Abstract
The epigenetic machinery plays a pivotal role in the control of many of the body's key cellular functions. It modulates an array of pliable mechanisms that are readily and durably modified by intracellular or extracellular factors. In the fast-moving field of neuroepigenetics, it is emerging that faulty epigenetic gene regulation can have dramatic consequences on the developing CNS that can last a lifetime and perhaps even affect future generations. Mounting evidence suggests that environmental factors can impact the developing brain through these epigenetic mechanisms and this report reviews and examines the epigenetic effects of one of the most common neurotoxic pollutants of our environment, which is believed to have no safe level of exposure during human development: lead.
Keywords: brain, development, environmental exposure, epigenetics, heavy metal, human, lead
Lead (Pb) toxicity remains a significant health problem in the USA and worldwide. Although the prevalence of childhood Pb poisoning nationally has decreased dramatically over the last 10 years, more than 250,000 children aged 1–5 years still have blood Pb levels (BLLs) greater than 10 μg/dl of blood, which was until recently the current CDC action level. The seriousness of the situation was emphasized in a 2012 report from the CDC Advisory Committee on Childhood Lead Poisoning Prevention stating that “no level of Pb appears to be safe” for children [1]. As a result, the CDC recently lowered the action level to a BLL of 5 μg/dl, increasing thereby the number of at-risk US children to >500,000 [101]. Because Pb interferes with developmental neuronal plasticity, it is especially toxic to the rapidly developing fetal and neonatal brain [2–4]. Thus, environmental exposure to Pb during prenatal life or in childhood, even at BLLs below the new CDC action level, may cause permanent neurological damage in exposed children. Despite the prevalence of Pb exposure in the USA and elsewhere, the mechanisms by which prenatal Pb exposure compromises human brain development and function are largely unknown. Several routes of action for Pb neurotoxicity have been proposed, such as oxidative stress, deregulation of calcium signaling and abnormal neural transmission/gene expression [3].
The fascinating world of epigenetics has opened a novel avenue of research for understanding how the environment can influence our genes and contribute to neurodevelopmental disorders. Indeed, a number of recent studies have implicated epigenetic mechanisms in the etiology of Pb-induced neurotoxicity, suggesting that by altering epigenetic determinants, the effects of Pb could be lifelong, or possibly even transgenerational. This is supported by recent studies identifying gestational Pb exposure as a risk factor for a variety of neurological diseases with onset in later life, including Alzheimer's disease and schizophrenia [5–8].
In this report, we will review the deleterious effects that environmental Pb exposure may have on the developing human brain and the insights gained from animal models. We will present evidence of the importance of epigenetic regulation in brain formation and maintenance and will examine evidence to date suggesting how Pb exposure could affect epigenetic marks and interfere with brain development and function. We will also discuss the key achievements and the challenges faced ahead, as researchers seek to understand the fragile balance between environmental Pb exposure, epigenetics and human neurodevelopment.
Pb is a pervasive environmental threat
Pb is a naturally occurring heavy metal that has been mined for thousands of years [9]. Natural and mostly anthropogenic sources of dispersion have made of Pb a ubiquitous nondegradable toxic pollutant of our environment, which can acutely or chronically affect human health. The most common routes of exposure are inhalation of air contaminated with Pb dust, ingestion of Pb-tainted food or water, or direct contact with Pb-polluted soil [102]. Even though Pb is now less of a contamination hazard in the USA owing to its removal from paints and gasoline, it nevertheless remains a serious problem since it can still be found in a variety of products used daily, including pipes, batteries, ceramics, water, food and toys. Pb exposure can be detrimental to every organ in the human body, but the brain is particularly sensitive to its harmful effects, especially in early developmental years [3,4,10]. Among the population, unborn and young children are the most sensitive to Pb neurotoxicity because their small size facilitates the absorption and retention of Pb, and their brains are actively developing. Also, because Pb can freely cross the placental barrier [11] and be mobilized from maternal bones during pregnancy, Pb-exposed pregnant women may in turn expose the developing embryo. Even though the neurotoxicity of high Pb concentrations is well documented, it is now emerging from animal and human studies that even low Pb concentrations may be harmful to early brain development [1,12], and each year there continues to be hundreds of new cases in the USA.
