Abstract
Cholesterol is a fundamental molecule for life. Located in the cell membrane, this sterol participates to the cell signaling of growth factors. Inside the cell it can be converted in hormones such as androgens or modulate the immune response. Such important functions could not be solely dependent of external supply by diet hence de novo synthesis could occur from acetate in almost all mammalian cells. If a deficiency in cholesterol sourcing leads to development troubles, overstocking has been associated to various diseases such as atherosclerosis and cancers. Cholesterol homeostasis should thus be tightly regulated at the uptake, de novo synthesis, storage and export processes. Various transcription factors have been described these last years as important to regulate cholesterol levels. Besides, synthetic molecules have been developed for many years to modulate cholesterol synthesis, such as statins. Many articles have associated prostate cancer, whose incidence is constantly increasing, to cholesterol disequilibrium. Targeting cholesterol could thus be a new pharmacological hit to counteract the initiation, development and/or progression of prostate cancer. Among the transcription factors regulating cholesterol homeostasis, the nuclear receptors Liver X Receptors (LXRs) control cholesterol uptake and export. Targeting the LXRs offers a new field of investigation to treat cancer. This review highlights the molecular relationships among LXRs, prostate cancer and cholesterol and why LXRs have good chance to be targeted one day in this tumor. LXRs, prostate cancer and cholesterol, more than a “Ménage à trois”, The Good, the Bad and the Ugly.
Keywords: LXR, cholesterol, prostate cancer, lipid raft, pharmacological modulation
Introduction
Prostate cancer is one of the most common malignancy [1], mainly affecting elders. Various risk factors have been involved including aging, ethnic origins, hormonal status and energy balance. Among the lipids, cholesterol has a particular position. This fundamental molecule is part of the cell membrane and thus plays an architectural role in its organization by maintaining the fluidity or by securing important proteins in the membrane when located in the socalled “lipid rafts”. Cholesterol is also involved in “ligand-type” signaling: as the precursor of androgen synthesis as well as in the production of oxysterols, which activate the nuclear receptors LXRα and LXRβ. Maintaining a tight regulation of cholesterol homeostasis is thus of primary importance since it could affect cell signaling and the proliferation/apoptosis balance. Reducing de novo cholesterol synthesis and/or uptake, or increasing reverse transport by exporting cholesterol from the cell could represent an efficient way to control prostate epithelial proliferation. This review is focused on the deleterious effect of a higher cholesterol (The Ugly) concentration on prostate cancer (the Bad) and the role of LXRs (The Good) in maintaining cholesterol homeostasis to avoid progression of prostate cancer (Figure 1). The Saga started in 1909 and is still going on.
Figure 1.
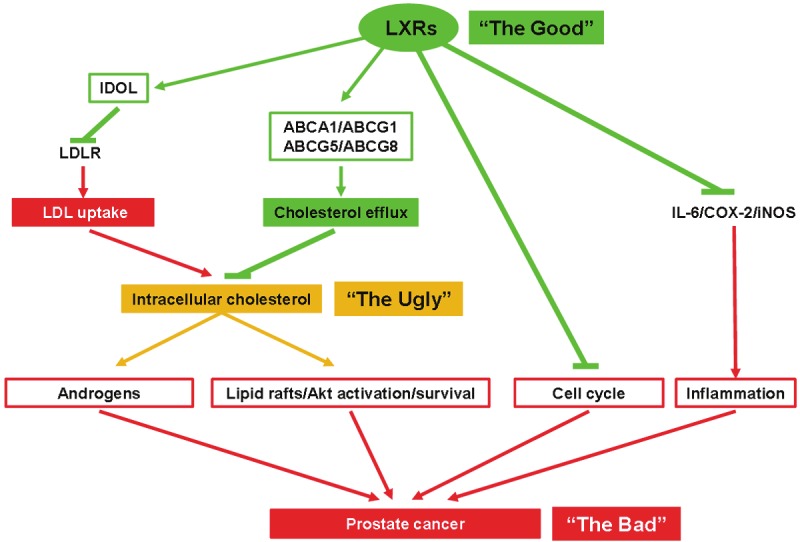
LXRs and prostate physiology: potential beneficial actions of LXRs over prostate cancer. LXR activity increases IDOL as well as various ABC transporters, which ultimately decreases LDL uptake and increases the efflux of cholesterol, altogether decreasing the intracellular pool of cholesterol. Consequently, this leads to the reduction of androgen synthesis and lipid raft/AKT/survival pathway. LXRs finally induce cell cycle arrests, and by inhibiting the expression of IL-6, COX-2 and iNOS limit the inflammation inside the tumor. Altogether, LXR activation may limit prostate cancer development.
