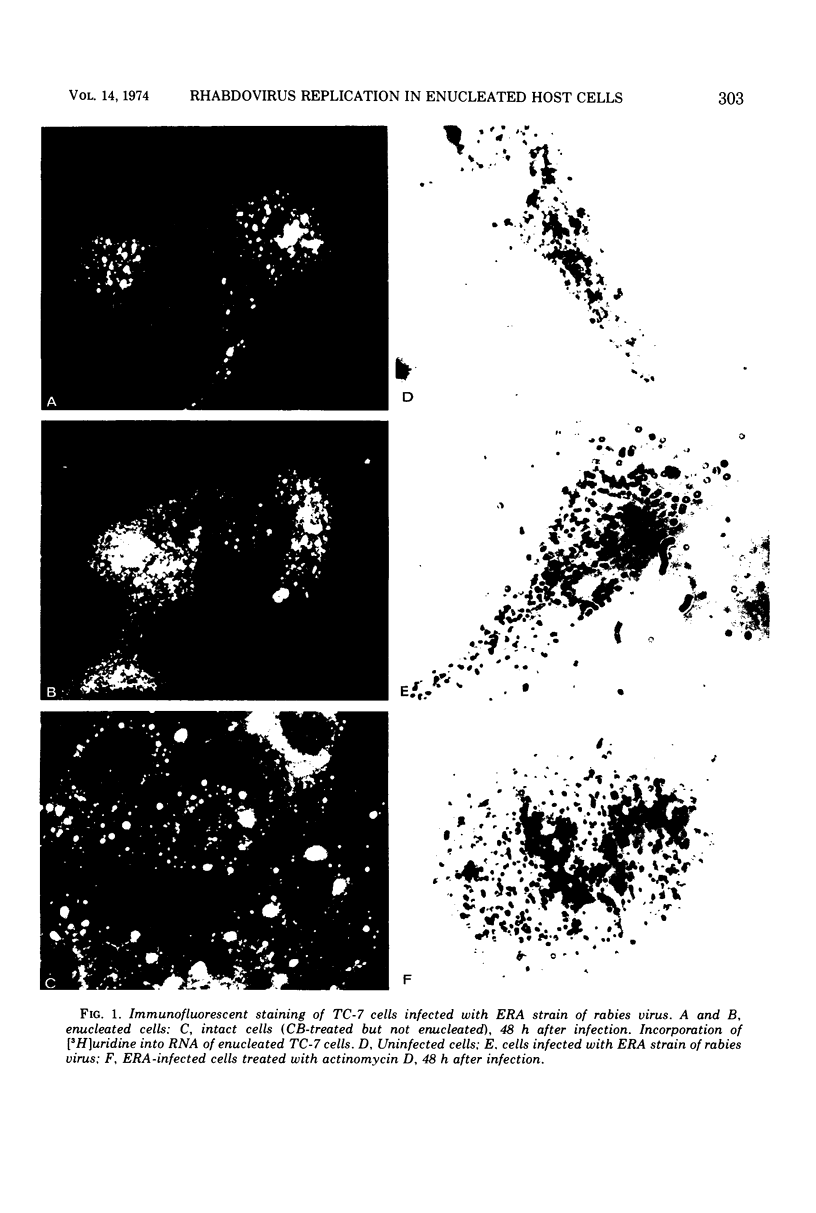
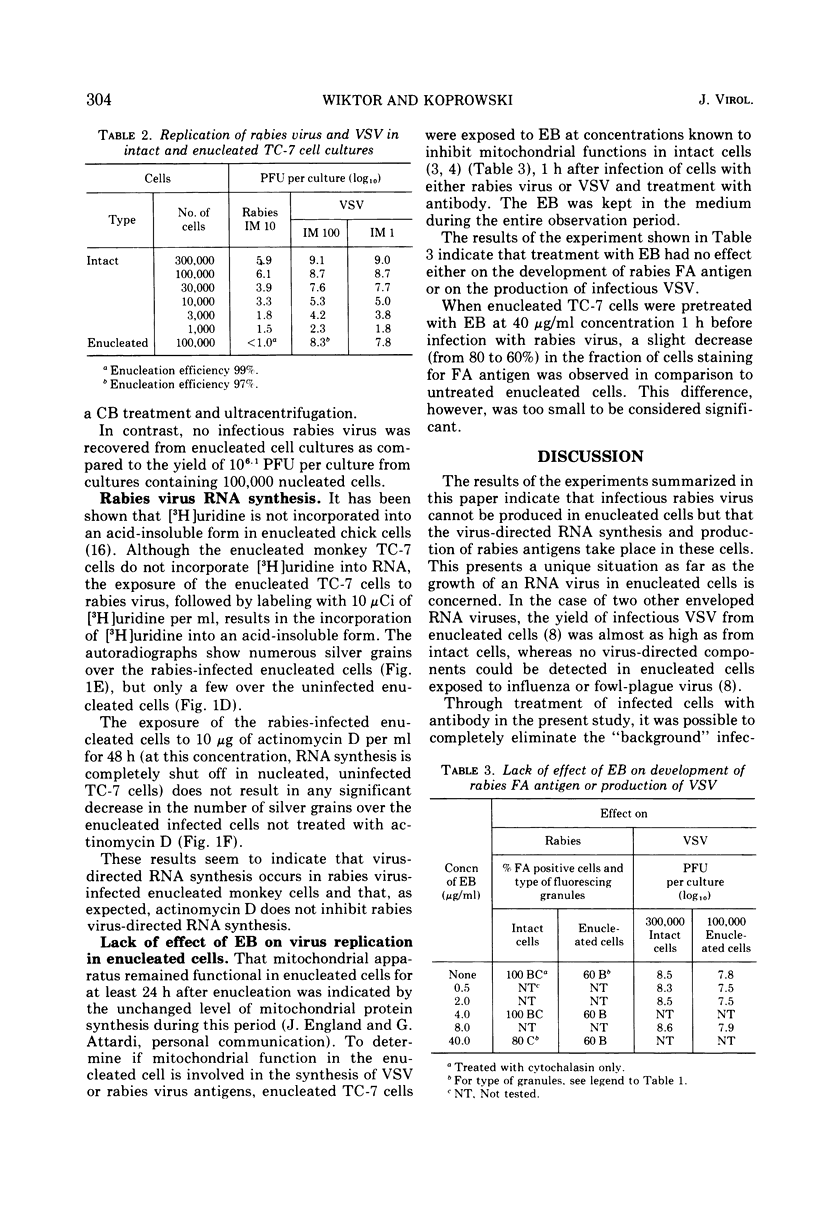


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Clark H. F., Bishop D. H., Koprowski H. Comparison of the ribonucleic acid polymerases of two rhabdoviruses, Kern Canyon virus and vesicular stomatitis virus. J Virol. 1971 Jun;7(6):726–735. doi: 10.1128/jvi.7.6.726-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasiu P. Quantitative assay and potency test of antirabies serum and immunoglobulin. Monogr Ser World Health Organ. 1973;(23):314–318. [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. B., Maes R. F., Wiktor T. J., Koprowski H. The inhibition of rabies virus by arabinosyl cytosine. Studies on the mechanism and specificity of action. Virology. 1968 Apr;34(4):701–708. doi: 10.1016/0042-6822(68)90091-3. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Enucleation of cells made simple and rescue of SV40 by enucleated cells made even simpler. Virology. 1973 Jan;51(1):227–229. doi: 10.1016/0042-6822(73)90382-6. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Wiktor T. J., Koprowski H. Early events of rabies virus replicaton in tissue cultures. An electron microscopic study. Lab Invest. 1973 Feb;28(2):142–148. [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C. Studies on influenza infection in ferrets by means of fluorescein-labelled antibody. II. The role of soluble antigen in nuclear fluorescence and cross-reactions. J Exp Med. 1955 Jun 1;101(6):677–686. doi: 10.1084/jem.101.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Pollack R., Goldman R. Synthesis of infective poliovirus in BSC-1 monkey cells enucleated with cytochalasin B. Science. 1973 Mar 2;179(4076):915–916. doi: 10.1126/science.179.4076.915. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kirkpatrick J. B. Mass enucleation of cultured animal cells. Methods Cell Biol. 1973;7:189–202. doi: 10.1016/s0091-679x(08)61777-x. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaginès R. Interference of ethidium bromide with the replication of herpes virus DNA. Brief report. Arch Gesamte Virusforsch. 1970;29(1):109–112. [PubMed] [Google Scholar]
- Wiktor T. J., Clark H. F. Chronic rabies virus infection of cell cultures. Infect Immun. 1972 Dec;6(6):988–995. doi: 10.1128/iai.6.6.988-995.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]