Abstract
Clear cell carcinoma of the endometrium (CCC) is an uncommon histotype whose analyses have generally been hampered by its rarity and issues of interobserver diagnostic variability. In this study, we analyzed the clinicopathologic features of 50 CCCs that were assembled from multiple institutions and which we considered to be morphologically unambiguous after a rigorous review process for diagnostic accuracy. Forty-four (88%) of the 50 CCC cases showed an admixture of the classic architectural patterns (glandular, papillary, solid and cystic in decreasing order of prevalence). Mitotic indices were variable but were generally low: 60% of cases had a mitotic index of 3 or lower. The predominant cell type lining glands and papillae was invariably hobnail and/or cuboidal. Stratification of nuclei (greater than 3 cells) or columnar cells on glands and papillae were uncommon and never diffusely present. 82% of cases showed an admixture of polygonal cells with clear and eosinophilic cytoplasm; only clear cells were present in 4% and only eosinophilic cells were present in 10%. Hobnail cells were common, being identifiable in 86% of cases, and being diffuse in 60%. Only 2 cases had a predominance of nuclear grade 3 cells. Psammoma, hyaline and targetoid bodies were identified in 32%, 52% and 20% of cases respectively. Clear cell endometrial intraepithelial carcinoma was identified in 41.7% of cases with evaluable background endometrium. The 5-year progression free survival (PFS) for the entire cohort was 61%, and was 88%, 75%, 22% and 28.6% for stages I to IV respectively. On univariate analyses, age >65 years, advanced FIGO stage, and the presence of any lymph node metastases were associated with reduced PFS (p=0.02, 0.002, and 0.002 respectively). On multivariate analyses, the only variable associated with reduced PFS was age >65 years. The 5-year overall survival (OS) for the entire cohort was 78%, and was 94%, 87.5%, 66.7%, and 42.8% for stages I to IV respectively. On univariate analyses, the following factors were associated with reduced OS: age >65 years (p=0.04), advanced FIGO stage (p=0.003), distant metastases (p=0.003), myometrial invasion >30% (p=0.01), a mitotic index >4 (p=0.014), and a specific architectural pattern (at least 10% of the tumor composed of solid masses or individual infiltrating tumor cells, p=0.02). On multivariate analyses, only age >65 years and advanced stage were associated with reduced OS (p=0.023 and 0.022 respectively). In summary, endometrial CCC has a wide morphologic spectrum that is detailed and illustrated herein, but also has core cytoarchitectural features that are of high diagnostic utility. Morphologically unambiguous CCC apparently have patient outcomes that are more favorable than has previously been reported, indicating that ambiguous tumors should be classified separately. The existence of morphologically ambiguous clear-cell rich carcinomas that do not fit the conventional histotypic groupings, is a likely reflection of the biologic complexity of endometrial carcinomas in general; these cases should be reported descriptively, and studied separately from conventional CCC.
Keywords: Clear cell carcinoma, endometrium, morphologic features
Introduction
Endometrial carcinoma represents the most frequently diagnosed malignancy of the female genital tract in the United States [1]. Most endometrial carcinomas are of the endometrioid histotype, are uterus-confined at presentation, and as such, are associated with favorable patient outcomes [2]. However, non-endometrioid carcinomas are associated with much poorer outcomes. This necessitates that the pathologic classification of cases be accurate and reproducible, as erroneous classifications in either direction may have significant clinical consequences [3]. Endometrial clear cell carcinoma (CCC) is an uncommon histotype of endometrial carcinoma that represented only 2.15-3% of endometrial cancers in two population-based analyses [2,4]. Patients with CCC have a lower overall and disease-free survival than patients with endometrioid carcinomas [2], and these differences are accordingly reflected in the more aggressive adjuvant treatment recommendations for patients with CCC [5]. Since its description, reports on CCC have been associated with notable levels of clinicopathologic heterogeneity and disparity on several levels, including the proportion of endometrial carcinomas that CCC represents [6], patient outcomes [5] and their molecular features [7]. For example, in 5 series on CCC, the proportion of cases with another admixed histotype ranged widely from 14 to 72% [8-12]. A possible contributor to this heterogeneity is the significant interobserver variability in how pathologists classify endometrial carcinomas with clear cells [13]. This suggests an absence of well-defined diagnostic criteria, poor delineation of its morphologic spectrum and exclusion criteria, suboptimal adherence to diagnostic criteria by practitioners, some combination of the above, or other factors.
The seminal descriptions of the morphology of endometrial CCC were largely based on the then-well known features of their ovarian counterparts [14,15], in keeping with the general principle that carcinoma histotypes arising from different anatomic derivatives of the mullerian duct exhibit broadly similar histologic features irrespective of location. However, this assumption may obscure the recognition of subtle location-related differences, and serve as an impediment to the definition of the true phenotypic spectrum of the histotype at each location. Serous carcinomas are exemplary of a histotype where location-related differences may exist, as there are known differences in the morphologic spectrum, immunophenotypic profiles and rate/pattern of coexistence with other histotypes between extrauterine high grade serous carcinomas and endometrial serous carcinomas, [15-19]. It is also possible that simple anatomy-related differences between the adnexa and endometrium, and/or their different hormonal environments, facilitate and select for clear cell carcinomas of the different phenotypic and molecular profiles at either location, their similar morphologic features notwithstanding. Therefore, morphologic profiles that are primarily defined by direct extrapolations from ovarian clear cell carcinoma, may over- or understate the pathologic spectrum of endometrial CCC.
In this study, we analyzed the clinical and pathologic features of a group of rigorously reviewed endometrial CCCs to 1) precisely define the morphologic spectrum of this tumor and to contribute data on the frequencies of various classic, new or previously underemphasized morphologic features, and 2) assess patient outcome data in relation to pathologic features.
Materials and methods
Case selection and central review
This study was approved by the institutional review board (IRB) at Vanderbilt University (IRB #12606), and was based on a review of archived pathologic material from the files of the authors’ institutions. Contributors, all gynecologic pathologists, were asked to contribute morphologically unequivocal cases of endometrial CCC, which they all retrieved by searching their respective computerized pathologic databases for cases signed out as CCC. As such, the cases received 2 levels of pre-central review, including the original assessment and the secondary assessment by contributors. An “original dataset” of 62 cases was thus generated. These cases were then subjected to a central review process by a panel of three authors (OF, JH, VP). Each of the three authors independently examined a representative tumor slide from all 62 cases, and classified each as CCC or non-CCC without any study-specific, agreed-upon criteria. The prerequisite for inclusion of a case into the final dataset, established prior to the review, was that a CCC diagnosis must be agreed upon by at least 2 of the 3 reviewers for every case. The kappa value for the central review of 62 cases was 0.846, indicative of an excellent level of interobserver agreement. 12 cases were removed as not representing true and/or pure CCC, which left a final dataset for subsequent analyses that was comprised of 50 cases. In 7 of the 12 cases that were ultimately removed, all 3 panelists agreed with a non-CCC classification. All cases were diagnosed between 2000 and 2011. Basic clinical data were retrieved from pathology reports and medical records for these patients, including patient age, nature of surgical procedures, neoadjuvant and adjuvant treatments, sites of recurrences, and patient status at follow-up.