LXRs and cholesterol: when the Good controls the Ugly
The liver X receptors
Liver X Receptors (LXRs) are transcription factors initially isolated in the liver [2,3], and activated by cholesterol derivatives, the oxysterols [4]. LXRα (NR1H3) and LXRβ (NR1H2) share 80% identity both in their DNA- and ligand-binding domains. Their structure is characteristic of the nuclear receptor superfamily, which possesses three functionally independent domains [5,6]. The N-terminal modulator domain contains an activating function of the transcription (AF1) independent from the presence of the ligand. This domain presents several putative sites of phosphorylation potentially important for LXR activity modulations [7,8]. The DNA-binding domain recognizes the LXR response elements (LXRE) characterized by two direct repeats of the hexanucleotide motif AGGTCA usually separated by four nucleotides. Part of this domain is also involved in the heterodimeri-sation with the Retinoid X Receptor RXR (NR2B1-3), which binds 9-cis retinoic acid, the requisite LXR partner [3]. The carboxy-terminal region is responsible for the ligand-binding and contains the AF2 region necessary for the transcriptional initiation of target genes [4]. This domain is masked by co-repressors in absence of ligand. For a review on LXR-functioning, see Viennois et al. 2011 [9].
LXRα and LXRβ are differentially expressed in tissues. While LXRβ expression is accepted to be rather ubiquitous, LXRα is more restricted and mainly found in liver, intestine, fat tissue, macrophages, kidney and gonads, suggesting their important function in the control of cholesterol homeostasis (for a view on LXR expression see www.nursa.org). The fundamental role of LXRs in lipid homeostasis is highlighted by the highly conserved function of these receptors among species [10], and has been continuously demonstrated since the first observation of a link between LXRα and cholesterol homeostasis by Peet et al. [11]. They observed that mice lacking LXRα and fed a high cholesterol diet rapidly accumulate large amount of cholesterol ester in the liver inducing a liver steatosis. Actually these mice are unable to sense and respond to dietary cholesterol and develop an impaired bile acid metabolism due to a default in the transcription of the cholesterol 7α-hydroxylase (Cyp7a1), encoding an enzyme essential in bile acid synthesis [11].
The discovery of the natural ligands of LXRs by Janowski et al. [4,12], largely improved our comprehension of the unique role of LXRs in controlling cholesterol homeostasis. In these studies, oxysterols, the natural derivatives of cholesterol, activated LXR at physiological concentrations. Following this finding the development of synthetic ligands of LXRs (e.g.T0901317 [13] and GW3965 [14]) and the generation of a mouse model lacking Lxrα and/or β, greatly contributed to the comprehension of the oxysterol/LXR dependent pathways in cells, and gave the opportunity to identify several target genes and therefore functions of the LXRs [9]. Thus, it has been admitted that LXR activities are associated with four schematic functions: 1) lipid metabolism, including cholesterol and fatty acids homeostasis; 2) steroidogenesis; 3) glucose homeostasis; 4) inflammation and immunity. Since in this review we will focus more specifically on the role of LXRs on cholesterol homeostasis, we will not develop further their other physiological functions. For more information about them, refer to Viennois et al. 2011 [9].
LXRs: two sensors of cholesterol homeostasis
Cholesterol is an essential structural component of mammalian cell membranes and is required to establish proper membrane permeability and fluidity. In addition cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acids, and vitamin D. Besides, this molecule is also part of the membrane signaling pathway by its specific distribution in lipid rafts (see above). Furthermore, cholesterol also functions in intracellular transport, cell signaling and nerve conduction. Hence, although cholesterol is important and necessary for human health, its intra- and extra-cellular concentrations have to be strictly controlled as high levels of cholesterol in the blood have been linked to damages to arteries and cardiovascular diseases.
Modulation of de novo synthesis and uptake of cholesterol
LXRs act at various levels to control the intracellular pool of cholesterol. The first possible source of cholesterol results from the enzymatic reaction leading to the transformation of Acetyl-CoA in mevalonate by the HMG-CoA reductase [15]. That reaction ultimately leads to the formation of de novo cholesterol. In mice lacking Lxr, higher expression of Srebp2, Hmgcoa and Squalene synthase has been observed [16], while the oral treatment of wild type mice with T0901317 led to a decrease in Hmgcoa synthase and Squalene synthase gene expression [17], suggesting a role of LXRs in the negative modulation of de novo cholesterol synthesis.
A second way to modulate the pool of intracellular cholesterol regards its cellular import via the LDL-receptor (LDLR). Even though a correlation was repeatedly observed between LXR activation and LDLR protein reduction, the mechanism has been described only recently. LXRs activate the expression of the E3 ubiquitin ligase Idol (Inducible Degrader of the LDLR), ultimately leading to the targeted degradation of LDLR, thus resulting in the reduction of the intracellular pool of cholesterol [18].
