Abstract
Extracellular ATP (eATP) is the most abundant among extracellular nucleotides and is commonly considered as a classical danger signal, which stimulates immune responses in the presence of tissue injury. In fact, increased nucleotide concentration in the extracellular space is generally closely associated with tissue stress or damage. However non-lytic nucleotide release may also occur in many cell types under a variety of conditions. Extracellular nucleotides are sensed by a class of plasma membrane receptors called P2 purinergic receptors (P2Rs). P2 receptors are expressed by all immunological cells and their activation elicits different responses. Extracellular ATP can act as an initiator or terminator of immune responses being able to induce different effects on immune cells depending on the pattern of P2 receptors engaged, the duration of the stimulus and its concentration in the extracellular milieu. Millimolar (high) concentrations of extracellular ATP, induce predominantly proinflammatory effects, while micromolar (low) doses exert mainly tolerogenic/immunosuppressive action. Moreover small, but significant differences in the pattern of P2 receptor expression in mice and humans confer diverse capacities of ATP in regulating the immune response.
Keywords: Extracellular nucleotides, P2 purinergic receptors, extracellular ATP, innate immunity
Extracellular nucleotides
Nucleotides (ATP, ADP, UTP and UDP) are among the most ancient biologic molecules and this is consistent with their multifunctional role in living organisms. Nucleotides are the constituents of nucleic acids, represent an intracellular energy source and serve as substrate in signal transduction pathways. Intracellular nucleotides can be also massively released in the extracellular space and play a role in intercellular communication [1-3].
ATP is the most abundant among nucleotides. Intracellular concentration of ATP ranges between 1 and 10 mM while, in normal conditions, the extracellular compartment contains ATP in the low nanomolar concentration range. Because of such steep concentration gradient, ATP small size and high mobility, a dramatic increase of ATP concentration can occur in the extracellular space around damaged cells leaking their cytoplasmic content [3-7].
ATP can be also actively released by many different cell types under certain conditions. Activated platelets represent one of the most abundant source of actively released adenine nucleotides [8-10]. ATP is also released from vascular endothelial cells under mechanical or shear stress [11-13]. In addition, ATP secretion from endothelial cells as well as from leukocytes can be induced by pathogen-associated molecules [7,14-17]. T lymphocytes secrete ATP in the early stages of activation [18]. Moreover commensal bacteria in the gut are able to secrete ATP exerting relevant modulatory effects on immune responses [19]. Finally ATP is released during the early stages of apoptosis inducing monocyte/macrophages recruitment acting as a “find me” signal to exert an efficient cell clearance [20]. Of note, different eukaryotic cells use different mechanisms to release ATP. For example, under proper stimulation, neurons and platelets secrete adenine nucleotides stored in cytoplasmic vesicles [21,22]. In other cell types, such as T lymphocytes, PMN neutrophils and monocyte/macrophages, ATP is released in response to increased cytosolic calcium concentration through pannexin (panx)-1 hemichannels [18,23-25]. Alternatively, ATP can be released through the opening of volume-sensitive channels [26], purinergic X receptors (P2X)-gated channels [27,28] or by the opening of connexin 43 channels upon mechanical stress [29]. Different secretion pathways are used by different cells of the immune system for ATP release depending on the nature of the activating stimulus and/or pathophysiological condition.
Purinergic receptors
Once in the extracellular space, nucleotides bind to specific plasma membrane receptors, named P2 receptors, widely distributed in a variety of different organisms such as mammals, plants, yeasts and bacteria, suggesting that nucleotides represent an archaic communication system [30,31]. All eukaryotic cells express P2 receptors and nucleotides trigger intracellular signaling pathways in almost every tissue. Intracellular signaling pathways activated by P2 receptors depend on cell type, pattern of P2 receptors expressed and type/quantity of released nucleotides. Two P2 receptor subfamilies have been described so far: P2X and P2Y [32-34]. P2 receptors signaling altogether cooperate in determining the basal level of cell activation for signal transduction pathways [35]. Moreover, a wide variety of physiological functions are regulated by P2 receptors, including the regulation of cell volume, tissue blood flow and inflammation.
The P2X subfamily is composed of seven members named P2X1-P2X7. P2X receptors are ligand-gated ion channels selective for monovalent and divalent cations. The amino- and carboxyl-terminal domains of the P2X subtypes are both cytoplasmic. Upon activation, P2X subunits aggregate to form homo- or in some cases hetero-multimers and determine Ca2+ and Na+ influx and K+ efflux [34]. The only known physiological agonist for P2X receptors is ATP. P2X receptors were originally identified in mammalian sensory neurons, and subsequently also found in several additional cell types such as smooth muscle cells, fibroblasts, megakariocytes, platelets, lymphocytes, macrophages, granulocytes, dendritic cells [36,37].
P2Y receptors are widely expressed, being present in platelets [38,39] mucosal cells [40,41], monocytes [42,43], macrophages [43,44], dendritic cells [45-47], NK cells [48], granulocytes [49-51], neurons [52,53], smooth and striated muscle cells [54-58]. Eight P2Y subtypes have been cloned (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) [59-62]. P2Y receptors are seven membrane-spanning, G-protein-coupled receptors whose activation exerts different effects depending on the G protein subtype involved. P2Y1, 2,4,6, and 11 are coupled to Gq/11 proteins that trigger the generation of inositol 1,4,5-trisphosphate and release of Ca2+ from the intracellular stores. P2Y12, 13 and 14 are associated with Gi/0 proteins, which inhibits adenylate cyclases [63]. Of note, P2Y11 activation is associated with increased intracellular cAMP concentration [45,64,65]. As each P2Y family member display different affinity for diverse ligands, each receptor is characterized by a distinct agonist rank of potency (see Table 1 and Figure 1). P2Y1, P2Y11, P2Y12 and P2Y13 are activated by ATP or ADP. P2Y2 is activated both by UTP and ATP; P2Y4 and P2Y6 have UTP and UDP as agonists, whereas UDP-glucose activates the P2Y14 subtype.