Review of morphologic features
All cases were assessed for a variety of morphologic features as outlined below. In 5 cases only biopsy slides were available for review, whereas sections from the resected primary tumor were evaluated in the remainder. A median of 3 tumor-representative slides per case were reviewed (range 1 to 16), but only 1 tumor-representative slide was available in 17 (34%) of the 50 cases. For each case, the presence and extent of architectural patterns of CCC were documented (glandular, papillary, cystic, and solid). In this study, the traditional “tubulocystic” pattern was divided into “glandular” and “cystic” groups for analytic purposes. Since the line between glandular and cystic is non-discrete, an arbitrary cut-off of 0.1 mm luminal diameter was established; formations exceeding this diameter were classified as cystic and all others were classified as glandular. The papillary areas were further subclassified into 2 groups 1) small and round papillae (SRP, i.e. rounded or oval with stromal cores that typically occupied more than 50% of the diameter of the papillae, and which was typically (but not invariably) lined by approximately 25 cuboidal or hobnail epithelial cells or less), and 2) papillary pattern not otherwise specified (which included any papillary pattern that could not be classified as SRP). Papillae were also classified based on the nature of their cores (hyalinized papillae, inflammatory papillae). Where present, architectural patterns were also quantified as follows: <33% of tumor (“minor”), 33 to 66% of tumor (“significant”), >66% of tumor (“predominant”). Cytologic features were assessed in detail, including an approximate clear to eosinophilic cell ratio for polygonal cells, presence and extent of hobnail cells, the nature of non-hobnail polygonal cells (columnar versus cuboidal) that lined papillae and glands, the presence and extent of nuclear/cellular stratification (greater than 3 cells) on glands and papillae, the presence and extent of cells with nucleolomegaly, and nuclear grade. Nuclear grade was assessed on a previously described 3-tiered scale centered on pleomorphism [13]: grade 1 nuclei show broad uniformity in size and shape, without any bizarre nuclear forms, grade 2 nuclei show an approximately 2-fold variation in nuclear shape and/or size, and grade 3 nuclei show severe pleomorphism, i.e. a ≥3-fold variability in nuclear shape and/or size, all as compared with the background tumor cells. For example, if a tumor was composed of cells displaying no more than a 1-fold variation in nuclear size and shape, all its constituent cells were classified as possessing grade 1 nuclei. The presence of any higher grade nuclei was noted. The “predominant” nuclear grade describes the nuclear grade of at least 50% of tumoral nuclei of a case. Other features that were documented included the nature of the tumoral stroma (inflammatory, hyalinized, myxoid, or non-specific: “fibroblastic”), psamomma bodies, targetoid bodies, open tumor rings, hyaline bodies, mitotic figures per 10 high power fields (HPF), assessed in the most mitotically active region (HPF: 40X objective/0.55 mm in diameter), background endometrium and any lesions therein. We also assessed the significance of a grading system, designated the “Architectural grade”, that has previously been proposed for ovarian CCC by Yamamoto et al [21]. The architectural grading system is a three-tiered system that is based solely on tumor architecture. Grade A tumors have ≥90% of a tumor composed of well-differentiated tubulocystic and/or papillary patterns; Grade C tumors have at least 10% of the tumor composed of solid masses or individual infiltrating tumor cells; Grade B tumors do not fit either of the aforementioned descriptions. This grading system has been found to be predictive of survival in ovarian CCC, with grade A being the most, and grade C being least prognostically favorable groups [21]. Information on the depth of myometrial invasion, presence of endometrial polyps, and lymphovascular invasion status were all retrieved from pathologic reports when these parameters were not evident on the reviewed slides.
Statistical analysis
Descriptive statistics were used to characterize the data. Kaplan-Meier survival curves were generated, and comparison between survival curves was done using log- rank tests to determine overall and progression/recurrence/relapse-free survival; Cox regression analyses were used to assess relationships between clinicopathologic factors and outcome using multivariate and univariate models. Univariate analyses using Fisher exact, Pearson Chi square, and Student t tests were also used to compare between subgroups depending on the variable being analyzed. A 2-tailed p value of <0.05 was considered to be statistically significant for all analyses.
Results
A. Pathologic features
i. Architectural patterns
Forty-four (88%) of the 50 CCC cases showed an admixture of architectural patterns. 6 (12%), 20 (40%), 21 (42%) and 3 (6%) cases had 1, 2, 3, and 4 architectural patterns respectively. In 40 cases, there was a predominant pattern. The 4 architectural patterns are detailed below. Cytological, architectural, and other morphologic features are illustrated in Figures 1, 2, 3, 4, 5, 6, 7, 8 and are outlined in Tables 1 and 2.
Figure 1.

Morphologic spectrum of the glandular pattern. A. confluent and small irregular glands; B. Non-confluent, large round glands lined by cuboidal cells; C. Confluent glands lined by flat to hobnail cells; D. Non-confluent, large round glands lined by cuboidal cells with eosinophilic cytoplasm; E. closed tubules; F. Glands with early intraglandular papillations (asterix); G. compressed glands in inconspicuous lumens; H. A rare findings of glands with nuclear stratification.
Figure 2.

Morphologic spectrum of the papillary pattern (small rounded papillae). A. small rounded papillae arisingfrom large fibrous stems; B. small rounded papillae arising from thin fibrous stems; C. small rounded papillae protrudingwithin a cystic unit; D. small rounded papillary pattern maintained in a focus of myometrial invasion; E. smallrounded papillae with non-specifically fibroblastic stromal cores lined by hobnail cells; F. small rounded papillae withhyalinized stroma lined by hobnail cells; G. small rounded papillae with inflammatory cores and lined by low cuboidaleosinophilic cells; H. open tumor rings (asterix) and small rounded papillae lined by clear cells.
Figure 3.

Other papillary patterns. A. micropapillary pattern; B. elongated rounded papillae with fibrous cores andlined by low cuboidal epithelium; C. complex, arborizing papillary pattern with minimal stromal cores and lined byhobnail cells; D. papillae with inflamed stromal cores and lined by clear cells; E. A rare focus of cellular stratification(asterix) on papillae; F. Variably sized papillary units with fibrous stromal cores lined by hobnail cells.
Figure 4.

Morphologic spectrum of the solid pattern. A. Solid patterns of predominantly eosinophilic cells with well-definedcell membranes and no more than a 2 fold variation in nuclear size and shape; B. solid pattern of clear cellswith well-defined cell membranes and scattered grade 3 nuclei; note thin fibrous septae; C. solid pattern of admixedclear and eosinophilic cells with well-defined cell membranes and rare grade 3 nuclei D. solid pattern of clear cellswith numerous grade 3 nuclei; thin fibrous septae are present; E. solid pattern of cells with poorly defined cell membranesand scattered grade 3 nuclei; F. solid pattern of cells with poorly defined cell membranes and no more thana 2 fold variation in nuclear size and shape.
Figure 5.
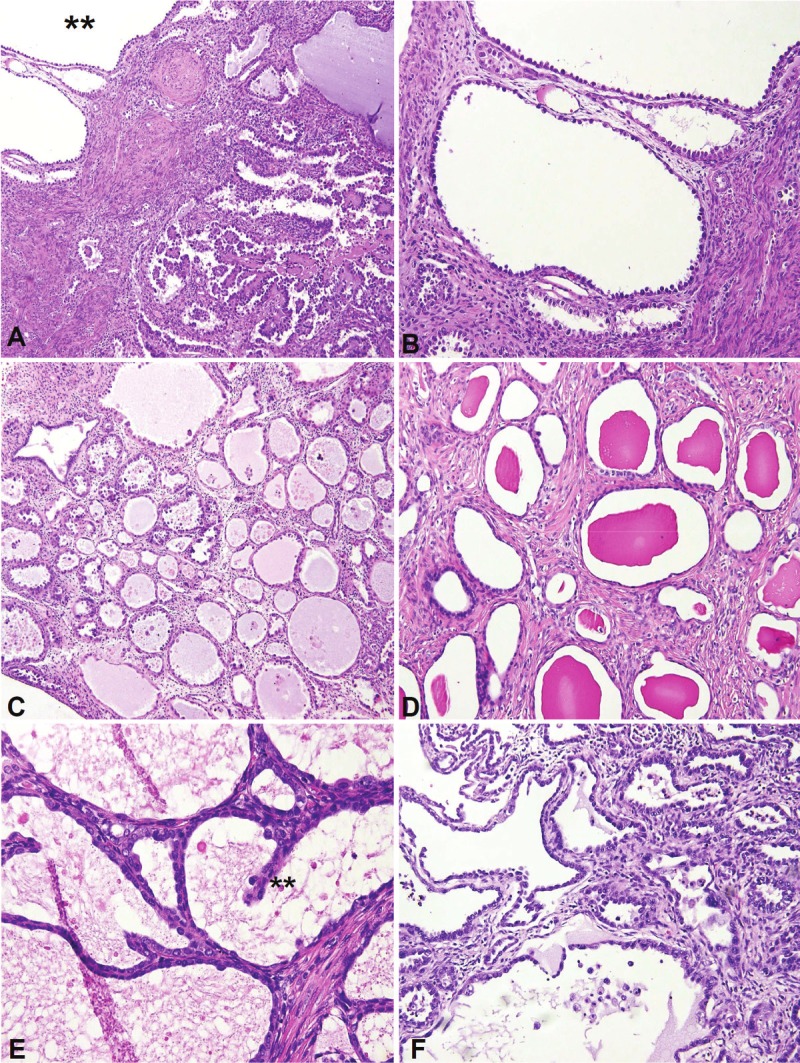
Morphologic spectrum of the cystic pattern, A. All cases with the cystic pattern (double asterix) also hadother architectural patterns; these patterns were admixed with the cysts in most cases; B. Non-confluent roundcysts lined by hobnail cells; C. Partially confluent round cysts lined by flat, hobnail and eosinophilc cells and withamphophilic luminal contents; D. Non-confluent round cysts with eosinophilic luminal contents, and lined by flatcells; E. Confluent cysts lined by low cuboidal epithelium and with foci of intracystic filiform papillations (doubleasterix); F. Irregularly contoured cysts.