Induction of bile acid synthesis
Cyp7a1 is the first and rate limiting enzyme that catalyzes the initial step of bile acid biosynthesis from cholesterol. Although it is not the primary function of bile acid synthesis, this reaction also allows the liver to reduce in rodent the excess of cholesterol in cells. Interestingly, while in wild type mice fed a high cholesterol diet Cyp7a1 expression increases, this induction is not observable in Lxrα-deficient mice fed similarly [11]. Additionally, in these mice the diet induces a hepatic steatosis due to an accumulation of cholesteryl esters in the liver [11,16].
Induction of reverse cholesterol transport
The last way LXRs use to control cholesterol levels is by exporting it outside the cells. Indeed, several ATP-binding cassettes encoding genes such as ABCA1 [19-21] and ABCG1 [22] are LXR bona fide targets. These ABC transporters actively efflux cholesterol to the extracellular acceptor HDL and increase the reverse cholesterol transport. In addition, LXRs have also been shown to modulate Apolipoprotein E level, an essential component of the VLDL particles [23]. Furthermore, LXRs modulate the expression of the genes encoding ABCG5 and ABCG8 that export sterols from the inner compartment of hepatocytes to the bile duct [24,25] and from the enterocytes into the gut lumen [26]. Altogether LXRs demonstrate a critical role in controlling the amount of intracellular cholesterol and in its processing outside the cells.
Steroid synthesis
We and others have shown that LXRs could regulate the rate of cholesterol transformation into steroids in various tissues such as testis [27]. A decrease in the amount of circulating testosterone can be detected after LXR activation by the synthetic agonist T0901317 [28]. That well identified mechanism is dependent on the activation by LXRs of Sulfotransferase 2a1 that deactivates androgens, and the inhibition by LXRs of the steroidsulfatase that activates androgens [28]. Interestingly, those hormones have a key role in prostate cancer development. LXRs might thus have also a role to play in this part of the anticancer journey.
Cholesterol and prostate cancer: when the Ugly plays with the Bad
Due to its different roles, cholesterol is hence linked to cell proliferation (see above). Indeed, its synthesis increases in tissue with high proliferation rate such as in cancer. On the other side, inhibition of HMGCoA-reductase blocks cell growth [29].
Prostate cancer: the Bad at a glance
Prostate cancer (PCa) is the second most diagnosed cancer and a leading cause of cancer related death [1]. The incidence of PCa is constantly increasing due in part to new methods of diagnostic, and also to the increase in life expectancy. Indeed, this cancer has a slow evolution and about 85% of diagnosed PCa are in patients older than 65 years old [30]. Interestingly, it is accepted that more men die with PCa than from it. Indeed, an American study performed after autopsy determined that 50% of the men of 50 years old have latent PCa [31]. However the development and the cause of the disease is still poorly understood, and various factors such as genetic/ethnical origin, diet, life style and environmental factors have been suggested to play a role on it [32].
As already stated, great differences in the incidence of PCa are observed depending on the ethnical origin or the country of the patients. A Caucasian American has 30% less risk to develop a PCa compared to an African American [30], but at the same time Asians develop twice less PCa than Americans. These differences are in part due to the ethnical factors, and thus to the genetic background and the lifestyle of the individuals. However it could also show disparities in the accessibility of the diagnostic tests and treatments.
Yet, the genetic background cannot explain everything since the first generation of Asian migrants living in the US have a more important risk of PCa than those leaving in Asia [33]. This unexpected observation is credited to be due to factors acting on PCa development rather than on PCa initiation, and presumably on the higher lipid consumption in the USA [34]. Additionally a comparable observation has been done with increased incidence to develop a PCa for Japanese population that moved to America [35]. In this study the authors also compared the migrants according to their age at arrival, and did not find any correlation with the risk to develop PCa. They therefore concluded that PCa risk may be increased by late rather than early life style event [35]. These two studies are therefore suggesting a potential lifestyle/diet parameter that can greatly influence the development of PCa.
Role of cholesterol in prostate cancer: the Ugly goes with the Bad!
Cholesterol accumulation in tumors is not a recent observation. White demonstrated in 1909 an accumulation of crystals of lipid nature in tumors [36]. Later Swyer and his coworkers showed for the first time an increase of cholesterol content in zone of the prostate affected by a mild hypertrophy [37] compared to healthy tissues. Afterward, similar observations were obtained on other types of cancer [38-40]. Two mechanisms are generally put forward to explain this intracellular cholesterol accumulation: a higher circulating cholesterol uptake, and the increase in the accumulation of the enzymes of the mevalonate pathway [41,42]
Moreover, increased uptake of LDL particles and therefore exogenous cholesterol attributable to a loss of modulation in the LDL receptor expression, and a higher de novo cholesterol synthesis due to the upregulation of the HGM-CoA reductase, have been suggested as key components of that accumulation [17,43]. The final result of that process could potentially give sufficient bricks for the membrane to expand and to the tumor to grow and develop [44].