Table 1.
Agonists binding affinity (EC50) for all P2 receptors and their main downstream signaling events
| Receptor | Agonists | Agonists binding affinities EC50(μM) | Main downstream signaling events |
|---|---|---|---|
| P2X Receptors | |||
| P2X1 | ATP | 0.05-1 | Ca2+ /Na+ influx |
| P2X2 | ATP | 1-30 | Ca2+ influx |
| P2X3 | ATP | 0.3-1 | Cations influx |
| P2X4 | ATP | 1-10 | Ca2+ influx |
| P2X5 | ATP | 1-10 | Ion influx |
| P2X6 | ATP | 1-12 | Ion influx |
| P2X7 | ATP | >100 | Cations influx and pore formation |
|
| |||
| P2Y Receptors | |||
| P2Y1 | ADP | 8 | PLCβ activation |
| P2Y2 | ATP, UTP | 0.1 (ATP), 0.2 (UTP) | PLCβ activation, cAMP inhibition |
| P2Y4 | UTP (ATP, UTP) | 2.5 | PLCβ activation cAMP inhibition |
| P2Y6 | UDP, UTP | 0.3 (UDP), 6 (UTP) | PLCβ activation |
| P2Y11 | ATP | 17 | cAMP increase, PLCβ activation |
| P2Y12 | ADP | 0.07 | cAMP inhibition |
| P2Y13 | ADP, ATP | 0.06 (ADP), 0.26 (ATP) | cAMP inhibition |
| P2Y14 | UDP-glucose | 0.1-0.5 | PLCβ activation |
Figure 1.
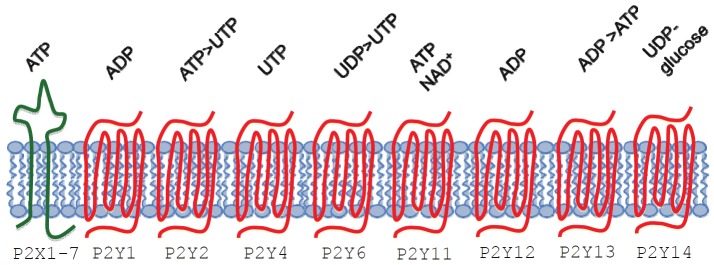
Type 2 purinergic receptors and their nucleotide agonists. Extracellular nucleotides bind to type 2 purinergic receptors exerting their effects on cells’ function. Two distinct P2 receptor subfamilies were described P2X and P2Y. P2X receptors are membrane cation channels gated exclusively by extracellular ATP. Seven P2X receptors have been cloned and named P2X1-7. They are oligomers of three subunits each composed by an extracellular loop, two transmembrane domains and an amino- and a carboxyl-terminal both cytoplasmic. ATP binding induce the subunits assembly to form omo- or etero-multimerc channels permeable to monovalent and divalent cations. P2Y receptors are seven membrane-spanning, G-protein-coupled receptors. Eight P2Y subtype receptors have been cloned so far named P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14. They can be subdivided into adenine nucleotide-preferring receptors (P2Y1, P2Y11, P2Y12 and P2Y13), uracil nucleotide-preferring receptors (P2Y4 and P2Y6), a receptor of mixed specificity (P2Y2) and a UDP-glucose-preferring receptor (P2Y14).
Extracellular metabolism of nucleotides
The nature and intensity of purinergic signaling depend on extracellular nucleotide/nucleoside concentrations, which are controlled by a family of ectoenzymes known as ecto-nucleoside triphosphate diphosphohydrolases (E-NTDPase 1, 2, 3 and 8). CD39/ENTPD1 ectonucleotidase (CD39) is expressed by monocytes, NK cells, T and B lymphocytes and dendritic cells [66,67]. It can hydrolyze tri- and di-phosphate nucleosides, but is not able to hydrolyze monophosphate nucleosides [68]. Regulation of extracellular ATP concentration by ATP scavenging CD39 has been shown to regulate immune cells function and inflammation in different settings [66,67,69,70].
Another important membrane-bound enzyme involved in the metabolism of extracellular nucleotides is CD73/ecto-5’-nucleotidase. It catalyzes the hydrolysis of adenosine–monophosphate (AMP) generating adenosine that is in turn recognized by P1 adenosine receptors. Interestingly CD39 and CD73 are simultaneously expressed on the same cellular population as occurs for example on murine T regulatory lymphocytes (Tregs) and on a subset of human Tregs [71] or on human monocytederived dendritic cells [72]. Effects due to ATP catabolites rather than to ATP itself can be distinguished by comparing the observations made using ATP with those obtained with nonhydrolyzable ATP analogues (e.g. ATP-γ-S), adenosine deaminase (ADA; that converts adenosine into inosine) or exogenous apyrase that hydrolyzes extracellular ATP.
Regulation of innate immunity by extracellular nucleotides
The innate immune system is the first line of defense against invading pathogens. Four major pattern recognition receptor (PRR) families, are involved in the recognition of a wide range of pathogen-associated molecular patterns (PAMPs): Toll-like receptors (TLRs), cytosolic RIG-I-like receptors (RLRs), Nod-like receptors and C-type lectins. It is now clear that detection of foreign microorganisms is not sufficient to induce inflammation, but recognition of a damage signal is also necessary.