Figure 6.

Cytologic features, A. Admixture of clear and eosinophilic cells showing relative nuclear uniformity andnucleolomegaly; B. Focus of clear cells with scattered grade 3 nuclei; C. Glands and papillae were predominantlylined by low cuboidal or hobnail cells, a rare focus lined by columnar cells is shown (long arrow); arrowheads depictexamples of higher grade nuclei relative to background cells; D. pattern of relatively uniform background cells withscattered admixed higher grade nuclei (arrwheads).
Figure 7.

Other features of clear cell carcinoma: A. Background stromal hyalinization; B. Background stromal inflammation;C. Hyaline bodies (arrow); D. targetoid bodies (arrow head); E. psammoma bodies (arrow); F. clear cell carcinomaassociated with an endometrial polyp; G. Myometrial invasion with stromal reaction; H. myometrial invasionwithout stromal reaction.
Figure 8.

Clear cell intraepithelial carcinoma and associated lesions. A. Intraepithelial carcinoma, with a surfacegrowth pattern on atrophic endometrium; B. Boxed inset from Figure 8A, showing hyperchromatic nuclei; C. intraepithelialcarcinoma, including foci (left panel) that are one-cell thick; D. Gland that is only partially involved byintraepithelial carcinoma; E. Intraepithelial carcinoma, characterized by an isolated cluster of non-confluent glandsand cysts lined by hobnail and eosinophilic cells; F. Boxed inset from Figure 8E.
Table 1.
Morphologic Features, Architectural features and background stroma
| Pathologic Features | Number of cases with feature |
|---|---|
| Glandular pattern | |
| -Present | 42 |
| -Predominant | 15 |
| -Significant | 12 |
| -Minor | 15 |
| -Absent | 8 |
|
| |
| Papillae | |
| -Present | 36 |
| -Predominant | 14 |
| -Significant | 8 |
| -Minor | 14 |
| -Absent | 14 |
| Small rounded papillae present | 26 |
| -Predominant/Significant proportion of papillae | 23 |
| -Minor proportion of papillae | 3 |
| -with stromal hyalinization | 18 |
| -without stromal hyalinization | 8 |
| Papillary pattern NOS (other than small rounded papillae) are present | 35 |
|
| |
| Solid Pattern | |
| -Present | 27 |
| -Predominant | 9 |
| -Significant | 7 |
| -Minor | 11 |
| -Absent | 23 |
|
| |
| Cystic Pattern | |
| -Present | 16 |
| -Predominant | 2 |
| -Significant | 3 |
| -Minor | 11 |
| -Absent | 34 |
|
| |
| Frequency of Architectural Patterns and Pattern Combinations | |
| Exclusively papillary pattern | 3 |
| Exclusively solid pattern | 1 |
| Exclusively glandular pattern | 2 |
| Exclusively cystic pattern | 0 |
| Glandular and papillary patterns only | 7 |
| Glandular and papillary and solid patterns only | 12 |
| Glandular and papillary and solid and cystic patterns only | 3 |
| Glandular and papillary and cystic patterns only | 7 |
| Glandular and solid patterns only | 5 |
| Glandular and cystic patterns only | 4 |
| Papillary and solid patterns only | 4 |
| Papillary and cystic patterns only | 0 |
| Solid and cystic patterns only | 0 |
| Papillary and Solid and cystic patterns only | 0 |
| Glandular and solid and cystic patterns present | 2 |
|
| |
| Background stromal inflammation | |
| -Diffuse | 9 |
| -Sporadic or focal | 12 |
| -Absent | 29 |
|
| |
| Background stromal Hyalinization | |
| -Diffuse | 3 |
| -Sporadic or focal | 12 |
| -Absent | 35 |
|
| |
| Background stromal myxoid change | |
| -Diffuse | 3 |
| -Sporadic or focal | 3 |
| -Absent | 44 |
Table 2.
Morphologic Features, Cytologic and other features
| Pathologic Features | Number of cases with feature |
|---|---|
| Predominant cell type lining glands and papillae | |
| - polygonal cuboidal and/or hobnail | 49 (100%) |
| - columnar | 0 (0%) |
| - Flat | 0 (0%) |
|
| |
| Cytoplasmic tincture | |
| -Exclusively clear cells | 2 |
| -Exclusively eosinophilic cells | 5 |
| -Both (clear>eosinophilic) | 19 |
| -Both (eosinophilic>clear) | 13 |
| -Both (approximately equal) | 11 |
|
| |
| Nuclear stratification(>3 cells) in glands and papillae | |
| -Diffuse | 0 |
| -Sporadic or focal | 3 |
| -Absent or N/A | 46 |
|
| |
| Nuclear pleomorphism | |
| Predominant nuclear grade 1 | 0 |
| Predominant nuclear grade 2 | 48 |
| Predominant nuclear grade 3 | 2 |
| Grade 1 nuclei are present | 22 |
| Grade 2 nuclei are present | 50 |
| Grade 3 nuclei are present | 40 |
|
| |
| Nucleolar prominence | |
| -Diffuse | 3 |
| -Sporadic or focal | 31 |
| -Absent | 16 |
|
| |
| Hobnail cells | |
| -Diffuse | 30 |
| -Sporadic or focal | 13 |
| -Absent | 7 |
| Necrosis | 19 |
| Psammoma bodies | 16 |
| Targetoid bodies | 10 |
| Hyaline bodies | 26 |
| Open tumor rings | 12 |
|
| |
| Mitotic Figures per 10 high power fields | |
| 0 | 6 |
| 1 | 14 |
| 2 | 7 |
| 3 | 6 |
| 4 | 4 |
| 5 | 4 |
| ≥6 | 9 |
| Mean | 4.42 |
| Median | 2 |
| Range | 0-32 |
|
| |
| Lymphovascular Invasion | |
| Present | 22 |
| Absent | 27 |
| Unknown | 1 |
|
| |
| Depth of Myometrial Invasion | |
| 0% (non-myoinvasive) | 8 |
| Greater than 0% but less than 50% | 21 |
| Greater than 50% | 20 |
| Unknown | 1 |
|
| |
| Associated with an Endometrial Polyp | |
| Yes | 6 |
| No | 33 |
| Unknown | 11 |
1. Glandular: The glandular pattern was the most commonly encountered, and was present at least focally in 84% of cases, was the predominant pattern in 30%, and was the exclusive pattern in 4%. There was a wide morphologic spectrum associated with the glandular pattern, and only vaguely recurring themes emerged (Figure 1A-H). In 27 of the 42 cases with a glandular pattern, the glands were not entirely confluent in most areas of the tumor, with individual glandular units being separated by stroma, or tumor cells of other architectural configurations. The glands were predominantly rounded, but in 7 cases were either irregularly shaped (Figure 1A) or had such a “compressed” appearance that their lumens were inconspicuous (Figure 1G). Glands ranged from small rounded units to large, ectatic, almost cystic lesions (Figure 1B, C, D). Luminal contents, where present, were eosinophilic to amphophillic. Closed tubules were admixed with open tubules in large areas of the tumor in 4 cases (Figure 1E). The glands were lined by either flat, hobnail or low cuboidal polygonal cells. Intraglandular papillations were present, typically as a focal finding, in 7 cases, all of which had a coexistent papillary pattern (Figure 1F). Nuclear stratification was present as a focal finding in 1 case (Figure 1H). Mitotic activity, cellular cytoplasmic tincture, and cytologic changes were as described below for the overall group.