Diet, cholesterol and PCa
Since the late 90’s, multiple lines of evidence have been highlighting the potential influence of diet on PCa appearance. First, intake of products from animal origin is correlated to a higher risk of developing metastatic PCa, but not on the initial development of PCa [45] as shown by the identical prevalence between vegetarians or meat eaters [46]. Second, the presence of dietary fat in the diet was shown to be a risk factor of PCa, although the exact contribution of fat was not clearly established [47]. Third, an increase in PCa incidence, angiogenesis and metastasis was observed in the TRAMP mouse model of PCa fed a western-type diet [48]. Finally aggressiveness of PCa was increased in elders having important dietary fat intake [49]. So far data linking excessive consumption of cholesterol, rates of circulating cholesterol and risk of PCa have been controversial [50], even though studies suggest an impact of cholesterol in the development of high grade PCa [51-53]. Inversely, Platz et al. pointed out that a “weak” level of circulating cholesterol (< 200mg/dL) was associated with a reduction of the risk of developing a prostate cancer of high grade [54]. Finally, circulating cholesterol increases tumor size of LNCaP xenografts in a mouse model, as well as intratumoral synthesis of androgens [55]. This suggests that the androgen dependent tumor growth could be under a deep association with circulating cholesterol. Likewise, high serum HDL is inversely correlated with PCa [53,56]. Since HDL formation is dependent on the export of cholesterol via the ABCA1 and ABCG1 transporters that are under the positive modulation of LXRs, it could be suggested a possible beneficial role to over activating LXRs in PCa, even though this needs to be demonstrated.
Modulation of circulating cholesterol and PCa: when reasoning the Ugly can block the Bad
Altogether the presented data raise the question of the molecular mechanisms by which the cholesterol can favor tumor progression. Some observations on cancer development after treatment with statin, a cholesterol-lowering drug that specifically inhibits the HMG-CoA reductase and therefore the formation of de novo cholesterol, partially answer that question. Indeed early investigations suggested a potent growth inhibitory effect as well as an anticancer potential of statins in vitro and in vivo [57,58], partially explained by their ability of inducing apoptosis via the activation of the Caspase-7 [59]. Moreover in the PC3 prostate cancer cell line, statins also prevent the cell migration potential therefore reducing the formation of metastatic prostate colonies [60]. Then it seems that these cholesterol-lowering agents can act at different level on PCa progression. The potential use of statins to prevent PCa is currently under active investigation mostly on prospective studies. Until now, numbers of studies have been published and extensively reviewed [61]. Statin treatments do not seem to have any beneficial effect on the rate of appearance of prostate cancer conversely to the incidence of advanced PCa [62-64]. Interestingly this effect even increases when statins are used for more than five years [65].
Androgen synthesis is dependent on the amount of circulating cholesterol; besides, PCa is linked to androgen synthesis; moreover statins are cholesterol-lowering drugs. Altogether what could be the potential impact of statins on the hormonal status in prostate cancer? Actually, statins do not seem to decrease the circulating androgen [66], even though a decreased synthesis of androgens cannot be excluded since statins users show a decline in serum PSA levels, an androgen regulated gene in prostate [67].
Cholesterol is not only used as a precursor of steroid synthesis. Indeed, it can be found enriched in cell membranes in regions called rafts essential for the activation of the kinase cascade Akt and consequently for tumor survival [68]. Zhuang et al. showed that simvastatin decreases the cholesterol content of lipid rafts, leading to a decrease in Akt phosphorylation and activation, and subsequently to an increase of LNCaP cells apoptosis [69]. These results improve our comprehension of the mechanism of statins in cancer progression, and also suggest lipid rafts as new players in PCa development. In accordance with that suggestion, the essential component of lipid rafts caveolin 1 is associated with the aggressive ness of the PCa tumor and therefore considered as a marker of poor prognosis in PCa [70,71]. Accordingly, the use of an antibody targeting the caveolin 1 can block the metastatic process in PCa [72]. Both observations then confirm the important role of lipid rafts in PCa progression.
LXRs and prostate cancer: a benefic effect of the Good over the Bad?