For example, DCs reside in peripheral tissues and serve as “sentinels”, and they are not only activated upon encounter with foreign pathogens recognized by Toll like receptors, but they also react to the presence of environmental molecules associated with tissue stress, the so-called damage-associated molecular patterns (DAMPs). Constitutively expressed endogenous molecules can function as danger signals as for example ATP, adenosine, high mobility box group 1 (HMBG1) and heat shock proteins, while other danger signal are inducible factors such as type I interferons [73]. The recognition of endogenous danger signals by cells of the immune system participate in determining the quality and the strength of the immune response and enables the immune system to distinguish between pathogenic or harmless/commensal organisms.
In order to maintain homeostasis the termination of the immune response and the resolution of inflammation is as important as its initiation and tissue damage might be caused by the intrinsic toxicity of sustained inflammation. Extracellular ATP can act as an initiator or terminator of immune responses. Relatively high concentrations of extracellular ATP (in the millimolar range) induce predominantly proinflammatory effects through the engagement of the low affinity receptor P2X7. On the other hand low (micromolar) doses exert mainly tolerogenic/immunosuppressive action (Figure 2) through the activation of the high affinity P2Y11 receptor [74,75].
Figure 2.
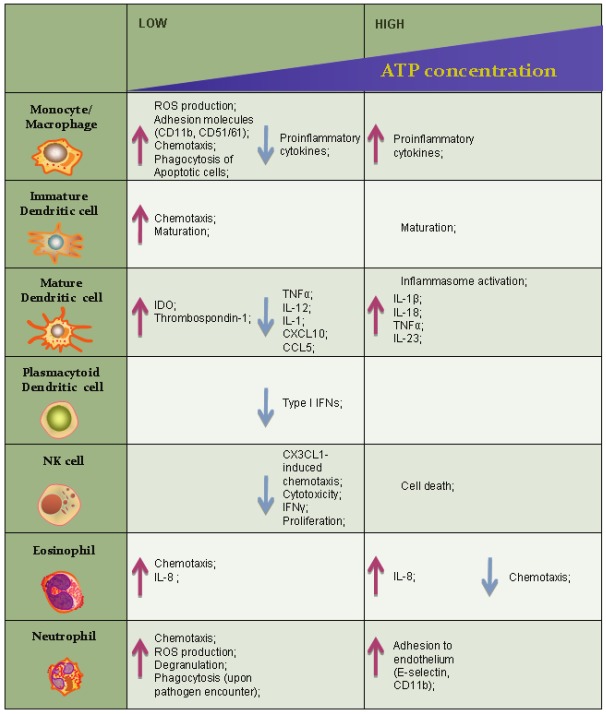
Extracellular ATP exerts different effects on cells of the innate immunity depending on its concentration. Low (1-250 μM) eATP concentrations activate high and intermediate ATP-binding affinity P2 receptors. High (1-10 mM) concentrations of eATP activate P2X7 receptor which displays low ATP-binding affinity.
Monocytes/macrophages
Macrophages continuously differentiate from monocytes that leave blood flow to reach the tissues throughout the body. When a potentially pathogenic microorganism crosses the epithelial barrier is immediately recognized by macrophages that reside in the host tissues and that are able to pahgocyte and kill it.
In macrophages, millimolar extracellular ATP engages P2X7 and trigger the activation of the inflammasome [16,76]. K+ efflux occurring through the opened P2X7 channel is a key event leading to the assembly of the Natch Domain-, Leucine-Rich Repeat-, PYD-Containing Protein 3 (NLRP3) inflammasome [77]. Two distinct triggering signals are necessary for macrophages to secrete IL-1 β and IL-18: the activation of Toll-like receptor pathway that determines the expression and accumulation of pro-IL-1β and pro-IL-18, and the engagement of P2X7 receptor that activate the inflammasome, composed by NLRP3, the ASC adaptor and procaspase1. Once activated, NLRP3 promotes the oligomerization of procaspase 1 and its subsequent proteolytic activation into active Caspase 1 that in turn cleaves pro-IL-1β and pro-IL-18 into active cytokines [78-81]. In keeping, macrophages from P2X7 KO mice display impaired NLRP3 inflammasome activation and reduced secretion of IL-1β and IL-18 after LPS stimulation [82]. As a consequence, in a monoclonal anti-collagen induced arthritis model, P2X7 KO mice develop less severe synovial inflammation as well as reduced cartilage destruction [83]. Moreover high levels (mM) of extracellular ATP increase macrophage secretion of inflammatory cytokines such as IL-1α [84], IL-1β [85-87], IL-6 [82], IL-18 [88,89], TNF-α [90,91], whereas low micromolar ATP concentrations sufficient to trigger the P2Y11 but not the P2X7 receptor, inhibit TNF-α and CCL-2 production while increasing the production of the immunoregulatory cytokine IL-10 [92]. Extracellular nucleotides have been shown to regulate several other cell functions in a P2X7 receptor-independent manner. For example macrophages exposed to micromolar levels of extracellular nucleotides, display increased ROS production, [44,93,94]. Such event in turn activates different signalling pathways leading to the production of macrophage inflammatory protein-2 (MIP-2), that promote migration of neutrophils toward inflamed tissues [95]. In addition micromolar levels of both extracellular ATP and ADP also induce chemotaxis of monocyte/macrophages [92,96-98]. Phagocytic activity of macrophages is also influenced by extracellular nucleotides. Clearance of apoptotic cells is a crucial task performed by macrophages. Removal of apoptotic cells normally does not lead to upregulation of co-stimulatory molecules or cytokine production by macrophages and therefore does not contribute to or stimulate immune responses [99]. On the contrary upon encounter with necrotic cells macrophage proinflammatory activity is stimulated while phagocytosis is not. As dying cells release nucleotides and macrophages express most of purinergic receptors, Marques-da-Silva and colleagues recently investigated whether extracellular nucleotides could influence phagocytosis of murine macrophages through the activation of purinergic receptors [100]. Pretreatment of macrophages with low concentrations of several extracellular nucleotides, induced increased expression of adhesion molecules such as CD11b/CD18 (Mac-1) and CD51/61 and consequent enhanced phagocytosis, possibly through the engagement of P2X1, P2X3 ,P2Y1 and/or P2Y6. This scenario is consistent with an homeostatic environment where low levels of nucleotides, released by apoptotic cells, stimulate macrophages to clear apoptotic bodies enhancing pahgocytosis. Higher concentrations of extracellular nucleotides, consistent with a necrotic environment, do not stimulate the upregulation of adhesion molecules, nor the clearance of necrotic cells, determinig the amplification of inflammatory effects exerted by necrotic debris.