2. Papillary: The papillary pattern was present in 36 (72%) of 50 cases, and was the exclusive pattern in 3 cases. Small rounded papillae (SRP), as previously described (Figure 2A-H), was the most common papillary pattern, as they were present in 26 (72%) of the 36 cases with papillae. All of the 26 cases, however, also had other papillary patterns (papillary pattern NOS). SRP were seen as end tributaries of larger, fibrous-cored stem papillae (Figure 2A), direct protrusions into round cystic/glandular spaces (Figure 2C), or tributaries from elongated slender papillae (Figure 2B). The SRP pattern was maintained in the foci of myometrial invasion only when they were extensive in the tumor (Figure 2D). Stromal hyalinization (Figure 2F) was present in 18 (69%) of SRP, but they were only focally present in 15, and in no case did hyalinized papillae constitute more than a third of the SRP. Open tumor rings (Figure 2H), which were identified in 12 cases, were strongly associated with hyalinized SRP, as all cases with open tumor rings also had hyalinized SRP nearby. Intermediate forms were also present that suggest that open tumor rings are hyalinized SRP at an early phase. Non-hyalinized SRP displayed inflamed stroma, myxoid stroma or most commonly, non-specifically fibroblastic stroma singly or in varying combinations. The predominant cell type lining SRP was hobnail in 20 cases, low cuboidal eosinophilic cells in 4 cases, and low cuboidal clear cells in the remainder. Papillary patterns NOS patterns were miscellaneous (Figure 3A-E), and included architecturally complex papillae with hierarchical branching or micropapillae, long and slender papillae (i.e. maze-like papillae, each >0.5 mm in length, fibrovascular core-laden, multiple times as long as it was wide), simple and complex thin papillae with variably prominent fibrous cores, and various other formations lined by hobnail, polygonal clear and/or eosinophilic cells (Figure 3A-D, Figure 3F) and other non-specific papillary patterns. Notably, no case showed the kind of significant cellular tufting or budding that is characteristic of serous carcinomas. For the overall group of papillary lesions, nuclear stratification was present in two cases (Figure 3E), and significant papillary stromal inflammation was present in 9 cases (Figure 2G). Mitotic activity and nuclear pleomorphism for the papillary pattern was as described below for the whole group.
3. Solid: The solid pattern, characterized by diffuse sheets of cells without any architectural patterns (Figure 4A-F), was present at least focally in 27 (54%) of 50 cases, but was predominant in 9, and was the exclusive pattern in only 1 case. Eosinophilic cells and clear cells were in approximately equal proportions in 9 cases; clear cells were predominant or exclusive in 15 cases, and eosinophilic cells were predominant or exclusive in the remaining 3. The mean mitotic index of cases with a solid component (6.56) was higher than cases without a solid component (2.7, p=0.028). Cell membranes were well-defined in 25 (92.6%) of the 27 cases. For cases with grade 3 nuclei, these cells were most clearly discernable, and displayed the highest degree of clustering, in the solid regions. Thin fibrous septae were diffusely distributed throughout the solid regions in 19 (70%) of 27 cases.
4. Cystic: The cystic pattern was the least common (Figure 5A-F). Although it was at least focally present in 16 (32%) of the 50 cases, it was predominant in only 2 (4%), and was not the exclusive pattern in any case. In 14 cases, the cystic units were interspersed with other architectural patterns, but small foci exclusively comprised of cystic units were present in 8 cases; the units were confluent in 5 (62.5%) of these 8 cases. The predominant cell type lining cystic units was flat in 5 cases, low cuboidal in 4 cases, and hobnail in the remainder. 14 (87.5%) of 16 cases, however, showed an admixture of these cell types. Mitotic figures were exceptionally rare, as were grade 3 nuclei. Cyst lumens were entirely empty in 7 cases and were at least focally filled with an eosinophilic material in 9 cases. The cysts mostly had rigid contours but occasionally were irregular, and few showed focal intracystic filiform papillations.
ii. Cytological features
Cytologic features exhibited less heterogeneity than architectural features, but the cases still displayed a substantial spectrum (Figures 1, 2, 3, 4, 5 and 6). A distinct pattern of nuclear pleomorphism was evident in 80% of cases. This pattern was characterized by a background of nuclei that display an approximately 2-fold variation in nuclear size and shape, and sporadically interspersed higher grade nuclei. This background of predominant grade 2 nuclei, with their relative uniformity in nuclear attributes, was present in all 50 cases (Figure 6A). However, in 40 cases there were interspersed grade 3 nuclei that were characterized by bizarre nuclear shapes, florid hyperchromasia, and notable enlargement relative to the background nuclei. The latter nuclei were variable in extent, but at least one was typically evident in most high power fields; however, they generally did not cluster to any significant extent and did not form diffuse sheets, with the exception of 2 cases where the predominant nuclear grade was 3 (Figure 6B). The scattered nature of the relatively enlarged cells gave the tumors an overall pleomorphic appearance at low or intermediate magnification.The greatest degree of clustering of these cells, when present, tended to be in the solid regions (Figures 4D, 6B). The 10 cases (20%) without these cells had a distinctly low grade appearance. In most cases (62%), nucleoli were prominent only focally or sporadically within the tumor, and both the pattern and extent of nucleolomegaly was variable from case to case. Regarding cellular cytoplasmic tincture, most cases (82%) showed an admixture of cells with clear and eosinophilic cytoplasm; only clear cells were present in 4% and only eosinophilic cells were present in 10%. Hobnail cells were common, being identifiable in 86% of cases, and being diffuse in 60% of cases. The predominant cell type lining glands and papillae was invariably hobnail and/or cuboidal. Columnar cells were identifiable in 24% of the 49 cases with glands and/or papillae (Figure 6C), but in no case was it predominant. Stratification of nuclei (greater than 3 cells) on glands and papillae were very uncommon (seen as a minor finding in 3 cases, Figures 1H, 3E), and entirely absent in all others.
iii. Mitotic index
The cases displayed significant variability in their mitotic indices, which ranged from 0 to 32 (median 2). The 9 most mitotically-active cases showed an average mitotic index of 15. However, in general, mitotic indices were low: 60% (30) of the 50 cases had a mitotic index of 3 or lower. In 6 (12%) of the 50 cases, mitotic figures were not discernable in the sections examined, but no more than 3 sections were available for review in all 6 cases. As previously noted, higher mitotic indices were associated with the presence of a solid component of any extent, as there was a statistically significant difference (p=0.028) between the average mitotic index of the cases with a solid pattern (6.56) and the cases without a solid pattern (2.7). Finally, there was a striking variability in the distribution of mitotic figures, with extraordinarily low mitotic indices on some slides, and higher indices in other slides of the same case.
iv. Miscellaneous “bodies”
Psammoma bodies (Figure 7E) were identified in 16 (32%) of 50 cases, but were numerous (>3/tissue section) in only one case. There was no discernable association between the presence of psammoma bodies and any architectural pattern; notably, psammoma bodies were present in 13 of the 36 cases with any papillary pattern as compared with 3 of 14 cases without a papillary pattern (p=0.5). Hyaline bodies (Figure 7C) were identified in 26 cases (52%) and targetoid bodies (Figure 7D) in 10 (20%). These bodies showed an apparent association: both were identified in 10 cases, and there was no case where a targetoid body was identified without a coexistent hyaline body elsewhere in the tumor. Neither exhibited any statistically significant associations with tumor architectural patterns. All of the aforementioned bodies were almost invariably identified in viable tissues and in direct association with tumoral epithelium (rather than tumoral stroma).
v. Other features
The patterns of myometrial invasion by the tumor, when present, were not noteworthy, with the exception of 2 cases in which there was no stromal reaction (Figure 7H). Six cases were associated with an endometrial polyp (Figure 7F). Background stromal inflammation (Figure 7B) and hyalinization (Figure 7A) were present in 42% and 30% of cases respectively, but these findings were extensive in 43% and 20% of those cases in which they were present. Among the 21 cases with background stromal inflammation, the infiltrate was lymphoplasmacytic in 18 cases, predominantly neutrophilic in 2 cases, and mixed in 1 case.
vi. Background endometrium and clear cell endometrial intraepithelial carcinoma
The non-neoplastic, background endometrium was available for evaluation in 24 cases, and this endometrium was immediately adjacent to the invasive cancer in 14 cases. The background endometrium in 70.8% (17/24) of cases was atrophic (AE); 4 were had a weakly proliferative pattern (WPE, i.e. non-cystic glands lined by pseudostratified columnar cells without mitotic activity), and 3 had a proliferative phase pattern (PE, i.e. non-cystic glands lined by pseudostratified columnar cells with mitotic activity). An intraepithelial growth pattern [Clear cell endometrial intraepithelial carcinoma [22,23]], characterized by endometrial surface growth (over benign endometrium), isolated (from the main malignancy) glands lined partially or wholly by cells that were identical (cytologically and in mitotic indices) to the associated invasive malignancy, or rare clusters of glands that exhibited no significant confluence, was identified in 41.7% (10/24) of cases (Figure 8A-F). These included 7 of the 17 atrophic endometria, 1 of the 3 proliferative pattern endometria, and 2 of the 4 weakly proliferative pattern endometria. The foci of intraepithelial carcinoma were multiple in 14 cases and focal in 11. These foci were characteristically in the region adjacent to the invasive malignancy, and displayed varying degrees of stratification and papillation.