LXR activation leads to cell cycle arrest in prostate cancer cell lines
Since LXRs control cholesterol homeostasis, these nuclear receptors have been considered as putative pharmacological targets in prostate cancer. Hence, activation of LXRs by natural (22 (R)-hydroxycholesterol, 24 (S)-hydroxycholesterol) or synthetic (T0901317) agonists led to cycle arrest of LNCaP cells via the lack of degradation of p27Kip1, an essential inhibitor of the cell cycle. Moreover, and as expected, treatment with LXR agonists also induced the protein accumulation of ABCA1, thus activating cholesterol efflux [73]. Conversely, targeted disruption of ABCA1 increases the proliferation rate of LNCaP cells [74]. Moreover, Chuu et al. observed that LXR-target genes were downmodulated during the tumor progression in mouse, while activation of LXRs by T0901317 delayed the progression of PCa [75]. Altogether, these studies are clearly in favor of an important protective role of LXRs in prostate cancer progression, even if no data are available in human yet.
How could LXRs be so good?
As presented above, activating LXRs will lead to the modulation of cholesterol concentration by their action on the various pathway involved.
LXRs antagonize the development of prostate tumor by interacting with the androgen pathway
Prostate cancer development is tightly associated with androgens. Indeed, it is frequent to treat PCa patients with anti-androgens in order to block the androgen response, and therefore the early development of PCa [76]. Interestingly, the androgen receptor (AR) modulates the expression of HMG-CoA synthase and reductase, and SREBP2, whose product controls genes involved in cholesterol homeostasis such as the LDL-Receptor (LDLR) [77]. The consequences of these modulations are: 1) an increase in intracellular cholesterol due to a higher de novo production and uptake via LDLR; 2) an increase in androgen synthesis from cholesterol. This may give an alternative explanation to the prostatic tumor growth dependence to cholesterol. Additionally, AR reduces LXR activation in prostate cancer, by competing for their coactivators [78].
There is also a mirror effect as LXRs reduce the proliferation of androgen-dependent cells via androgen deprivation [28], and inhibit tumor growth and slow down the passage of androgen dependent to androgen independent prostate cancer [75]. Furthermore T0901317 has also been suggested to act as an AR antagonist, even though the Kd found is highly questionable [79].
LXRs block cancer development through their transcriptional activity
Controlling the expression of key genes of cholesterol homeostasis is of primary importance to block cancer progression. Modulation of IDOL by LXRs [18] should decrease the amount of LDLR on the cell surface and then LDL uptake. Moreover as described previously, LXRs also modulate ABCA1 and ABCG1 two transporters responsible for the export of endogenous cholesterol. Associated to the crucial role of cholesterol on prostate cancer development, LXR activation thus reduces the potential pathogenicity of over accumulation of cholesterol, and therefore may limit the development of PCa.
LXRs induce apoptosis of prostate cancer cell line through lipid raft signaling
Activation of LXRs is associated with an important decrease of phosphorylation of Akt, a key player in the mechanism of cell survival, at the level of lipid rafts [68]. When LXRs are liganded, the pool of cholesterol is decreased in prostate cancer cells in parallel with lipid raft size and number. The consequences are a decrease in activated Akt and the induction of cells apoptosis [80,81]. Then the effect of LXR activation on lipid rafts and PCa cells is very similar to that observed after statin treatment, thus highlighting LXRs as a potential therapeutic target in PCa.
LXRs down-modulate inflammatory molecules correlated with cancer
Cancers are often associated with increased inflammation inside and surrounding the tumor [82]. This phenomenon is also characteristic of PCa, since a strong iNOS accumulation is found in tumor compared to peripheral tissue in PCa [83,84] and is associated with high Gleason score [83,85]. Associated to iNOS, COX-2, another pro-inflammatory enzyme, is highly expressed in tumor associated macrophages [86]. In the mouse model of prostate cancer TRAMP, Cox-2 expression increases with progression of carcinogenesis and the use of a COX-2 inhibitor increases the survival of mice with prostate cancer [87]. Additionally, IL-6, which promotes tumor growth [88], and activates the PI-3K/Akt signal transduction pathway [89], is highly expressed and associated with morbidity in PCa [90-92]. These observations are of particular interest since LXRs are known to down-modulate the accumulation of inflammatory molecules as iNOS, COX-2 and IL-6 [93], another argument making them good targets in PCa.
Pharmacologically targeting LXRs in PCa: Are the Good always trustable?