Dendritic cells
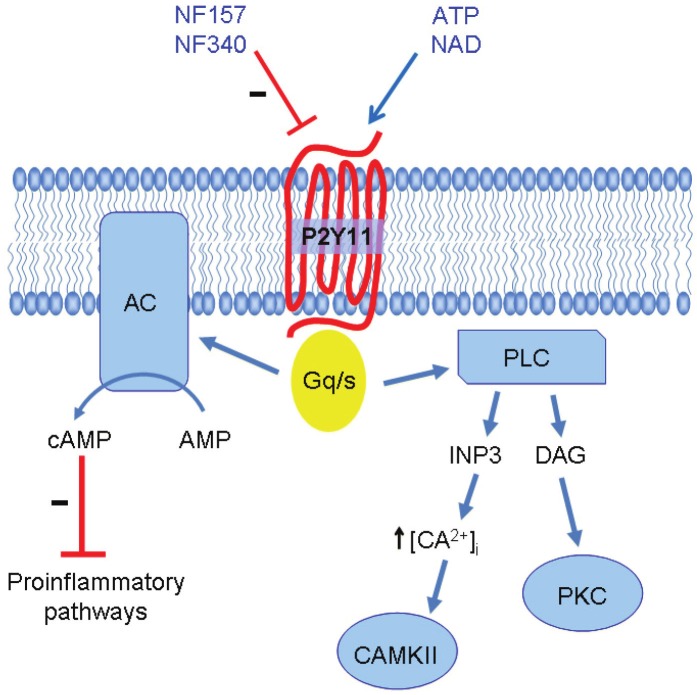
Dendritic cells (DCs) are professional antigen presenting cells. They reside in tissues where they uptake the antigen and then migrate to lymph nodes toward cytokines gradients, to stimulate T cells. Extracellular ATP is able to induce immature (but not mature) DCs migration [101]. P2X7 activation on DCs is able to induce inflammasome activation as well as secretion of proinflammatory cytokines such as IL-1β, IL-18, TNF-α and IL-23. On the other hand, dendritic cells maturing in the presence of micromolar concentrations of extracellular ATP display impaired production of TNF-α, IL-1β and IL-12 as well as reduced secretion of inflammatory chemokines such as CXCL-10, CCL-5, CCL-2 and CCL-3, while the expression of IL-10, IL-1 receptor antagonists or of CCL-17 and CCL-22 are either unaffected or upregulated [102-104]. In the same experimental setting, pharmacological inhibition of P2Y11 receptor restores the production of TNFα and IL-12 by DCs (la Sala et al., unpublished). Moreover extracellular ATP has been shown to induce the expression of two important immunosuppressive proteins: indoleamine 2,3-dioxygenase (IDO) and thrombospondin-1 via P2Y11 activation [105]. Of note the P2Y11 receptor is the only P2YR coupled to a Gs protein that in turn is able to activate adenylate cyclase determining an increase in intracellular cAMP concentration as depicted in Figure 3. Interestingly the treatment of DCs with different cyclic AMP elevating agents or cell-permeable cAMP analogs produce similar modifications of DC maturation process resulting in impaired capacity of DC to promote type 1 T cell responses [102,106-108]. Depending on the microenvironment, extracellular ATP can promote immunogenic or tolerogenic activity of DCs. For example micromolar concentrations of eATP that block IL-12 expression elicited by LPS, synergize with TNF for the induction of IL-12p70 [45,108]. Moreover, eATP has been shown to inhibit IL-27 secretion via P2Y11 activation and upregulate IL-23 mRNA expression through a P2Y11-independent mechanism [109].
Figure 3.
Peculiarities of the P2Y11 receptor. P2Y11 is expressed on human cells but has no ortholog in rodents. ATP is the P2Y11 preferred physiological ligand, it can also be activated by extracellular NAD+ and NAADP. The P2Y11 is the only P2Y receptor coupled to a Gq/s protein and upon activation stimulates adenylate cyclase (AC) as well as phospholypase C (PLC) activities. Consequent increased concentration of cAMP mediates several inhibitory effects of eATP on human cells of the innate immunity.
The “dualism” between P2X7 as low affinity ATP receptor exerting mainly proinflammatory effects and P2Y11 an high affinity receptor triggering cyclic AMP-mediated immune suppression, determines a complex regulation of immune functions also in other leukocyte subpopulations. Importantly as no orthologue gene of the human P2Y11 receptor have been identified in rodents, murine cells converge in delineating a marked proinflammatory role for ATP, while in the human system both pro and antiinflammatory effect have been documented [110]. Adding to such complex scenario, the duration of the stimulus must be taken into consideration as well. While P2X7 opening for a short time leads to the activation of the proinflammatory pathway to sustain inflammation, prolonged P2X7 receptor stimulation causes the enlargement of the pore that leads to cell death.
