Abstract
Although a mild degree of anemia is common in the third trimester of pregnancy, it remains a challenge to establish whether a decrease in hemoglobin (Hb) concentration is physiological or pathological. The World Health Organization suggested a Hb concentration of 110 g/L to discriminate anemia. Several European investigators recommended Hb cut-off values of between 101–110 g/L. The aim of this study was to establish short-term effects of iron supplementation on the hemoglobin content of reticulocytes (Ret-He) and red blood cells (RBC-He) in case of suspected iron deficient erythropoiesis (IDE) in the third trimester of pregnancy. Twenty-five subjects with suspected IDE during pregnancy (Hb ≤110g/L, Ret-He <29.6 pg, zinc protoporphyrin >75 mol/mol hem) participated in the study. After iron supplementation, reticulocyte counts increased from 0.061±0.015×1012/L to 0.079±0.026×1012/L and Ret-He increased from 23.6±2.8 pg to 28.3±2.6 pg (P=<0.001). RBC-He increased from 26.9±1.9 pg to 27.4±1.8 pg (not significant, NS) and Ret-He/RBC-He ratio increased from 0.97±0.06 towards 1.07±0.05 (P=<0.001). Hb concentrations demonstrated an obvious increase from 105±6 g/L towards 115±5 g/L (P≤0.001) after supplementation. An obvious increase in RBC distribution width was observed from 45.0±3.6 fL towards 52.3±7.0 fL (P≤0.001). We recommend that Ret-He and Ret-He/RBC-He ratio be integrated into the protocols for anemia screening and for monitoring effects of iron supplementation during pregnancy. In particular, the parameters should be considered in subjects with Hb results in the controversial range of 101–108 g/L.
Key words: anemia, IDE, pregnancy, reticulocyte haemoglobin.
Introduction
A high prevalence of anemia during pregnancy has been reported worldwide, ranging from 2% to 30% in developed countries.1–3 Anemia during pregnancy is partly due to physiological hemodilution and insufficient availability of essential nutrients for hemoglobin (Hb) synthesis and red blood cell (RBC) production in the erythron, such as iron, folic acid and vitamin B12.4 In the last trimester of pregnancy, decreased Hb concentrations as a result of functional iron deficiency may be associated with complications such as maternal infection, low birth weight and premature delivery.5,6
Although a mild degree of anemia is common in the third trimester of pregnancy, it remains a challenge to establish whether a decreased Hb concentration is a physiological or pathological phenomenon due to iron deficient erythropoiesis (IDE).4,5,7
Disagreement in diagnostic guidelines in obstetric practice illustrates the complexity of establishing discriminating Hb levels for screening anemia during pregnancy. The World Health Organization (WHO) suggested a Hb concentration of 110 g/L to discriminate anemia.8 European investigators recommend Hb cut-off values of between 101–110 g/L.4,9–13
The aim of this study was evaluate the possible beneficial effects of iron supplementation on the hemoglobin content of reticulocytes and RBCs in subjects with inconclusive Hb concentrations in the third trimester of pregnancy.