Considering the various beneficial effect of LXR activation on PCa ex vivo and in mouse models, using LXR modulators to treat patients seems very promising [9,94]. Unfortunately, most of the LXR agonists also have a consistent deleterious effect since they lead to transient hypertriglyceridemia ([95] and reviewed in [96]). The screening of new LXR-ligands is currently under active investigations to limit this inconvenience [97]. The first tissue specific LXR ligand identified has been the (22E)-ergost-22-ene-1α,2βdiol (YT-32). In mice, oral gavage with YT-32 decreased the amount of cholesterol present in the plasma and led to the intestinal accumulation of the LXR-target genes ABCA1, ABCG5 and ABCG8 without any modification of the expression of these genes in the liver [98]. More recently another intestine specific ligand of LXRs, GW6340, leads to LXR- target gene accumulation in the small intestine without increasing neither the liver triglycerides content or the hepatic LXR target genes expression [99]. Besides this side effect due to a hepatic activity of LXRs on the triglyceride synthesis, the fact that LXRs are also highly expressed in many tissues has to be taken into account. Hence, LXR-623 agonist was tested in healthy volunteers [100]. The authors showed a higher accumulation of the expression of ABCA1 and ABCG1 in blood cells in parallel with lowering the LDL and cholesterol levels in the serum [101]. Unfortunately, an important adverse effect on the central nervous system was observed, which ended the trial [100]. As for the other nuclear receptors targeted in breast (ER) and prostate (AR) cancers, the development of selective Liver X modulators (SLiM) [96] could be a very promising treatment option in numbers of different pathology where cholesterol is involved, including in cancer. Considering the potential important role of LXRs and cholesterol in prostate cancer, the use of SLiM may slow down the evolution into high grade PCa, although more investigations will be necessary.
Acknowledgments
Chester’s lab is supported by grants from Association de Recherche sur les Tumeurs Prostatiques, Ligue contre le Cancer (Allier committee), Fondation pour la Recherche Médicale (FRM), Fondation BNP-Paribas, Association pour la Recherche contre le Cancer (ARC) and Cancéropôle Lyon Rhône-Alpes Auvergne (CLARA). A. Pommier and J. Dufour were funded by MNERT and ARC grants. H. De Boussac was funded by Région Auvergne “Nouveau Chercheur” program.
Declaration of conflicts of interest
The authors have nothing to declare.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Teboul M, Enmark E, Li Q, Wikstrom AC, Pelto-Huikko M, Gustafsson JA. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci U S A. 1995;92:2096–100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–45. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 4.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 5.Williams S, Bledsoe RK, Collins JL, Boggs S, Lambert MH, Miller AB, Moore J, McKee DD, Moore L, Nichols J, Parks D, Watson M, Wisely B, Willson TM. X-ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine-tryptophan switch. J Biol Chem. 2003;278:27138–43. doi: 10.1074/jbc.M302260200. [DOI] [PubMed] [Google Scholar]
- 6.Hoerer S, Schmid A, Heckel A, Budzinski RM, Nar H. Crystal structure of the human liver X receptor beta ligand-binding domain in complex with a synthetic agonist. J Mol Biol. 2003;334:853–61. doi: 10.1016/j.jmb.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Bradley MN, Beaven SW, Tontonoz P. Phosphorylation of the liver X receptors. FEBS Lett. 2006;580:4835–41. doi: 10.1016/j.febslet.2006.07.074. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Shimano H, Inoue N, Nakagawa Y, Matsuzaka T, Takahashi A, Yahagi N, Sone H, Suzuki H, Toyoshima H, Yamada N. Protein kinase A suppresses sterol regulatory elementbinding protein-1C expression via phosphorylation of liver X receptor in the liver. J Biol Chem. 2007;282:11687–95. doi: 10.1074/jbc.M611911200. [DOI] [PubMed] [Google Scholar]
- 9.Viennois E, Pommier AJ, Mouzat K, Oumeddour A, El Hajjaji FZ, Dufour J, Caira F, Volle DH, Baron S, Lobaccaro JM. Targeting liver X receptors in human health: deadlock or promising trail? Expert Opin Ther Targets. 2011;15:219–32. doi: 10.1517/14728222.2011.547853. [DOI] [PubMed] [Google Scholar]
- 10.Reschly EJ, Ai N, Welsh WJ, Ekins S, Hagey LR, Krasowski MD. Ligand specificity and evolution of liver X receptors. J Steroid Biochem Mol Biol. 2008;110:83–94. doi: 10.1016/j.jsbmb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 12.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A. 1999;96:266–71. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, Plunket KD, Morgan DG, Beaudet EJ, Whitney KD, Kliewer SA, Willson TM. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–6. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 16.Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest. 2001;107:565–73. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41–5. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–4. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 20.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–30. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkateswaran A, Repa JJ, Lobaccaro JM, Bronson A, Mangelsdorf DJ, Edwards PA. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J Biol Chem. 2000;275:14700–7. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 23.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98:507–12. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JE, Basso F, Shamburek RD, Amar MJA, Vaisman B, Szakacs G, Joyce C, Tansey T, Freeman L, Paigen BJ, Thomas F, Brewer HB Jr, Santamarina-Fojo S. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J Biol Chem. 2004;279:22913–22925. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–70. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 26.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 27.Volle DH, Mouzat K, Duggavathi R, Siddeek B, Déchelotte P, Sion B, Veyssière G, Benahmed M, Lobaccaro J-MA. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol. 2007;21:1014–1027. doi: 10.1210/me.2006-0277. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Gong H, Khadem S, Lu Y, Gao X, Li S, Zhang J, Xie W. Androgen deprivation by activating the liver X receptor. Endocrinology. 2008;149:3778–88. doi: 10.1210/en.2007-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MS, Goldstein JL. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974;249:7306–14. [PubMed] [Google Scholar]