B. Clinical features and patient outcomes
Clinical information regarding patient age, stage distribution, treatments and follow-up are summarized in Table 3.
Table 3.
Clinical features
| Clinicopathologic Parameters | Values |
|---|---|
| Patient Age At Diagnosis (years) | |
| Mean | 67.8 |
| Median | 67 |
| Range | 50-85 |
|
| |
| Surgical/Pathologic Stage Distribution | |
| IA | 17 |
| IB | 1 |
| II | 8 |
| IIIA | 6 |
| IIIB | 1 |
| IIIC | 7 |
| IV* | 9 |
| 1A (pT0: no residual malignancy in resection) | 1 |
|
| |
| Lymphadenectomy | |
| Only pelvic lymph nodes obtained | 10 |
| Only paraaortic lymph nodes obtained | 0 |
| Both pelvic and paraaortic lymph nodes obtained | 23 |
| No lymph nodes obtained | 7 |
| Lymphadenectomy status unknown | 10 |
| Lymph nodes positive for metastatic carcinoma | 10 |
| Pelvic nodes only positive | 6 |
| Paraaortic nodes only positive | 2 |
| Both pelvic and paraortic lymph nodes positive | 2 |
|
| |
| Treatment | |
| Chemotherapy only (no surgery) | 1 |
| Neoadjuvant chemotherapy, SR, adjuvant chemotherapy | 1 |
| TH/BSO with adjuvant chemotherapy and EBRT | 3 |
| TH/BSO with adjuvant chemotherapy and EBRT and VBT | 0 |
| TH/BSO with adjuvant chemotherapy and VBT | 6 |
| TH/BSO with VBT and EBRT | 3 |
| TH/BSO with VBT only | 2 |
| TH/BSO with EBRT only | 7 |
| TH/BSO with adjuvant chemotherapy only | 10 |
| TH/BSO without further treatment | 7 |
| Unknown | 10 |
|
| |
| Follow-up | |
| Number with follow up | 43 |
| Follow-up duration, Median/Mean/Range (months) | 31/21/1-104 |
| No evidence of Disease | 25 |
| Dead of Disease | 9 |
| Alive with Disease | 8 |
| Dead of other causes | 1 |
| Number of cases with recurrences/relapses | 11 |
|
| |
| Recurrences/Relapses | |
| Number of patients with recurrences/relapses | 11 |
| Duration to relapse | 1-27 months (mean 11.2 months) |
| Original Stage distribution for cases with relapses | III (n=8), I (n=2), II (n=1) |
| Relapse sites | Vagina (n=2), pleura, inguinal/groin region (n=2), supraclavicular lymph node, kidney, bone (n=2), abdominal soft tissue, lungs |
Stage assigned by FIGO (international Federation of Gynecology and Obstetrics criteria); TNM designation pT0 (assigned by 2009 American Joint Cancer Committee criteria);
Stage in one case (a stage IV patient ) was assigned clinically; TH/BSO: total hysterectomy and bilateral salpingo-oophorectomy; VBT vaginal cuff brachytherapy; EBRT external beam radiotherapy.
i. Survival
The mean/median follow-up duration for stages I, II, III and IV patients were 39.5/36 months, 41/30.5 months, 19/15 months and 14.1/14 months respectively. The PFS for the entire cohort at 5 years was 61%, and was 88%, 75%, 22% and 28.6% for stages I to IV respectively (Figure 9). On univariate analyses, age greater than 65 years, extrauterine disease (FIGO stages III and IV), and the presence of any lymph node metastases were associated with reduced PFS (p=0.02, 0.002, and 0.002 respectively). There was a trend to reduced PFS for patients with architectural grade C [21], but this was not statistically significant (p=0.09). On multivariate analyses, the only variable associated with reduced PFS was patient age greater than 65 years (Table 4). The overall survival for the entire cohort at 5 years was 78%, and was 94%, 87.5%, 66.7%, and 42.8% for stages I to IV respectively. On univariate analyses, the following factors were associated with reduced overall survival: age >65 years (p=0.04), extrauterine disease (FIGO stages III and IV, p=0.003), distant metastases (p=0.003), myometrial invasion >30% (p=0.01), a mitotic index of greater than 4 (p=0.014), and architectural grade C (p=0.02) (Figure 10). The presence of any relapses also approached statistical significance regarding an association with reduced overall survival (p=0.07). On multivariate analyses, only age >65 years and advanced FIGO stage were associated with reduced overall survival (p=0.023 and 0.022 respectively), see Table 5. The significance of stage was maintained when using a dichotomous classification (FIGO stages I and II versus III and IV) as well as when each stage is considered in isolation relative to the others.
Figure 9.

Progression free (left panel) and Overall (right panel) survival curves for the entire cohort.
Table 4.
Relationship of Clinicopathologic factors and Progression Free Survival
| Parameter | Number of patients | Survival (months) Median ± SE | 95% CI | P value* (univariate) | P value* (multivariate) |
|---|---|---|---|---|---|
| Age | |||||
| > 65 years | 25 | 70.6 ± 9.3 | 52.2 – 88.8 | 0.02 | 0.02 |
| ≤ 65 years | 18 | 75.6 ± 10.7 | 54.7 – 96.5 | ||
|
| |||||
| FIGO stage | |||||
| 1 and II | 26 | 91.2 ± 7.1 | 77.3 – 104.8 | 0.002 | NS |
| III and IV | 17 | 16.5 ± 2.0 | 12.5 – 20.5 | ||
|
| |||||
| Architectural pattern In >50% of tumor | |||||
| Glandular | 17 | 71.8 ± 10.5 | 51.2 – 92.5 | NS | NS |
| papillary | 12 | 64.4 ± 15.8 | 33.3 – 95.4 | ||
| Solid | 10 | 37.4 ± 3.4 | 30.5 – 44.1 | ||
| cystic | 4 | 60.3 ± 18.5 | 24.1 – 96.6 | ||
|
| |||||
| Lymph nodes | |||||
| positive | 8 | 13.7 ± 2.8 | 8.2 - 19.1 | 0.002 | NS |
| negative | 29 | 84.1± 8.1 | 96.1- 101.4 | ||
|
| |||||
| Lymphovascular Invasion | |||||
| positive | 20 | 70.1 ± 11.1 | 48.4- 91.8 | NS | NS |
| negative | 22 | 76.4 ± 8.9 | 58.9-93.9 | ||
|
| |||||
| Myometrium invasion | |||||
| >30% | 19 | 61.7 ± 11.1 | 40.1 – 83.3 | NS | NS |
| ≤30% | 24 | 83.9 ± 9.1 | 66.1 – 101.7 | ||
|
| |||||
| Mitotic index | |||||
| >4 | 12 | 68.9 ± 13.4 | 42.7 – 95.2 | NS | NS |
| ≤4 | 31 | 77.3 ± 7.6 | 61.3 – 91.2 | ||
|
| |||||
| Architectural grade** | |||||
| A+B | 24 | 77.1 ± 9.4 | 58.6 – 95.5 | 0.09 | NS |
| C | 19 | 32.6 ± 3.6 | 25.6 – 39.6 | ||
NS: not statistically significant(all p values between 0.05 and 0.09999 are listed); CI confidence interval; SE standard error; FIGO international Federation of Gynecology and Obstetrics.
Architectural grade per Yamamoto et al criteria [21].
Figure 10.

Assessment of an architectural grading system. Lower overall survival for patients with architectural grade C.
Table 5.