Hemocytometric parameters such as Hb concentration and mean corpuscular volume (MCV) demonstrate poor sensitivity for the detection of short-term disturbances in ery-thropoiesis during pregnancy.5–7 In addition, biomarkers reflecting iron status, i.e. serum concentrations of ferritin, transferrin receptor (TfR) and transferrin saturation (TfSat), reveal serious limitations concerning clinical interpretation.4,5 It should be emphasized that functional IDE and definite IDE are not mutually exclusive phenomena. Both phenomena may co-exist, particularly during the last trimester of pregnancy when low-level inflammation together with depleted iron stores is likely to occur.10 It is still a challenge to establish appropriate cut-off limits for evaluating the shift of hemoglobin content of RBCs in the course of pregnancy. A suitable biomarker for detection of long-term IDE is the zinc protoporphyrin hem ratio (ZPP/Hb ratio). In subjects without iron supplementation, ZPP will clearly increase in the last trimester of pregnancy.10,14,15 Recently, new hemocytometric parameters such as erythrocyte hemoglobin content (RBC-He), reticulocyte hemoglobin content (Ret-He) and Ret-He/RBC-He ratio have been demonstrated to yield useful biomarkers for the detection of insufficient hemoglobinization in the third trimester of pregnancy.5,16 In healthy subjects, Ret-He results exceed those of RBC-He, amounting to 5–15%. From corresponding shifts in decreased values for Ret-He and Ret-He/RBC-He ratios, respectively, a temporarily decreased degree of hemoglobinization is achieved. The RET-He/RBC-He ratio provides accurate and sensitive information concerning the deviation in hemoglobin content between the (normocytic) RBC population (RBC-He) and the (hypochromic) reticulocyte population (Ret-He).17
In contrast to ZPP and RBC-He, Ret-He reflects a short-term indication corresponding to a lifespan of reticulocytes in the blood circulation of several days.18
Materials and Methods
Study design
Subjects with inconclusive Hb concentrations in the range 101–110 g/L in the third trimester of pregnancy were selected for inclusion in the screening program. The subjects were subsequently supplemented with ferrous fumarate (200 mg 2 times a day, approx. 200 mg iron a day) according to local practice.12 From the first trimester of pregnancy, 400 g folic acid was given as supplement in a multi-vitamin tablet (Centrum® Materna). After four weeks of iron supplementation, blood samples were drawn to establish hemocytometric parameters to evaluate RBC and reticulocyte hemoglobin content.
Hemocytometry
Hemocytometric analyses were performed within 4 h after collection of blood samples (K2EDTA, Becton Dickinson, Plymouth, UK) on a Sysmex XE2100 hematology analyzer (Sysmex Corporation, Kobe, Japan). Reticulocyte methodology of measurement is based on automated fluorescent flow cytometry utilizing a polymethine dye for binding cytoplasmic RNA. The mean forward light scatter intensity in the reticulocyte channel is estimated as a measure that reflects particle volume and Hb content of RBCs and reticulocytes, respectively. Hb content was initially reported as RBC-Y for RBCs and RET-Y for reticulocytes. Subsequently, algorithms y=6.4*e0.0009*Ret-Y and y= 6.4*e0.0009*RBC-Y were applied in order to transform arbitrarily reported channel numbers of the RET-Y and RBC-Y into hemoglobin content equivalents.19 Hb content in reticulocytes and RBCs is expressed in pg and denoted as RET-He and RBC-He, respectively.
Zinc protoporphyrin hem ratio
Measurements of zinc protoporphyrin hem ratio (ZPP/Hb ratio) were performed on a hematofluorometer (AVIV Biochemical Inc., Lakewood, NJ, USA) using front surface illumination fluorometry.20
Statistical analysis
SPSS/PC statistical software, version 14.0 for Windows, was applied for statistical analysis of results (SPSS, Chicago, IL, USA). Paired-sample t-tests were performed to detect statistically significant deviations between results before and after iron supplementation. P<0.05 was considered statistically significantly different. Data are expressed as mean values±SD, unless specified otherwise.
Results
The study included a group of 25 subjects during the third trimester of pregnancy. On suspicion of IDE, we selected parameters to discriminate subjects with Hb ≤110g/L. Additionally, MCV 80–100 fL, Ret-He <29.6 pg and ZPP >75 mol/mol hem were applied as initial screening parameters.16 Results indicating deviations of erythropoiesis activity are listed in Table 1. Reticulocyte counts demonstrated a tendency towards increased levels after iron supplementation (0.079±0.026×1012/L) compared with those before supplementation (0.061±0.015×1012/L) (P<0.001). Individual hemoglobin content of reticulocytes (Ret-He and Ret-He/RBC-He ratio) before and after iron supplementation are shown in Figure 1A and B. After iron supplementation, there was a clear increase in Ret-He content of 20% from 23.6±2.8 pg to 28.3±2.6 pg (P<0.001) and Ret-He/RBC-He ratio was increased by 10% from 0.97±0.06 to 1.07±0.05 (P<0.001).