- 30.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 31.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150:379–85. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 32.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters DJ, Timms B. Human prostate cancer risk factors. Cancer. 2004;101:2371–490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 33.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161:152–5. [PubMed] [Google Scholar]
- 34.Watanabe M, Nakayama T, Shiraishi T, Stemmermann GN, Yatani R. Comparative studies of prostate cancer in Japan versus the United States. A review. Urol Oncol. 2000;5:274–283. doi: 10.1016/s1078-1439(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White C. The occurence of crystals in tumours. J Pathol Bacteriol. 1909;13:3–10. [Google Scholar]
- 37.Swyer G. The cholesterol content of normal and enlarged prostates. Cancer Res. 1942;2:372–375. [Google Scholar]
- 38.Dessi S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, Costelli P, Baccino FM, Aroasio E, Pani P. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–8. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Rudling M, Collins VP. Low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA levels are coordinately reduced in human renal cell carcinoma. Biochim Biophys Acta. 1996;1299:75–9. doi: 10.1016/0005-2760(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka Y, Sasaki J, Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and cholinecontaining lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13:377–83. doi: 10.1002/1099-1492(200011)13:7<377::aid-nbm658>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Graziani SR, Igreja FA, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, Amancio RF, Pinotti JA, Maranhao RC. Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol. 2002;85:493–7. doi: 10.1006/gyno.2002.6654. [DOI] [PubMed] [Google Scholar]
- 42.Tatidis L, Masquelier M, Vitols S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem Pharmacol. 2002;63:2169–80. doi: 10.1016/s0006-2952(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 43.Caruso MG, Notarnicola M, Santillo M, Cavallini A, Di Leo A. Enhanced 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human colorectal cancer not expressing low density lipoprotein receptor. Anticancer Res. 1999;19:451–4. [PubMed] [Google Scholar]
- 44.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 45.Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001;12:557–67. doi: 10.1023/a:1011256201044. [DOI] [PubMed] [Google Scholar]
- 46.Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang-Claude J, Frentzel-Beyme R, Kuzma JW, Mann J, McPherson K. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70:516S–524S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 47.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–28. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 48.Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, Frank PG. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol. 2010;177:3180–91. doi: 10.2353/ajpath.2010.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs EJ, Gapstur SM. Cholesterol and cancer: answers and new questions. Cancer Epidemiol Biomarkers Prev. 2009;18:2805–6. doi: 10.1158/1055-9965.EPI-09-1027. [DOI] [PubMed] [Google Scholar]
- 51.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kok DE, van Roermund JG, Aben KK, den Heijer M, Swinkels DW, Kampman E, Kiemeney LA. Blood lipid levels and prostate cancer risk; a cohort study. Prostate Cancer Prostatic Dis. 2011;14:340–5. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 53.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22:1545–52. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Kristal AR. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7:e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, Albanes D. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–21. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maltese WA, Defendini R, Green RA, Sheridan KM, Donley DK. Suppression of murine neuroblastoma growth in vivo by mevinolin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Clin Invest. 1985;76:1748–54. doi: 10.1172/JCI112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata MA, Kavanaugh C, Shibata E, Abe H, Nguyen P, Otsuki Y, Trepel JB, Green JE. Comparative effects of lovastatin on mammary and prostate oncogenesis in transgenic mouse models. Carcinogenesis. 2003;24:453–9. doi: 10.1093/carcin/24.3.453. [DOI] [PubMed] [Google Scholar]
- 59.Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, Denner L. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer Res. 1998;58:76–83. [PubMed] [Google Scholar]
- 60.Brown M, Hart C, Tawadros T, Ramani V, Sangar V, Lau M, Clarke N. The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer. 2012;106:1689–96. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy M, Kung HJ, Ghosh PM. Statins and prostate cancer: role of cholesterol inhibition vs. prevention of small GTP-binding proteins. Am J Cancer Res. 2011;1:542–61. [PMC free article] [PubMed] [Google Scholar]
- 62.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 63.Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U. S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 65.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 66.Hall SA, Page ST, Travison TG, Montgomery RB, Link CL, McKinlay JB. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16:1587–94. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 67.Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010;105:1222–5. doi: 10.1111/j.1464-410X.2009.09007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–31. [PubMed] [Google Scholar]