Relationship of Clinicopathologic factors and Overall Survival
| Parameter | Number of patients | Overall Survival (months) Median ± SE | 95% CI | P value* (univariate) | P value (multivariate) |
|---|---|---|---|---|---|
| Age | |||||
| > 65 years | 25 | 85.2 ± 11.7 | 62.2 – 108.7 | 0.04 | 0.023 |
| ≤ 65 years | 18 | 59.7 ± 9.5 | 41.1 – 78.2 | ||
|
| |||||
| FIGO stage | |||||
| 1 and II | 26 | 90.2 ± 7.4 | 75.6 – 104.7 | 0.003 | 0.022 |
| III and IV | 17 | 32.6 ± 6.7 | 19.9 – 45.3 | ||
|
| |||||
| Architectural pattern In >50% of tumor | |||||
| Glandular | 17 | 71.6 ± 10.2 | 51.5 – 91.7 | NS | NS |
| papillary | 12 | 76.6 ± 13.6 | 49.8 – 103.3 | ||
| Solid | 10 | 36 ± 4.33 | 27.5 – 44.5 | ||
| cystic | 4 | 52.4 ± 15.5 | 22.1 – 82.6 | ||
|
| |||||
| Lymph nodes | |||||
| positive | 8 | 29.3 ± 5.4 | 18.7- 39.8 | NS | NS |
| negative | 29 | 85.3± 8.3 | 96.1- 101.4 | ||
|
| |||||
| Distant Metastases | |||||
| positive | 8 | 17.4 ± 2.4 | 12.7 – 22.1 | 0.003 | NS |
| negative | 35 | 82.5 ± 7.8 | 67.2 – 97.8 | ||
|
| |||||
| Lymphovascular Invasion | |||||
| positive | 20 | 69.9 ± 11.1 | 48- 91.7 | NS | NS |
| negative | 22 | 77.9 ± 9.5 | 59.2-96.6 | ||
|
| |||||
| Myometrium invasion | |||||
| >30% | 24 | 49.9 ± 10.5 | 29.4 – 70.5 | 0.01 | NS |
| ≤30% | 19 | 94.2 ± 6.6 | 81.1 – 107.2 | ||
|
| |||||
| Any Relapse | |||||
| positive | 11 | 42.5 ± 7.3 | 28.1 – 56.9 | 0.07 | NS |
| negative | 32 | 78.4 ± 8.4 | 61.9 - 94.7 | ||
|
| |||||
| Mitotic index | |||||
| >4 | 12 | 47.9 ± 12.9 | 22.8 – 73.2 | 0.014 | NS |
| ≤4 | 31 | 85.6 ± 8.6 | 68.8 – 102.4 | ||
|
| |||||
| Architectural grade** | |||||
| A+B | 24 | 82.3 ± 8.6 | 65.5 – 99.1 | 0.02 | NS |
| C | 19 | 38.7 ± 6.1 | 26.7 – 50.7 | ||
NS: not statistically significant (all p values between 0.05 and 0.09999 are listed for informational purposes); CI confidence interval; SE standard error; FIGO international Federation of Gynecology and Obstetrics.
Architectural grade per Yamamoto et al criteria [21].
ii. Recurrences
Eleven patients experienced a recurrence at an average of 11.2 months (range 1-27) after their primary surgical resections. Relapse sites included the vaginal apex (n=2), pleura, inguinal/groin region (n=2), supraclavicular lymph node, kidney, bone (n=2), abdominal soft tissue, and lungs. As expected, recurrences were significantly associated with stage: 70% of the recurring patients originally had extrauterine disease (Stage III or IV). There were 3 recurrences in patients with early stage disease. The latter group included a 55-year-old with a stage IA, non-myoinvasive, and non vascular-invasive tumor that recurred at the vaginal apex within 2 months of the original resection; also in the group was a 78-year-old with a stage 1A tumor that exhibited lymphovascular invasion and 30% myometrial invasion, and which recurred in the abdominal soft tissue at 9 months; the patient was DOD shortly thereafter. Morphologic features that may be predictive of recurrence in early stage cases could not be assessed since an adverse outcome was so infrequent in this group. There was no significant difference between the ages of the recurring (64.1 years) and the non-recurring (68.7) patients. These 2 groups also showed no significant differences regarding the frequency of the various architectural patterns, depth of myometrial invasion, mitotic index, depth of myometrial invasion and frequency of lymphovascular invasion. As previously noted, the presence of any positive lymph nodes was associated with an increased risk of recurrence on univariate analyses (p=0.002).
Discussion
Our previous analysis of clear cell-rich endometrial carcinomas suggested a lack of diagnostic reproducibility even among experienced gynecologic pathologists in cases with non-classical morphology [13]. A similar problem for ovarian clear cell carcinoma has largely been resolved by adoption of an immunophenotypic gold standard (hepatocyte nuclear factor 1-beta (HNF1-β) positive, estrogen receptor negative, progesterone receptor negative, WT1 negative and p53 wild type) [25]. No such gold standard has been validated for endometrial CCC, and HNF1-β immunoreactivity has proven to be suboptimally specific for the clear cell carcinoma histotype in the uterus [26-28]. As such, the diagnosis of CCC in the endometrium, and their distinction from other clear cell-rich, morphologically ambiguous carcinomas, may pose a significant challenge.
In this study, we have detailed the morphologic spectrum of a group of endometrial CCC cases that we considered to be morphologically unequivocal. By necessity, case selection by gynecologic pathologists and further selection via central review, with all their attendant potential limitations, served as gold standard. Our analysis showed that although CCC has a wider morphologic spectrum than has previously been reported, there are several cytoarchitectural features that assist with its diagnosis, especially when compared with the known morphologic profiles of serous and endometrioid carcinomas. The classic architectural patterns remain the diagnostic foundation for this histotype, but only when the appropriate cytologic features are concurrently present. The papillary and glandular patterns were the most frequently seen in our cohort. The papillary pattern has a near protean spectrum in CCC, but the small rounded papillary pattern as previously described was the most common papillary pattern. SRP were invariably coexistent with other papillary patterns but existed in varying amounts, which suggests that in a small biopsy unequivocal SRP may be entirely absent. Glandular patterns also showed a wide spectrum regarding shape, size, degree of confluence, and configuration of constituent units, and it was our impression that glandular patterns were in and of themselves not diagnostic. However, in both the papillary and glandular patterns, the cytological features of the lining cells were relatively consistent. These cells were flat, cuboidal or hobnail, and perhaps more importantly, were neither columnar nor stratified (more than 3 cells) in a significant proportion of any tumor. These cytologic features allowed the aforementioned architectural patterns to be classified as being CCC-related in the vast majority of cases, and should facilitate their distinction from the principal diagnostic consideration, endometrioid carcinoma, which are typically lined by columnar and stratified cells. The distinction of CCC from serous carcinomas, which may be lined by hobnail, columnar, cuboidal, stratified or non-stratified cells is clearly a greater diagnostic challenge when the former has a significant clear cell component. Differences in architectural patterns between these 2 lesions would probably be of more diagnostic utility in this scenario.
Solid proliferations of clear cells have previously been identified as a cause of interobserver diagnostic variability [13]. The constituent cells of most, but not all solid areas in this study had well defined membranes, but this was the only feature that was identified with a high level of consistency. Therefore, solid patterns should be evaluated within the context of other architectural patterns that may be present, as their features are not optimally diagnostic in isolation. Fortunately, in this study, the solid pattern was uncommon, representing the predominant pattern in only 18% of cases and the exclusive pattern in a single case. The cystic pattern is cytoarchitecturally noteworthy when present, but its diagnostic utility may be limited by the fact that it is uncommon. When present, it is highly suggestive of the diagnosis. In a small sample, cystic pattern CCC can be distinguished from cystic atrophy by the cytologic features, foci of confluence, and the invariable admixture with other patterns of CCC.
The distinctive pattern of nuclear pleomorphism is a useful adjunct to making the diagnosis of CCC, especially when the architectural pattern mimics another variant of endometrial carcinoma. This pattern, marked by isolated atypical nuclei in a background of “uniformly atypical grade 2 nuclei” contrasts with the florid pleomorphism that typifies serous carcinomas. Likewise, the relative low mitotic index may help bolster the diagnosis for CCC when making the distinction from serous carcinomas.