Table 1. Hemocytometric parameters before and after iron supplementation. Results are established in 25 subjects with Hemoglobin values in the range of 101–110 g/L.
| Iron supplementation | P | ||
|---|---|---|---|
| Before | After | ||
| Parameter | mean±SD (min-max) | mean±SD (min-max) | |
| Hb (g/L) | 105±6 | 114±5 | <0.001 |
| (87–111) | (108–122) | ||
| MCV (fL) | 83.3±4.2 | 85.0±3.3 | 0.118 (NS) |
| (72.5–90.9) | (78.7–89.7) | ||
| MCHC (mmol/L) | 20.3±0.5 | 20.4±0.6 | 0.599 (NS) |
| (19.3–21.2) | (19.4–21.8) | ||
| RDW-SD (fL) | 45.0±3.6 | 52.3±7.0 | <0.001 |
| (37.8–52.0) | (40.1–69.2) | ||
| Reti (×1012/L) | 0.061±0.015 | 0.079±0.026 | <0.001 |
| (0.024–0.089) | (0.025–0.150) | ||
| Ret-He (pg) | 23.6±2.8 | 28.3±2.6 | <0.001 |
| (18.6–28.7) | (21.8–32.0) | ||
| RBC-He (pg) | 26.9±1.9 | 27.4±1.8 | 0.197 (NS) |
| (22.2–29.6) | (24.2–30.2) | ||
| Ret-He / RBC-He ratio | 0.97±0.06 | 1.07±0.05 | <0.001 |
| (0.83–1.05) | (0.97–1.18) | ||
| ZPP (µMol/Mol haem) | 124±44 | 116±34 | 0.359 (NS) |
| (77–246) | (63–207) | ||
SD, standard deviation; NS, non significant; Hb, Hemoglobin; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; RDW-SD, red blood cell distribution width; Ret-He, hemoglobin content of reticulocytes: RBC-He, hemoglobin content of red blood cell; ZPP, zinc protoporphyrin.
Figure 1.
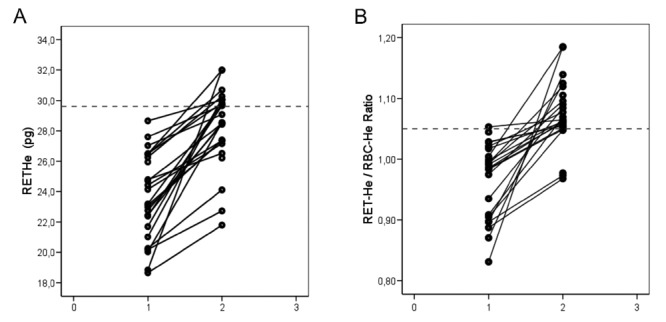
Individual results for reticulocyte hemoglobin content (RET-He, pg, A) and Ret-He/RBC-He ratio (B) established in 25 subjects during pregnancy before (1) and after (2) four weeks of iron supplementation. The horizontal line indicates the lower level of the reference range for apparently healthy subjects.
There was only a slight (2%) increase in RBC-He from 26.9±1.9 pg to 27.4±1.8 pg (NS). Hb concentrations showed a tendency to increase from 105±6 g/L (mean±SD) to 115±5 g/L (P<0.001) after iron supplementation.
In order to evaluate the effect of iron supplementation on RET-He, deviations in Hb, RET-He and RET-He/RBC-He ratio, respectively, are shown in Figure 2A and B. Evaluation of RET-He and RET-He/RBC-He ratio provides a more sensitive measurement of shifts in values as a result of iron supplementation when compared with traditional Hb measurements. This is expected, because RET-He and RET-He/RBC-He ratio parameters reflect short-term deviations. No statistically significant deviations in MCV or mean corpuscular hemoglobin concentration (MCHC) were observed.
Figure 2.
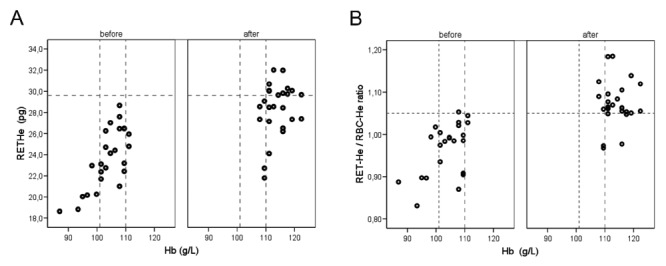
Changes in hemoglobin (Hb, g/L) and reticulocyte hemoglobin content (RET-He, pg, A), respectively, RET-He/RBC-He ratio (B) established in 25 subjects during pregnancy before (1) and after (2) four weeks of iron supplementation. The horizontal line indicates the lower level of the reference range for apparently healthy subjects. The vertical lines indicate the controversial Hb range of 101–110 g/L.