NK cells
Natural killer cells are bone marrow-derived circulating lymphocytes that contribute to the innate immune response by exerting cytolytic activity against virally infected and neoplastic cells and by secreting cytokines, especially IFN-γ. Extracellular ATP is a modulator of the activity of NK cells as well. It inhibits NK cells proliferation and IFN-γ production [111]. In addition NK cells cytotoxic activity and chemotaxis elicited by CX3CL1 is blocked by eATP, an effect that is mediated by the P2Y11 receptor [48]. CX3CL1 might have a role in the crosstalk between leukocytes and endothelial cells. Soluble CX3CL1 is released by activated ECs during early stages of inflammation, and is able to induce the recruitment of leukocytes expressing its cognate receptor CX3CR1. In addition, CX3CL1 triggers interferon-γ production by NK cells that reinforces CX3CL1 expression by ECs [112]. Moreover, activated ECs can express both the soluble and the membrane-bound form of CX3CL1, the latter acting as an adhesion molecule thus reinforcing the strength of leukocyte-endothelial cell interaction [113]. In addition CX3CL1 can stimulate the cytolytic activity of NK cells toward ECs [114]. Noteworthy ECs represent a major source of actively secreted ATP [115], pointing it out as an important player in the regulation of NK-EC interaction. It has been proposed that activated NK cells may mediate vascular injury in different pathological conditions such as vascular leak syndrome, allograft rejection, and cytomegalovirus infection [113,114,116]. In the presence of ATP, CX3CL1 failed to enhance NK cell–mediated cytolysis of endothelial cells. Most importantly increased degradation of extracellular ATP by exogenous apyrase significantly increased NK cells capacity to kill endothelial cell. In addition ATP influences NK chemotaxis by inhibiting CX3CL1-induced cell migration. Such effect is not due to a general inhibition of the capacity of NK cell to migrate because in the same experimental settings extracellular ATP has proven able to increase chemotaxis toward CXCL12 and enhance chemokinesis [48].
Polymorphonuclear cells (PMN)
Eosinophils are bone marrow-derived granulocityc leukocytes. Only few of these cells are normally present in the circulation the majority of them residing in connective tissues, just under epithelium. Eosinophils exert two effector functions: upon activation they release highly toxic granule proteins and free radicals, and secrete several cytokines and chemical mediators to attract and activate other immunological cells. Extracellular ATP in low (micromolar) concentration enhances eosinophil migration toward inflamed tissues [117,118].
In eosinophils, extracellular ATP increases intracellular calcium concentration through the opening of ion channels allowing Ca2+ influx from the extracellular space and by triggering Ca2+ release from the intracellular stores as well [119]. As actin reorganization is preceded by increased intracellular Ca2+ concentration, extracellular nucleotides, especially ATP, UTP and ADP, are able to induce a rapid and transient actin polymerization, in a concentration dependent manner [119,120]. In addition, extracellular nucleotides trigger the secretion of eosinophil cationic protein, and IL-8, two potent chemoattractants recruiting other eosinophils and neutrophils [121,122].
Polymorphonuclear neutrophilic leukocytes are short living cells very abundant in blood, but normally not present in healthy tissues. They share with macrophages a key role in innate immunity because they are able to recognise, ingest and kill many pathogens without an aid of adaptive immune response. Extracellular ATP enhances chemotactic response of neutrophils [24,123].
Noteworthy, neutrophils express P2 receptors [50,124,125], and they are able to actively secrete ATP [126]. Neutrophils can transiently but rapidly secrete ATP through panx1 and connexin 43 channels, from the protruding edge of the cell during migration. ATP activate P2Y2 receptors through an autocrine pathway and at the same time ATP is hydrolyzed by ectonucleotidases to adenosine that engages the Gi-coupled A3 receptor. These two concomitant mechanisms determine an amplification of chemotaxis [123]. Neutrophils also express a maxianion channel known as human tweety homolog 3 (hTTYH3), which upon cell activation by N-formil-Met-Leu-Phe bacterial peptide receptors (FPRs), is able to secrete ATP. Noteworthy panx1 hemichannels colocalize with FPRs and hTTYH3 at the leading edge of migrating neutrophils, delimiting an area for active ATP release [24]. ATP secretion by neutrophils is induced not only upon FPRs stimulation, but also after activation by IL-8, leukotriene B4 (LTB4), the complement component C5α and FcγR receptor, pointing out the importance of this autocrine purinergic pathway [127].
Neutrophil adhesion to endothelium [128-132], the production of reactive oxygen species (ROS) [50,133-138] and degranulation are also increased by extracellular ATP [134,136,139,140]. Interestingly extracellular nucleotides regulate neutrophils phagocytosis in a complex manner. It has been previously shown that both ATP and ADP at micromolar concentrations stimulate phagocytosis via activation of Mac-1 [141,142], but recently Kudo and colleagues have shown that low micromolar concentration of the same nucleotides can inhibit neutrophil phagocytosis until pathogen stimulation [143]. It is possible that ATP and ADP can enable phagocytic cup formation thus inihibiting the binding/uptake of antigens [144]. The inhibition of phagocytic activity by neutrophils should might be important for limiting excessive phagocytosis that occurs in pathological conditions such as hemophagocytic syndrome [145]. However neutrophil bactericidal activity is unaffected by this regulatory mechanism as the inhibition of phagocytosis by ATP and ADP is abrogated by stimulation with fMLP or LPS [143].
Plasmacytoid dendritic cells
The regulation of plasmacytoid dendritic cells (pDCs) function by extracellular nucleotides has been only partially investigated. Plasmacytoid DCs are a subpopulation of dendritic cells playing a crucial role in antiviral immunity. These cells are specialized in the rapid and abundant production type I interferons (IFN-α, -β, -ω) in response to viral infection [146,147].