- 69.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–80. [PubMed] [Google Scholar]
- 71.Satoh T, Yang G, Egawa S, Addai J, Frolov A, Kuwao S, Timme TL, Baba S, Thompson TC. Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer. 2003;97:1225–33. doi: 10.1002/cncr.11198. [DOI] [PubMed] [Google Scholar]
- 72.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–5. [PubMed] [Google Scholar]
- 73.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–9. doi: 10.1158/0008-5472.CAN-04-2332. [DOI] [PubMed] [Google Scholar]
- 74.Fukuchi J, Hiipakka RA, Kokontis JM, Hsu S, Ko AL, Fitzgerald ML, Liao S. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 2004;64:7682–5. doi: 10.1158/0008-5472.CAN-04-2647. [DOI] [PubMed] [Google Scholar]
- 75.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66:6482–6. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 76.Zumsteg ZS, Zelefsky MJ. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: the standard of care? Lancet Oncol. 2012;13:e259–269. doi: 10.1016/S1470-2045(12)70084-0. [DOI] [PubMed] [Google Scholar]
- 77.Segawa T, Nau ME, Xu LL, Chilukuri RN, Makarem M, Zhang W, Petrovics G, Sesterhenn IA, McLeod DG, Moul JW, Vahey M, Srivastava S. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene. 2002;21:8749–58. doi: 10.1038/sj.onc.1205992. [DOI] [PubMed] [Google Scholar]
- 78.Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. J Biol Chem. 2011;286:20637–47. doi: 10.1074/jbc.M111.227082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chuu CP, Chen RY, Hiipakka RA, Kokontis JM, Warner KV, Xiang J, Liao S. The liver X receptor agonist T0901317 acts as androgen receptor antagonist in human prostate cancer cells. Biochem Biophys Res Commun. 2007;357:341–6. doi: 10.1016/j.bbrc.2007.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, Baron S, Lobaccaro JM. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–23. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 81.Dufour J, Viennois E, De Boussac H, Baron S, Lobaccaro JM. Oxysterol receptors, AKT and prostate cancer. Curr Opin Pharmacol. 2012;12:724–8. doi: 10.1016/j.coph.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Current pharmaceutical design. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 83.Aaltoma SH, Lipponen PK, Kosma VM. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate cancer. Anticancer Res. 2001;21:3101–6. [PubMed] [Google Scholar]
- 84.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–903. [PubMed] [Google Scholar]
- 85.Baltaci S, Orhan D, Gogus C, Turkolmez K, Tulunay O, Gogus O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001;88:100–3. doi: 10.1046/j.1464-410x.2001.02231.x. [DOI] [PubMed] [Google Scholar]
- 86.Tsai CS, Chen FH, Wang CC, Huang HL, Jung SM, Wu CJ, Lee CC, McBride WH, Chiang CS, Hong JH. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68:499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 87.Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–43. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 88.Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogenactivated protein kinase pathway. Am J Pathol. 2003;162:655–63. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wegiel B, Bjartell A, Culig Z, Persson JL. Interleukin- 6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int J Cancer. 2008;122:1521–9. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- 90.Siegsmund MJ, Yamazaki H, Pastan I. Interleukin 6 receptor mRNA in prostate carcinomas and benign prostate hyperplasia. J Urol. 1994;151:1396–9. doi: 10.1016/s0022-5347(17)35267-9. [DOI] [PubMed] [Google Scholar]
- 91.Twillie DA, Eisenberger MA, Carducci MA, Hseih WS, Kim WY, Simons JW. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–9. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 92.Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Murai M. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69:113–7. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 93.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 94.Chuu CP, Kokontis JM, Hiipakka RA, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci. 2007;14:543–53. doi: 10.1007/s11373-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 95.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–25. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 96.Viennois E, Mouzat K, Dufour J, Morel L, Lobaccaro JM, Baron S. Selective liver X receptor modulators (SLiMs): what use in human health? Mol Cell Endocrinol. 2012;351:129–41. doi: 10.1016/j.mce.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 97.Jakobsson T, Treuter E, Gustafsson J-Å, Steffensen KR. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends in pharmacological sciences. 2012;33:394–404. doi: 10.1016/j.tips.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 98.Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J Biol Chem. 2003;278:36091–8. doi: 10.1074/jbc.M304153200. [DOI] [PubMed] [Google Scholar]
- 99.Yasuda T, Grillot D, Billheimer JT, Briand F, Delerive P, Huet S, Rader DJ. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–6. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katz A, Udata C, Ott E, Hickey L, Burczynski ME, Burghart P, Vesterqvist O, Meng X. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–9. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- 101.Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, Clerin V, Resmini C, Keith JC, Berrodin TJ, Feingold I, Zhong W, Hartman HB, Evans MJ, Gardell SJ, DiBlasio-Smith E, Mounts WM, LaVallie ER, Wrobel J, Nambi P, Vlasuk GP. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res. 2009;50:2358–70. doi: 10.1194/jlr.M900037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]