A statistically significant tendency towards increased red blood cell distribution width (RDW-SD) was observed showing a rise from 45.0±3.6 fL before supplementation to 52.3±7.0 fL after supplementation (P<0.001). An example of a representative shift in RBC histogram before and after iron supplementation is shown in Figure 3A and Figure B. After iron supplementation, the RBC histogram shows a dimorphic population, with a shoulder indicating the new RBC population on the right side.
Figure 3.
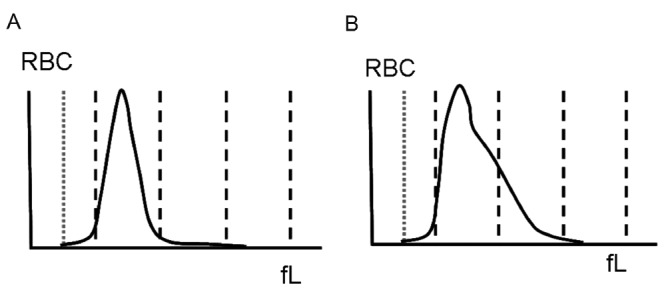
An example of an red blood cell (RBC) histogram before (A) and after (B) iron supplementation. The newly formed RBC population (B) is demonstrated on the right side of the curve. The x-axis demonstrates the RBC-volume (fL). The vertical dashed line (gray) reflects the lower discriminator of the RBC-volume (fL).
There was a slight decrease in ZPP from 124±44 mol/mol hem to 116±34 mol/mol hem (NS) after iron supplementation.
Discussion
A mild degree of anemia is common in the third trimester of pregnancy. Additional supplementation of iron is a question for debate.14–21 The aim of this study was to establish short-term effects of iron supplementation on Ret-He and RBC-He of women with inconclusive Hb concentrations with suspected IDE in the third trimester of pregnancy.
Our study demonstrated that Ret-He levels clearly increased after four weeks of iron supplementation towards levels within the lower region of the reference interval 30.4±36.8 pg.17 Ret-He/RBC-He ratio demonstrated a similar trend when compared with Ret-He. The observed shifts in Ret-He and Ret-He/RBC-He ratio reflect short-term alterations concerning the quality of erythropoiesis.17–25 Our study revealed a clear increase in Hb concentrations and absolute reticulocyte counts after iron supplementation, in particular in the group of subjects with Hb in the controversial range of 101–110 g/L. During pregnancy, it is difficult to assess whether an increase in Hb concentration and reticulocytes is the effect of increased activity of erythropoiesis after supplementation, or if this is due to a less rapid increase in plasma volume in late pregnancy. Several investigators reported a 6 g/L increase in Hb in late pregnancy without iron supplementation.4,5,7 However, in our study, after iron supplementation, an increase in Hb of approximately 10 g/L was observed.
According to an evaluation made in previous studies, nutrient supplementation did not reveal any significant changes in RBC-He content or ZPP/Hb ratio.10,14,15 The lack of effect may be explained by the fact that RBC-He and ZPP reflect long-term impact on shifts in hemoglobinization, corresponding with the lifespan of circulating mature RBCs (100 days).18 An increased degree of heterogeneity in the size of RBCs (anisocytosis), amongst others due to increased erythropoiesis, is reflected in RDW. After iron supplementation, RDW-SD increased in 88% of the subjects. In this study, increased RDW values are indicative of enhanced erythropoiesis as a response to iron supplementation. However, despite a positive response to erythropoiesis after iron supplementation, no definitive conclusions can be drawn concerning depletion of iron stores. During pregnancy, the need for micronutrients, in particular iron and folate, is increased. Maternal body stores and dietary intake may be insufficient for adequate erythropoiesis. Supplementary iron is needed for erythropoiesis to enhance increased production of RBC and to fulfill the additional iron demands of the fetus. The physiological mechanism for covering additional iron requirements is to release iron from the body stores. However, many Western European women have an inadequate dietary iron intake which can not fulfill the increased demands in middle and late pregnancy.2,9,26 Therefore, IDE is a frequent cause of anemia during pregnancy.