The presence of nucleotides such as ATP, ADP, UTP, UDP and UDP-glucose in the extracellular milieu inhibits type I IFN production by pDCs in response to influenza virus or the TLR9 agonist CpG. Nucleotides that exert the most potent inhibitory effect include UDP, UTP and UDPglucose. This finding suggests the involvement of P2Y4, P2Y6 and P2Y14 receptors [148]. Because type I IFNs enhance cytotoxic activity and IFN-γ production by NK and CD8+ T lymphocytes, their inhibition may reduce immune surveillance against virally infected and neoplastic cells.
Conclusions
Extracellular nucleotides can modulate the function of cells of the innate immune system as well as of T lymphocytes. The role of extracellular ATP in the regulation of immune responses and inflammation appears to be different in humans as compared to that established in mice. While several observations point out ATP as a signal that induces the innate immune system to trigger and sustain inflammation, other evidences suggest that ATP might represent a negative feedback signal to limit detrimental inflammation in the surrounding of stressed or damaged cells. Several of such regulatory effects of extracellular ATP are mediated by the P2Y11 receptor expressed in humans but not in rodents and linked to increased intracellular cAMP levels that play a major role as immunosuppressive signal.
Acknowledgements
This work has been supported in part by the Commission of European Union 2012-2014, ERA-NET NEURON program, “Role of danger signals in stroke and therapeutic targeting by nanobodies”.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 3.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 4.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 5.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 7.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Mills DC. ADP receptors on platelets. Thromb Haemost. 1996;76:835–856. [PubMed] [Google Scholar]
- 9.Gachet C. Identification, characterization, and inhibition of the platelet ADP receptors. Int J Hematol. 2001;74:375–381. doi: 10.1007/BF02982079. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 14.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 15.Warny M, Aboudola S, Robson SC, Sévigny J, Communi D, Soltoff SP, Kelly CP. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari D, Chiozzi P, Falzoni S, Dal SM, Melchiorri L, Baricordi OR, Di VF. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 17.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 19.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 20.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a findme signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 22.Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF. The platelet ATP and ADP receptors. Curr Pharm Des. 2006;12:859–875. doi: 10.2174/138161206776056029. [DOI] [PubMed] [Google Scholar]
- 23.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schon P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 26.Sabirov RZ, Dutta AK, Okada Y. Volumedependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 28.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka K, Gilroy S, Jones AM, Stacey G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010;20:601–608. doi: 10.1016/j.tcb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esther CR Jr, Sesma JI, Dohlman HG, Ault AD, Clas ML, Lazarowski ER, Boucher RC. Similarities between UDP-glucose and adenine nucleotide release in yeast: involvement of the secretory pathway. Biochemistry. 2008;47:9269–9278. doi: 10.1021/bi800855k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 33.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 34.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 35.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 37.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 38.Mills DC. ADP receptors on platelets. Thromb Haemost. 1996;76:835–856. [PubMed] [Google Scholar]
- 39.Greco NJ. Functional expression of a P2T ADP receptor in Xenopus oocytes injected with megakaryocyte (CMK 11-5) RNA. Arterioscler Thromb Vasc Biol. 1997;17:769–777. doi: 10.1161/01.atv.17.4.769. [DOI] [PubMed] [Google Scholar]
- 40.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 41.Degagne E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sevigny J, Gendron FP. P2Y2 receptor transcription is increased by NF-kappa B and stimulates cyclooxygenase-2 expression and PGE2 released by intestinal epithelial cells. J Immunol. 2009;183:4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid-Antomarchi H, Schmid-Alliana A, Romey G, Ventura MA, Breittmayer V, Millet MA, Husson H, Moghrabi B, Lazdunski M, Rossi B. Extracellular ATP and UTP control the generation of reactive oxygen intermediates in human macrophages through the opening of a charybdotoxin-sensitive Ca2+-dependent K+ channel. J Immunol. 1997;159:6209–6215. [PubMed] [Google Scholar]
- 45.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 46.Idzko M, Dichmann S, Ferrari D, Di VF, la SA, Girolomoni G, Panther E, Norgauer J. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxinsensitive P2y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- 47.Idzko M, Panther E, Sorichter S, Herouy Y, Berod L, Geissler M, Mockenhaupt M, Elsner P, Girolomoni G, Norgauer J. Characterization of the biological activities of uridine diphosphate in human dendritic cells: Influence on chemotaxis and CXCL8 release. J Cell Physiol. 2004;201:286–293. doi: 10.1002/jcp.20070. [DOI] [PubMed] [Google Scholar]
- 48.Gorini S, Callegari G, Romagnoli G, Mammi C, Mavilio D, Rosano G, Fini M, Di Virgilio F, Gulinelli S, Falzoni S, Cavani A, Ferrari D, la Sala A. ATP secreted by endothelial cells blocks CXCL 1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y receptor activation. Blood. 2010;116:4492–4500. doi: 10.1182/blood-2009-12-260828. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di VF, Luttmann W. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217–224. doi: 10.1016/s0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 51.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di VF, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 52.Allen TG, Burnstock G. The actions of adenosine 5’-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990;100:269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Illes P, Norenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993;14:50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- 54.Haggblad J, Eriksson H, Heilbronn E. Cell surface ATP (P2y) purinoceptors trigger and modulate multiple calcium fluxes in skeletal muscle cells. Prog Brain Res. 1990;84:111–116. doi: 10.1016/s0079-6123(08)60894-8. [DOI] [PubMed] [Google Scholar]