We report an overall survival of 78% and a PFS of 61% for patients of all stages. The OS was 94%, 87.5%, 66.7%, and 42.8 for patients with stage I, II, III and IV disease respectively. The corresponding PFS values were 88%, 75%, 22% and 28.6% respectively. These represent some of the highest survival figures ever reported for this histotype, as outlined in Table 6 [3,8,10,14,29-36]. Differences in patient population, sample sizes, detail of staging and improvements in adjuvant treatments, may account for these differences. However, we feel that a significant contributor to these differences is the variability amongst pathologists in the diagnosis CCC [13]. Our survival figures likely reflect our selection of morphologically unequivocal cases. In our experience, a diagnosis of CCC is frequently entertained not only due to the abundance of clear cells, but because of high levels of nuclear pleomorphism. Some of these cases likely represent poorly differentiated or undifferentiated endometrial carcinomas whose inclusion in datasets of CCC may serve to falsely decrease the overall survival for the entire group. These cases may be better reported descriptively (e.g “poorly differentiated carcinoma with clear cells” rather than “CCC”) to facilitate their segregation and analysis. Our cases, provide insight into the prognosis for patients with morphologically unambiguous CCC.
Table 6.
5-year Overall Survival rates for patients with CCC, limited to series with ≥15 patients**
| References | Overall survival (percentage) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | All stages | Stage I | Stage II | Stage III | Stage IV | |
| Kurman et al (1976) [14] | 21 | 55.3 | NA | NA | NA | NA |
| Christopherson et al (1982) [8] ¶ | 54 | 35.2 | 44 | NA | NA | NA |
| Webb and Lagios (1987) [30] | 29 | 64 | NA | NA | NA | NA |
| Kanbour-Shakir et al (1991) [29]* | 20 | 60 | NA | NA | NA | NA |
| Carcangiu and Chambers ¶(1995) [31] | 29 | NA | 72 | 59.2 | NA | NA |
| Malpica et al (1995) [36]¶ | 17 | NA | 75 | 67 | NA | NA |
| Abeler et al (1996) [10,35] | 181 | 46 | NA | NA | NA | NA |
| Thomas et al (2007) [34] | 99 | 55 | 79 | 77 | 47 | 21 |
| FIGO Data (Creasman et al, 2003) [32] ◙ | 173 | 62.5 | 85.1 | 66.7 | 48.5 | NA |
| FIGO Data (Creasman et al, 2004) [33] ◙ | 59 | NA | 81 | NA | NA | NA |
| SEER Data (Hamilton et al, 2006) [3] | 391 | 68 | NA | NA | NA | NA |
|
| ||||||
| Average | 97.5 (total 1073) | 55.75 | 72.7 | 53.32 | 47.8 | 21 |
| Current study | 50 | 78 | 94 | 87.5 | 66.7 | 42.8 |
Excludes any series with mixed carcinomas and without delineation of survival data for the histologically pure cases; excludes series of mixed clear cell/serous carcinomas; includes both surgically and clinically staged patients; includes patients that received a wide variety of treatments; SEER: Surveillance, Epidemiology and End Results; FIGO International Federation of Gynecology and Obstetrics;
crude overall survival;
Overlapping datasets; NA information not available or not applicable;
includes series of CCC with a minor (<50%) endometrioid carcinoma component.
We assessed a variety of clinicopathologic factors for potential prognostic significance. On multivariate analysis, only age and stage were significant prognostic factors, which is identical to the findings reported by Abeler et al [10] in the largest series of CCC reported to date. Our findings affirm the numerous studies that have previously found that stage is an important determinant of prognosis for CCC patients (Table 6). Age greater than 60 or 65 also appears to be an independently negative prognostic factor in both Type I and II endometrial carcinomas, including CCC [37-42]. Our dataset was heterogeneous regarding adjuvant treatments, which precluded any meaningful subsidiary analyses of the effects of surgical and treatment factors on outcome. However, in the only study that has specifically examined the issue in a substantial dataset, predictors of local control in advanced stage disease included absence of residual disease after cytoreduction, and chemotherapy. Adjusting for chemotherapy, absence of residual disease and adjuvant radiotherapy were predictive of PFS whereas the presence of residual disease was predictive of reduced OS [34].
The prognostic significance of pathologic factors is unclear and has previously been evaluated to varying extents by other authors in smaller datasets. Christopherson et al [8] assessed the significance of 3-tiered, nuclear pleomorphism-based grading system, and found no association with patient outcomes. The prognostic significance of nuclear grading in our dataset could not be assessed, since approximately 80% of cases had the same nuclear patterns. We found that a mitotic index of greater than 4 MF/10HPF was associated with reduced overall, but not progression free survival on univariate analyses, however Photopulus et al [43] reported no such association. Interestingly, in ovarian CCC, a mitotic index of less than 10 is one of the morphologic features that have been associated with a longer progression free survival [44]. The inter- and intra-tumor variability in mitotic index that is associated with CCC, as documented herein, indicates that any analyses of the significance of this parameter requires a sizable dataset.
Tumor architectural patterns represent another pathologic feature whose prognostic significance various authors have attempted to assess. No prognostic significance was identified by studies with either two-tier [31] or three-tier classification systems [43], although a small study did report a higher survival of patients with papillary patterns [8] In the ovary, more promising results have been found for architecture-based grading schemes [21,44-46]. When we tested an architectural grading system that was recently proposed by Yamamoto et al [21] for ovarian clear cell carcinoma on our dataset, grade C tumors were associated with reduced OS on univariate analysis, and reduced PFS that approached statistical significance (vs grades A and B combined). These findings suggest that grading may be feasible in endometrial CCC and should be evaluated in larger datasets.
In summary, although endometrial CCC has a wide morphologic spectrum, it has core cytoarchitectural features that are of high diagnostic utility. Awareness of the full morphologic spectrum of CCC, as well as how focal or extensive individual features may be within a given tumor, should allow an accurate diagnosis to be rendered in most cases. Morphologically unambiguous CCC have patient outcomes that are more favorable than has previously been reported, indicating that ambiguous tumors should be classified separately. The existence of morphologically ambiguous clear-cell rich carcinomas that do not fit the conventional histotypic groupings is probably a reflection of the biologic complexity of endometrial carcinomas in general [47,48]. These cases should be reported descriptively and studied separately from conventional CCC, as classifying such cases as CCC would probably contribute to the ongoing problems of diagnostic irreproducibility, biologic heterogeneity of CCC datasets, and accordingly, the contamination of clinical trials and translational research studies that in aggregate, impede advancements in understanding the histotype.