The Hb cut-off level for suspected IDE has been the subject of frequent discussion.4,9–11 Approximately 10% of the pregnant women showed inconclusive Hb concentrations in the range between 101–110 g/L (Margreet Schoorl, 2012 personal communication). Anemia screening and monitoring based exclusively on Hb concentrations is considered to be inappropriate. It has been recommended ZPP and Ret-He should both also be assessed.5,10,16
The appropriate dosage of prophylactic iron supplementation needs to be considered. Several investigators reported wide deviations in daily doses in the range of 40–200 mg. Regimens with less frequent iron supplementation, such as once or twice weekly, have been described as promising.11,13,21,24,27
Only marginal effects of iron supplementation on the newborn's birth weight or on prenatal morbidity or mortality in mother and child have been reported. However, positive effects have also been reported, such as increased physical fitness and well-being in pregnant women, prevention of postpartum iron deficiency due to blood loss at delivery, and enhanced iron reserves in the newborn to prevent iron deficiency in the first years of life.9,28 In summary, clinical practice needs simple and reliable strategies for screening and monitoring IDE during pregnancy.
Study limitations
In the present study, subjects with thalassemia were excluded. Although Ret-He is decreased in these subjects, results for Ret-He/RBC-He ratio are within the reference range. Corresponding shifts in decreased values for Ret-He and Ret-He/RBC-He ratios, respectively, lead to the conclusion that there is a temporarily decreased degree of hemoglobinization.17 In subjects with thalassemia, we also recommend measurement of Ret-He and Ret-He/RBC-He in IDE screening during pregnancy.
Conclusions
We recommend that Ret-He and Ret-He/RBC-He ratio parameters should be integrated into the protocol for anemia screening and monitoring during pregnancy. Ret-He and Ret-He/RBC-He ratio are sensitive markers for screening when a decrease in red blood cell hemoglobin content is observed and for monitoring short-term effects of iron supplementation. The recommended parameters should be considered in particular in the group of subjects with Hb in the controversial range of 101–110 g/L. Ret-He and Ret-He/RBC-He ratio may in future be a useful measurement to help optimize the dosage of prophylactic iron supplementation during pregnancy.
References
- 1.Lindsay H. Pregnancy and iron deficiency: unresolved issues. Nutr Rev. 1997;55:91–101. doi: 10.1111/j.1753-4887.1997.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 2.Hercberg S, Preziosi P, Galan P. Iron deficiency in Europe. Public Health Nutr. 2001;4:537–45. doi: 10.1079/phn2001139. [DOI] [PubMed] [Google Scholar]
- 3.Nybo M, Friis-Hansen L, Felding P. Higher prevalence of anemia among pregnant immigrant women compared to pregnant ethnic Danish women. Ann Hematol. 2007;86:647–51. doi: 10.1007/s00277-007-0305-7. [DOI] [PubMed] [Google Scholar]
- 4.Milman N, Bergholt T, Byg K, et al. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur J Haematol. 2007;79:39–46. doi: 10.1111/j.1600-0609.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 5.Ervasti M, Kotisaari S, Heinonen S, Punnonen K. Use of advanced red blood cell and reticulocyte indices improves the accuracy in diagnosing iron deficiency in pregnant women at term. Eur J Haematol. 2007;79:539–45. doi: 10.1111/j.1600-0609.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 6.Sant-Rayn P. Should we screen for iron deficiency anaemia? A review and recent recommendations. Pathology. 2012;44:139–47. doi: 10.1097/PAT.0b013e32834e8291. [DOI] [PubMed] [Google Scholar]