- 55.Vassort G. Adenosine 5’-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 56.Tsim KW, Barnard EA. The signaling pathways mediated by P2Y nucleotide receptors in the formation and maintenance of the skeletal neuromuscular junction. Neurosignals. 2002;11:58–64. doi: 10.1159/000057322. [DOI] [PubMed] [Google Scholar]
- 57.Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–44. doi: 10.1111/j.1749-6632.1990.tb37659.x. [DOI] [PubMed] [Google Scholar]
- 58.Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- 59.von KI, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 60.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 61.Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to G(i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 62.Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen TD, Meichle S, Kim US, Wong T, Moody MW. P2Y(11), a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G795–G804. doi: 10.1152/ajpgi.2001.280.5.G795. [DOI] [PubMed] [Google Scholar]
- 65.Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 66.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 69.Friedman DJ, Kunzli BM, Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 71.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 73.Hwang PF, Porterfield N, Pannell D, Davis TA, Elster EA. Trauma is danger. J Transl Med. 2011;9:92. doi: 10.1186/1479-5876-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di VF, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9:507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boeynaems JM, Communi D. Modulation of inflammation by extracellular nucleotides. J Invest Dermatol. 2006;126:943–944. doi: 10.1038/sj.jid.5700233. [DOI] [PubMed] [Google Scholar]
- 76.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 77.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 78.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 80.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 81.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 82.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 83.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 84.Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 85.Hogquist KA, Unanue ER, Chaplin DD. Release of IL-1 from mononuclear phagocytes. J Immunol. 1991;147:2181–2186. [PubMed] [Google Scholar]
- 86.Ferrari D, Pizzirani C, Adinolfi E, Forchap S, Sitta B, Turchet L, Falzoni S, Minelli M, Baricordi R, Di VF. The antibiotic polymyxin B modulates P2X7 receptor function. J Immunol. 2004;173:4652–4660. doi: 10.4049/jimmunol.173.7.4652. [DOI] [PubMed] [Google Scholar]
- 87.Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of beta-amyloid- and LPSinduced cytokine secretion from human macrophages and microglia. J Neuroimmunol. 2004;147:56–61. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 89.Muhl H, Hofler S, Pfeilschifter J. Inhibition of lipopolysaccharide/ATP-induced release of interleukin-18 by KN-62 and glyburide. Eur J Pharmacol. 2003;482:325–328. doi: 10.1016/j.ejphar.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 90.Tonetti M, Sturla L, Giovine M, Benatti U, De FA. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-alpha in lipopolysaccharide-treated RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- 91.Guerra AN, Fisette PL, Pfeiffer ZA, Quinchia-Rios BH, Prabhu U, Aga M, Denlinger LC, Guadarrama AG, Abozeid S, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- 92.Kaufmann A, Musset B, Limberg SH, Renigunta V, Sus R, Dalpke AH, Heeg KM, Robaye B, Hanley PJ. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem. 2005;280:32459–32467. doi: 10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- 93.Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 94.Nakanishi M, Takihara H, Minoru Y, Yagawa K. Extracellular ATP itself elicits superoxide generation in guinea pig peritoneal macrophages. FEBS Lett. 1991;282:91–94. doi: 10.1016/0014-5793(91)80451-8. [DOI] [PubMed] [Google Scholar]
- 95.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–458. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- 97.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 98.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216:1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 102.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 103.la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715–1722. doi: 10.1182/blood.v99.5.1715. [DOI] [PubMed] [Google Scholar]
- 104.Horckmans M, Marcet B, Marteau F, Bulte F, Maho A, Parmentier M, Boeynaems JM, Communi D. Extracellular adenine nucleotides inhibit the release of major monocyte recruiters by human monocyte-derived dendritic cells. FEBS Lett. 2006;580:747–754. doi: 10.1016/j.febslet.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 105.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 106.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol. 2000;30:2394–2403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 107.Ferrari D, Gorini S, Callegari G, la Sala A. Shaping immune responses through the activation of dendritic cells’ P2 receptors. Purinergic Signal. 2007;3:99–107. doi: 10.1007/s11302-006-9024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 109.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 110.Vitiello L, Gorini S, Rosano G, la SA. Immunoregulation through extracellular nucleotides. Blood. 2012;120:511–518. doi: 10.1182/blood-2012-01-406496. [DOI] [PubMed] [Google Scholar]
- 111.Miller JS, Cervenka T, Lund J, Okazaki IJ, Moss J. Purine metabolites suppress proliferation of human NK cells through a lineage-specific purine receptor. J Immunol. 1999;162:7376–7382. [PubMed] [Google Scholar]
- 112.Yoneda O, Imai T, Nishimura M, Miyaji M, Mimori T, Okazaki T, Domae N, Fujimoto H, Minami Y, Kono T, Bloom ET, Umehara H. Membrane-bound form of fractalkine induces IFN-gamma production by NK cells. Eur J Immunol. 2003;33:53–58. doi: 10.1002/immu.200390007. [DOI] [PubMed] [Google Scholar]