Acknowledgement
This work was supported by a Translational Research and Enhancement Award from the Department of Pathology, Microbiology and Immunology at Vanderbilt University Medical Center, and by a financial grant from the Ernest W. Goodpasture Endowed Professorship in Pathology, Microbiology and Immunology held by Cheryl M. Coffin, M.D. It was also supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. This study was presented in part at the United States & Canadian Academy of Pathology’s 102nd Annual Meeting, Baltimore, MD, March 2-8, 2013.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Uterine Neoplasms. Version 2.2012.; http://www.nccn.org; Accessed December 2011. [Google Scholar]
- 5.Olawaiye AB, Boruta DM 2nd. Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;113:277–283. doi: 10.1016/j.ygyno.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117–142. doi: 10.1097/00125480-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fadare O. The molecular pathogenesis of endometrial clear cell carcinoma: unclear, uncertain and possibly heterogeneous. Expert Rev Obstet Gynecol. 2012;7:109–112. [Google Scholar]
- 8.Christopherson WM, Alberhasky RC, Connelly PJ. Carcinoma of the endometrium: I. A clinicopathologic study of clear-cell carcinoma and secretory carcinoma. Cancer. 1982;49:1511–1523. doi: 10.1002/1097-0142(19820415)49:8<1511::aid-cncr2820490802>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Yamawaki T, Teshima H, Takeshima N, Yamauchi K, Hasumi K. [A clinicopathological study in clear cell adenocarcinoma of the endometrium] . Nihon Sanka Fujinka Gakkai Zasshi. 1996;48:328–34. [PubMed] [Google Scholar]
- 10.Abeler VM, Vergote IB, Kjørstad KE, Tropé CG. Clear cell carcinoma of the endometrium. Prognosis and metastatic pattern. Cancer. 1996;78:1740–1747. doi: 10.1002/(sici)1097-0142(19961015)78:8<1740::aid-cncr14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I-IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1272–1276. doi: 10.1016/s0360-3016(02)04404-8. [DOI] [PubMed] [Google Scholar]
- 12.Varughese J, Hui P, Lu L, Yu H, Schwartz PE. Clear cell cancer of the uterine corpus: the association of clinicopathologic parameters and treatment on disease progression. J Oncol. 2011;2011:628084. doi: 10.1155/2011/628084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadare O, Parkash V, Dupont WD, Acs G, Atkins KA, Irving JA, Pirog EC, Quade BJ, Quddus MR, Rabban JT, Vang R, Hecht JL. The Diagnosis of Endometrial Carcinomas With Clear Cells by Gynecologic Pathologists: An Assessment of Interobserver Variability and Associated Morphologic Features. Am J Surg Pathol. 2012;36:1107–1118. doi: 10.1097/PAS.0b013e31825dd4b3. [DOI] [PubMed] [Google Scholar]
- 14.Kurman RJ, Scully RE. Clear cell carcinoma of the endometrium: an analysis of 21 cases. Cancer. 1976;37:872–882. doi: 10.1002/1097-0142(197602)37:2<872::aid-cncr2820370236>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg SG, De Giorgi LS. Clear cell carcinoma of the endometrium. Clinical, pathologic, and ultrastructural findings. Cancer. 1973;31:1127–40. doi: 10.1002/1097-0142(197305)31:5<1127::aid-cncr2820310514>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Carcangiu ML, Chambers JT, Voynick IM, Pirro M, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: Clinical and histologic correlations. Am J Clin Pathol. 1990;94:247–254. doi: 10.1093/ajcp/94.3.247. [DOI] [PubMed] [Google Scholar]
- 17.Ordóñez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol. 2005;36:1163–1167. doi: 10.1016/j.humpath.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Bárcena C, Oliva E. WT1 expression in the female genital tract. Adv Anat Pathol. 2011;18:454–65. doi: 10.1097/PAP.0b013e318234aaed. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B, Huntsman DG. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis- associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, Tsuda H, Shimazaki H, Takano M, Yoshikawa T, Kuzuya K, Tsuda H, Kurachi H, Kigawa J, Kikuchi Y, Sugiyama T, Matsubara O. Histological grading of ovarian clear cell adenocarcinoma: proposal for a simple and reproducible grouping system based on tumor growth architecture. Int J Gynecol Pathol. 2012;31:116–124. doi: 10.1097/PGP.0b013e3182285c90. [DOI] [PubMed] [Google Scholar]
- 22.Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30:1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- 23.Moid F, Berezowski K. Pathologic quiz case: a 70-year-old woman with postmenopausal bleeding. Endometrial intraepithelial carcinoma, clear cell type. Arch Pathol Lab Med. 2004;128:e157–e158. doi: 10.5858/2004-128-e157-PQCAYW. [DOI] [PubMed] [Google Scholar]
- 24.Fadare O, Zheng W. Endometrial Glandular Dysplasia (EmGD): morphologically and biologically distinctive putative precursor lesions of Type II endometrial cancers. Diagn Pathol. 2008;3:6. doi: 10.1186/1746-1596-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLair D, Oliva E, Köbel M, Macias A, Gilks CB, Soslow RA. Morphologic spectrum of immunohistochemically characterized clear cell carcinoma of the ovary: a study of 155 cases. Am J Surg Pathol. 2011;35:36–44. doi: 10.1097/PAS.0b013e3181ff400e. [DOI] [PubMed] [Google Scholar]
- 26.Fadare O, Liang SX. Diagnostic Utility of Hepatocyte nuclear factor 1 beta immunoreactivity in endometrial carcinomas: Lack of specificity for endometrial clear cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:580–7. doi: 10.1097/PAI.0b013e31824973d1. [DOI] [PubMed] [Google Scholar]
- 27.Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 28.Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- 29.Kanbour-Shakir A, Tobón H. Primary clear cell carcinoma of the endometrium: a clinicopathologic study of 20 cases. Int J Gynecol Pathol. 1991;10:67–78. doi: 10.1097/00004347-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Webb GA, Lagios MD. Clear cell carcinoma of the endometrium. Am J Obstet Gynecol. 1987;156:1486–1491. doi: 10.1016/0002-9378(87)90021-4. [DOI] [PubMed] [Google Scholar]
- 31.Carcangiu ML, Chambers JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: comparison of clinicopathologic features and survival. Int J Gynecol Pathol. 1995;14:30–38. doi: 10.1097/00004347-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. Int J Gynaecol Obstet. 2003;83(Suppl 1):79–118. doi: 10.1016/s0020-7292(03)90116-0. [DOI] [PubMed] [Google Scholar]
- 33.Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–596. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Thomas M, Mariani A, Wright JD, Madarek EO, Powell MA, Mutch DG, Podratz KC, Dowdy SC. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma: a multi-institutional review. Gynecol Oncol. 2008;108:293–297. doi: 10.1016/j.ygyno.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Abeler VM, Kjørstad KE. Clear cell carcinoma of the endometrium: a histopathological and clinical study of 97 cases. Gynecol Oncol. 1991;40:207–217. doi: 10.1016/0090-8258(90)90279-t. [DOI] [PubMed] [Google Scholar]
- 36.Malpica A, Tornos C, Burke TW, Silva EG. Lowstage clear-cell carcinoma of the endometrium. Am J Surg Pathol. 1995;19:769–774. doi: 10.1097/00000478-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Vance S, Yechieli R, Cogan C, Hanna R, Munkarah A, Elshaikh MA. The prognostic significance of age in surgically staged patients with Type II endometrial carcinoma. Gynecol Oncol. 2012;126:16–9. doi: 10.1016/j.ygyno.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A, Zamba G, DeGeest K, Lynch CF. The impact of surgery on survival of elderly women with endometrial cancer in the SEER program from 1992-2002. Gynecol Oncol. 2008;111:35–40. doi: 10.1016/j.ygyno.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Jolly S, Vargas CE, Kumar T, Weiner SA, Brabbins DS, Chen PY, Floyd W, Martinez AA. The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol. 2006;103:87–93. doi: 10.1016/j.ygyno.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 41.Fader AN, Starks D, Gehrig PA, Secord AA, Frasure HE, O’Malley DM, Tuller ER, Rose PG, Havrilesky LJ, Moore KN, Huh WK, Axtell AE, Kelley JL, Zanotti KM UPSC Consortium. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2009;115:244–248. doi: 10.1016/j.ygyno.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 43.Photopulos GJ, Carney CN, Edelman DA, Hughes RR, Fowler WC Jr, Walton LA. Clear cell carcinoma of the endometrium. Cancer. 1979;43:1448–1456. doi: 10.1002/1097-0142(197904)43:4<1448::aid-cncr2820430435>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Crozier MA, Copeland LJ, Silva EG, Gershenson DM, Stringer CA. Clear cell carcinoma of the ovary: a study of 59 cases. Gynecol Oncol. 1989;35:199–203. doi: 10.1016/0090-8258(89)90043-7. [DOI] [PubMed] [Google Scholar]
- 45.Montag AG, Jenison EL, Griffiths CT, Welch WR, Lavin PT, Knapp RC. Ovarian clear cell carcinoma. A clinicopathologic analysis of 44 cases. Int J Gynecol Pathol. 1989;8:85–96. doi: 10.1097/00004347-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto S, Tsuda H, Shimazaki H, Takano M, Yoshikawa T, Kuzuya K, Tsuda H, Kurachi H, Kigawa J, Kikuchi Y, Sugiyama T, Matsubara O. Clear cell adenocarcinoma with a component of poorly differentiated histology: a poor prognostic subgroup of ovarian clear cell adenocarcinoma. Int J Gynecol Pathol. 2011;30:431–441. doi: 10.1097/PGP.0b013e3182165eba. [DOI] [PubMed] [Google Scholar]
- 47.Yeramian A, Moreno-Bueno G, Dolcet X, Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J, Matias-Guiu X. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2012 Mar 19; doi: 10.1038/onc.2012.76. doi: 10.1038/onc.2012.76. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Soslow RA. Endometrial carcinomas with ambiguous features. Semin Diagn Pathol. 2010;27:261–273. doi: 10.1053/j.semdp.2010.09.003. [DOI] [PubMed] [Google Scholar]


