Abstract
The appearance of Sindbis virus-envelope glycoproteins in the surfaces of chicken embryo fibroblasts was studied by an indirect labeling technique. This technique involved treating infected cells sequentially with rabbit immunoglobulin G (IgG) specific for Sindbis virus followed by hemocyanin-conjugated goat (anti-rabbit IgG) IgG; surface replicas of these cells were then prepared and examined in the electron microscope. As early as 2 h after infection (and at least 1 h before mature virions were released), newly synthesized virus-envelope glycoproteins were detected at the cell surface. By 3 h after infection, cell surface membranes were extensively modified by the insertion of the Sindbis glycoproteins. When infected cells were prefixed with glutaraldehyde before labeling, the glycoproteins were distributed fairly evenly over the cell surface, although a slight clustering was observed on cells labeled early in infection. However, no evidence for large-scale clustering of virus glycoproteins corresponding to patches of budding virus was observed. Similar results were found with unfixed cells labeled at 4 C. However, when unfixed cells were labeled at 37 C, the glycoproteins were shown to be in discrete clusters, demonstrating that these glycoprotein antigens can diffuse laterally through the cell membrane at this temperature.
Full text
PDF

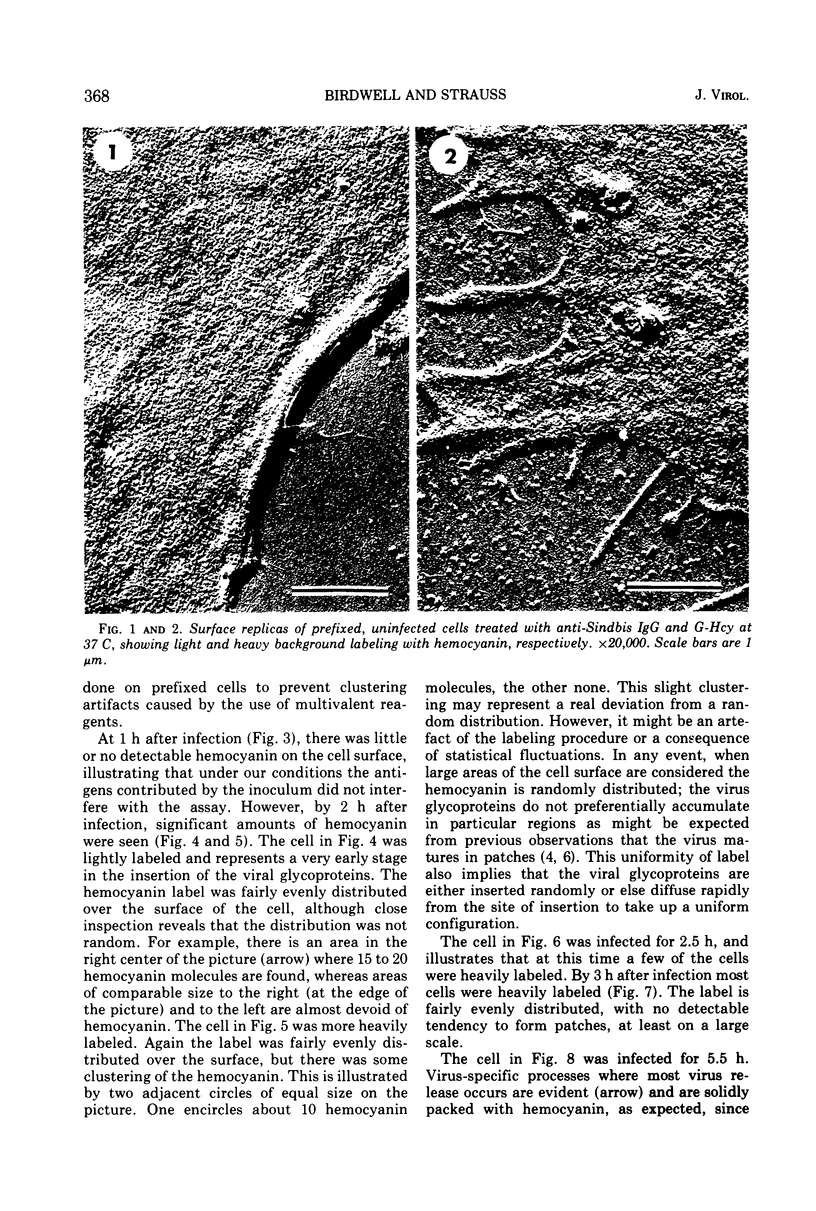
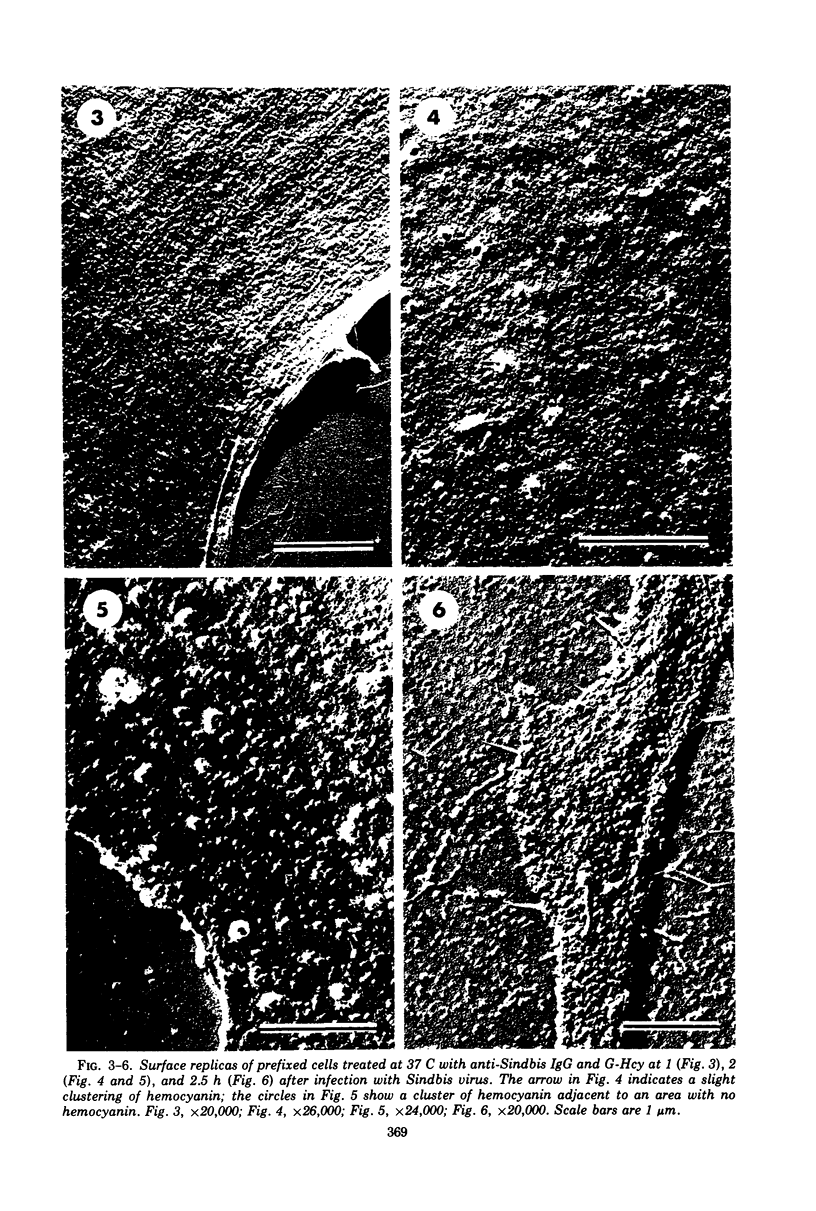

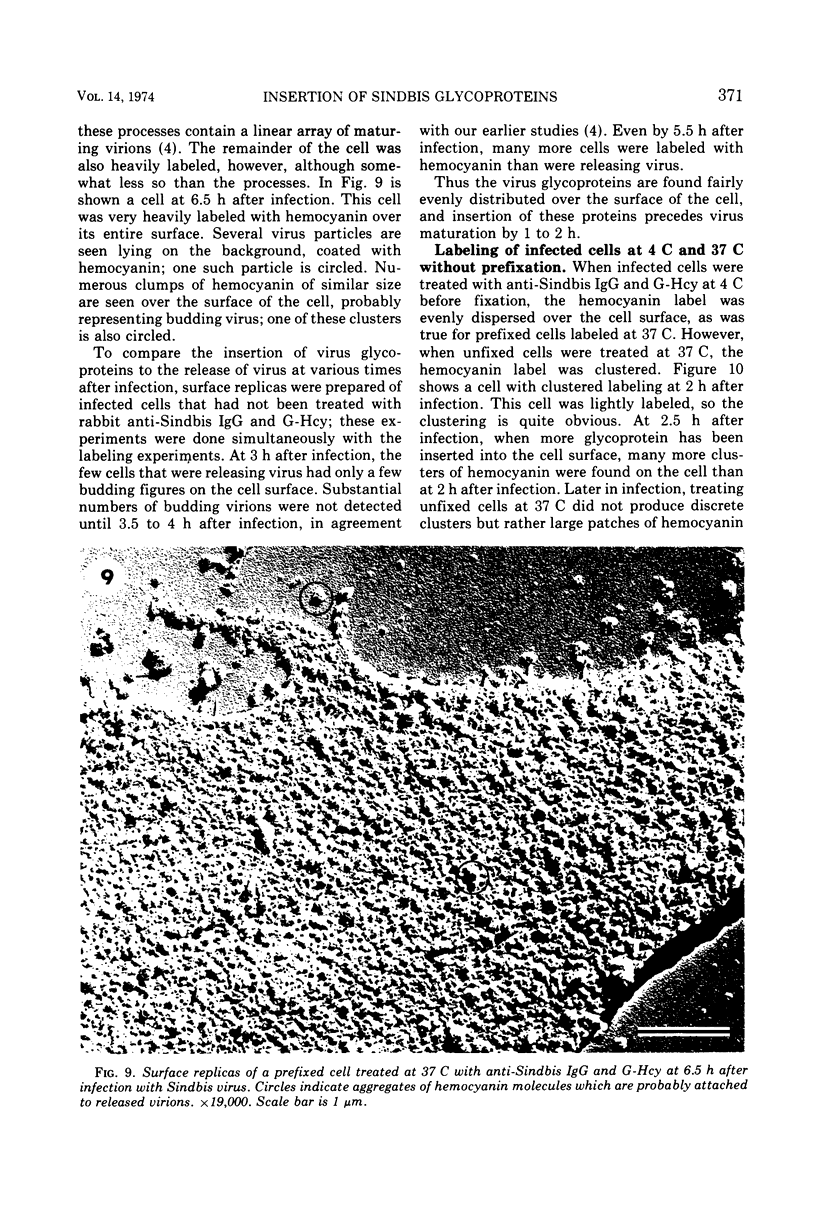



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss E. G., Strauss J. H. Replication of Sindbis virus. 3. An electron microscopic study of virus maturation using the surface replica technique. Virology. 1973 Dec;56(2):429–438. doi: 10.1016/0042-6822(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss J. H. Agglutination of Sindbis virus and of cells infected with Sindbis virus by plant lectins. J Virol. 1973 Apr;11(4):502–507. doi: 10.1128/jvi.11.4.502-507.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Temperature-sensitive mutants of Sindbis virus: biochemical correlates of complementation. J Virol. 1967 Oct;1(5):956–962. doi: 10.1128/jvi.1.5.956-962.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. THE SOURCE OF THE RIBONUCLEIC ACID AND PHOSPHOLIPID OF SINDBIS VIRUS. Virology. 1963 Jul;20:446–456. doi: 10.1016/0042-6822(63)90093-x. [DOI] [PubMed] [Google Scholar]
- Pierce J. S., Strauss E. G., Strauss J. H. Effect of ionic strength on the binding of Sindbis virus to chick cells. J Virol. 1974 May;13(5):1030–1036. doi: 10.1128/jvi.13.5.1030-1036.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Wolken K. Electronmicroscope investigations of the underside of cells in culture. Exp Cell Res. 1973 Mar 30;78(1):1–14. doi: 10.1016/0014-4827(73)90031-1. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Basic structure of group A arbovirus strains Middelburg, Sindbis, and Semliki Forest examined by negative staining. Virology. 1968 Feb;34(2):358–361. doi: 10.1016/0042-6822(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Revel J. P. Mapping of concanavalin A binding sites on the surface of several cell types. Dev Biol. 1972 Mar;27(3):434–441. doi: 10.1016/0012-1606(72)90183-2. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]