- 113.Umehara H, Bloom E, Okazaki T, Domae N, Imai T. Fractalkine and vascular injury. Trends Immunol. 2001;22:602–607. doi: 10.1016/s1471-4906(01)02051-8. [DOI] [PubMed] [Google Scholar]
- 114.Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- 115.Burnstock G. Vessel tone and remodeling. Nat Med. 2006;12:16–17. doi: 10.1038/nm0106-16. [DOI] [PubMed] [Google Scholar]
- 116.Bolovan-Fritts CA, Spector SA. Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction. Blood. 2008;111:175–182. doi: 10.1182/blood-2007-08-107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saito H, Ebisawa M, Reason DC, Ohno K, Kurihara K, Sakaguchi N, Ohgimi A, Saito E, Akasawa A, Akimoto K. Extracellular ATP stimulates interleukin-dependent cultured mast cells and eosinophils through calcium mobilization. Int Arch Allergy Appl Immunol. 1991;94:68–70. doi: 10.1159/000235327. [DOI] [PubMed] [Google Scholar]
- 118.Ferrari D, la SA, Panther E, Norgauer J, Di VF, Idzko M. Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J Leukoc Biol. 2006;79:7–15. doi: 10.1189/jlb.0505286. [DOI] [PubMed] [Google Scholar]
- 119.Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttmann W, Virchow C Jr, Di VF, Norgauer J. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–978. [PubMed] [Google Scholar]
- 120.Idzko M, Dichmann S, Panther E, Ferrari D, Herouy Y, Virchow C Jr, Luttmann W, Di VF, Norgauer J. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188:329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 121.Idzko M, Panther E, Bremer HC, Sorichter S, Luttmann W, Virchow CJ Jr, Di VF, Herouy Y, Norgauer J, Ferrari D. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br J Pharmacol. 2003;138:1244–1250. doi: 10.1038/sj.bjp.0705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Myrtek D, Idzko M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 2007;3:5–11. doi: 10.1007/s11302-006-9032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 124.Sak K, Boeynaems JM, Everaus H. Involvement of P2Y receptors in the differentiation of haematopoietic cells. J Leukoc Biol. 2003;73:442–447. doi: 10.1189/jlb.1102561. [DOI] [PubMed] [Google Scholar]
- 125.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 126.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 127.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 128.Freyer DR, Boxer LA, Axtell RA, Todd RF 3rd. Stimulation of human neutrophil adhesive properties by adenine nucleotides. J Immunol. 1988;141:580–586. [PubMed] [Google Scholar]
- 129.Oryu M, Sakamoto H, Ogawa Y, Tanaka S, Sakamoto N. Effects of released products from platelets on neutrophilic adhesion to endothelial cells and nylon fibers. J Leukoc Biol. 1996;60:77–80. doi: 10.1002/jlb.60.1.77. [DOI] [PubMed] [Google Scholar]
- 130.Akbar GK, Mills DC, Kunapuli SP. Characterization of extracellular nucleotide-induced Mac-1 (alphaM beta2 integrin) surface expression on peripheral blood leukocytes. Biochem Biophys Res Commun. 1997;233:71–75. doi: 10.1006/bbrc.1997.6396. [DOI] [PubMed] [Google Scholar]
- 131.von AM, Palmetshofer A, Kaczmarek E, Koziak K, Stroka D, Grey ST, Stuhlmeier KM, Robson SC. Extracellular ATP and ADP activate transcription factor NF-kappa B and induce endothelial cell apoptosis. Biochem Biophys Res Commun. 1998;248:822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 132.Goepfert C, Imai M, Brouard S, Csizmadia E, Kaczmarek E, Robson SC. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 133.Ward PA, Walker BA, Hagenlocker BE. Functional consequences of interactions between human neutrophils and ATP, ATP gamma S, and adenosine. Ann N Y Acad Sci. 1990;603:108–118. doi: 10.1111/j.1749-6632.1990.tb37665.x. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Palmblad J, Fredholm BB. Biphasic effect of ATP on neutrophil functions mediated by P2U and adenosine A2A receptors. Biochem Pharmacol. 1996;51:957–965. doi: 10.1016/0006-2952(95)02403-4. [DOI] [PubMed] [Google Scholar]
- 135.Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351–360. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 136.Aziz KA, Zuzel M. Regulation of polymorphonuclear leukocyte function by platelets. Saudi Med J. 2001;22:526–530. [PubMed] [Google Scholar]
- 137.Kaneider NC, Mosheimer B, Reinisch N, Patsch JR, Wiedermann CJ. Inhibition of thrombininduced signaling by resveratrol and quercetin: effects on adenosine nucleotide metabolism in endothelial cells and platelet-neutrophil interactions. Thromb Res. 2004;114:185–194. doi: 10.1016/j.thromres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 138.Tuluc F, Bredetean O, Brailoiu E, Meshki J, Garcia A, Dun NJ, Kunapuli SP. The priming effect of extracellular UTP on human neutrophils: Role of calcium released from thapsigarginsensitive intracellular stores. Purinergic Signal. 2005;1:359–368. doi: 10.1007/s11302-005-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Flaherty JT, Cordes JF. Human neutrophil degranulation responses to nucleotides. Lab Invest. 1994;70:816–821. [PubMed] [Google Scholar]
- 140.Meshki J, Tuluc F, Bredetean O, Ding Z, Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am J Physiol Cell Physiol. 2004;286:C264–C271. doi: 10.1152/ajpcell.00287.2003. [DOI] [PubMed] [Google Scholar]
- 141.Zalavary S, Grenegard M, Stendahl O, Bengtsson T. Platelets enhance Fc(gamma) receptor-mediated phagocytosis and respiratory burst in neutrophils: the role of purinergic modulation and actin polymerization. J Leukoc Biol. 1996;60:58–68. doi: 10.1002/jlb.60.1.58. [DOI] [PubMed] [Google Scholar]
- 142.Miyabe K, Sakamoto N, Wu YH, Mori N, Sakamoto H. Effects of platelet release products on neutrophilic phagocytosis and complement receptors. Thromb Res. 2004;114:29–36. doi: 10.1016/j.thromres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 143.Kudo F, Nishiguchi N, Mizuike R, Sato H, Ito K, Nakano M, Ito K. Neutrophil phagocytosis is down-regulated by nucleotides until encounter with pathogens. Immunol Lett. 2012;144:24–32. doi: 10.1016/j.imlet.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 144.Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6:601–608. doi: 10.3201/eid0606.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 147.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 148.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood. 2008;111:3062–3069. doi: 10.1182/blood-2007-02-071910. [DOI] [PubMed] [Google Scholar]