- 7.Klajnbard A, Szecsi PB, Colov NP, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin Chem Lab Med. 2010;48:237–48. doi: 10.1515/CCLM.2010.033. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Integrated management of pregnancy and childbirth (IMPAC) Geneva: World Health Organisation; 2006. Department of making pregnancy safer. Standards for maternal and neonatal care: iron and folate supplementation. [Google Scholar]
- 9.Milman N, Bergholt T, Byg K, et al. Iron status and iron imbalance during pregnancy. A critical reappraisal of iron supplementation. Acta Obstet Gyn Scan. 1999;78:749–57. [PubMed] [Google Scholar]
- 10.Wheeler S. Assessment and interpretation of micronutrient status during pregnancy. Proc Nutr Soc. 2008;67:437–50. doi: 10.1017/S0029665108008732. [DOI] [PubMed] [Google Scholar]
- 11.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156:588–600. doi: 10.1111/j.1365-2141.2011.09012.x. [DOI] [PubMed] [Google Scholar]
- 12.Koninklijke Nederlandse Organisatie van Verloskundigen (Royal Dutch Organisation of Midwives) Praktijkkaart behorende bij de gelijk-namige KNOV-standaard. Bilthoven: 2000. Anemie in de eerstelijns verloskundige praktijk. [Google Scholar]
- 13.Koninklijke Nederlandse Organisatie van Verloskundigen (Royal Dutch Organisation of Midwives) Praktijkkaart behorende bij de gelijknamige KNOV-standaard. Utrecht: 2010. Anemie in de verloskundige praktijk. [Google Scholar]
- 14.Romslo I, Haram K, Sagan N, Augensen K. Iron requirement in normal pregnancy assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaec. 1983;90:101–7. doi: 10.1111/j.1471-0528.1983.tb08891.x. [DOI] [PubMed] [Google Scholar]
- 15.Harthoorn EJ, Lindemans J. Langenhuijsen MMAC. Erythrocyte zinc protoporphyrin testing in pregnancy. Acta Obstet Gyn Scan. 2000;79:660–6. [PubMed] [Google Scholar]
- 16.Schoorl M, van der Gaag D, Schoorl M, Bartels PCM. Changes in red blood cell hemoglobinization during pregnancy. Ned Tijdschr Klin Chem Labgeneesk. 2010;35:206–8. [Google Scholar]
- 17.Bartels PCM, Schoorl M, Schoorl M. Hemoglobinization and functional availability of iron for erythropoiesis in case of thalassemia and iron deficiency anemia. Clin Lab. 2006;52:107–14. [PubMed] [Google Scholar]
- 18.Thomas C, Thomas L. Biochemical markers and haematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066–76. [PubMed] [Google Scholar]
- 19.Franck S, Linssen J, Messinger M, Thomas L. Potential utility of Ret-Y in the diagnosis of iron restricted erythropoiesis. Clin Chem. 2004;50:1240–2. doi: 10.1373/clinchem.2004.030254. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg WE, Eisinger J. Principles and applications of haematofluorometry. J Clin Lab Autom. 1984;4:29–42. [Google Scholar]
- 21.Milman N. Iron prophylaxis in pregnancy-general or individual and in which dose? Ann Hematol. 2006;85:821–8. doi: 10.1007/s00277-006-0145-x. [DOI] [PubMed] [Google Scholar]
- 22.Milman N. Targeted (not global) iron supplementation/fortification is the issue! Ann Hematol. 2012;91:959–60. [Google Scholar]
- 23.Ghio AJ. Anemia and global iron fortification and supplementation. Ann Hematol. 2012;91:957–8. doi: 10.1007/s00277-011-1335-8. [DOI] [PubMed] [Google Scholar]
- 24.Ribot B, Aranda N, Viteri F, et al. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birth-weight even when supplemented daily with moderate iron. Hum Reprod. 2012;27:1260–6. doi: 10.1093/humrep/des026. [DOI] [PubMed] [Google Scholar]
- 25.Brugnara C. Reticulocyte haemoglobin equivalent (Ret-He) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303–8. doi: 10.1111/j.1365-2257.2006.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svandberg B. Absorption of iron in pregnancy. Acta Obstet Gyn Scan. 1975;48(Suppl):1–108. [PubMed] [Google Scholar]
- 27.Beentjes M, Jans S. Revised practice Guideline Anaemia in midwifery practice. Ned Tijdschr Geneeskd. 2012;156:A3711–A3711. [PubMed] [Google Scholar]
- 28.Wiegerinck MM, Mol BWJ. Insufficient evidence supporting iron supplementation in anaemia during pregnancy. Ned Tijdschr Geneeskd. 2012;156:A4293–A4293. [PubMed] [Google Scholar]