Prenatal exposure to Pb affects brain development
Epidemiological studies have provided compelling evidence that, even at BLLs below the current CDC action level, early-life Pb exposure has harmful effects on the developing brain. Thus, chronic exposure to Pb during prenatal life or in childhood, can result in disrupted neuropsychiatric function and reduced cognitive ability, behaviors associated with attention deficit hyperactivity disorders, lowered intellectual quotients, increased likelihood of delinquency, reduced activity in brain areas involved in language function and altered sensory function [3,4,12–16]. Pb-exposed children have a decreased amount of gray matter and exhibit cognitive deficits as adults [17–19]. In human primary fetal neuronal cultures, Pb modifies the activity of protein phosphatases known to regulate synaptic plasticity [20]. While both males and females are vulnerable to the adverse effects of Pb exposure, there is significant evidence that sex can influence the severity of Pb neurotoxicity. Multiple studies describe more severe Pb-induced cognitive deficits in girls relative to boys, whereas prenatal Pb exposure appears to induce poorer cognitive performances in boys, even at very low BLLs (see [18,21,22]).
Animal model studies have provided invaluable insights into the mechanisms of Pb neurotoxicity by showing that chronic exposure to Pb can interfere with neurogenesis and reduce the proliferation, differentiation and survival of newly generated neurons (see [4,23]). Reports that chronic Pb exposure can lower the density of dendritic spines and synapses, decrease the levels of synaptic/neural proteins and alter presynaptic function and vesicular release, were landmark discoveries that identified the synapse as a major target of Pb neurotoxicity [4,24–28]. Indeed, Altmann et al. showed that chronic Pb exposure inhibits long-term potentiation (a model for learning) in the rat visual cortex and hippocampus [29]. As in human studies, sex-specific differences in the effects of Pb exposure have also been documented in spatial learning and memory, motor behavior, dopamine metabolism and brain gene expression [4,30,31], emphasizing the importance of taking sex into account when considering the epigenetic basis of Pb effects. Although described in both humans and animal models, sex differences in the severity of Pb-induced neurotoxicity have surprisingly been little explored, and their molecular mechanisms are largely unknown. The development of specific brain regions differs between males and females, and sex-biased response to Pb exposure may be due to the differential effects of Pb on sex chromosome genes or genes on autosomal chromosomes that are imprinted and dependent on maternal or paternal inheritance [30,32]. Sex-based effects of Pb could also be attributed to differences in the concentrations of sex hormones and their receptors during development [30,33]. For example, pretreatment with 17-β-estradiol was shown to protect cultured human neuroblastoma cells from Pb-induced toxicity [33]. Finally, sex-related differences in the pharmacokinetics of absorption, metabolism and excretion of Pb may account for sex different Pb neurotoxicity [34].
Recent advancements in the generation of cultured primary neurons and other neural cell types from embryonic stem cells [35], induced pluripotent stem cells [36], or even ‘induced’ neurons or neural progenitors produced by the direct reprogramming of skin fibroblasts [37,38], have the potential to revolutionize our understanding of the molecular and cellular mechanisms underlying Pb neurotoxicity.
Epigenetic mechanisms regulate brain development
Epigenetics are commonly defined as the study of heritable changes in gene activities that are not related to alterations in the genetic code. Epigenetic modifications are mediated through a series of interconnected pathways that include DNA methylation, histone modifications and ncRNAs [39–41]. DNA methylation, undoubtedly the most studied epigenetic mechanism, corresponds to the addition of a methyl group to the 5 carbon position on the cytosine pyrimidine ring (5-methylcytosine [5mC]) via DNA methyltransferases (DNMTs) [39]. CpG sites, which can cluster in so-called ‘CpG islands’, are the target sites of DNA methylation. By altering the binding of transcriptional modulators, DNA methylation plays an important role in regulation of transcription. DNA methylation is most commonly associated with gene silencing and plays a critical role in central developmental events, such as chromosome X inactivation and gene imprinting [42,43]. The recent discovery that TET proteins can oxidize 5mC into 5-hydroxymethylcytosine (5hmC) has added another layer of regulation to the DNA methylation process [44].
In addition to changes in DNA methylation status to control neural gene expression and subsequent brain function, histone modifications are another epigenetic means of regulating gene transcription [40]. Histones are proteins around which the DNA wraps itself to form the nucleosome, the basic unit of chromatin organization. By controlling how tight or loose chromatin is during transcription, histones influence gene expression. The terminal tails of histones can be subjected to various post-translational modifications, including acetylation, phosphorylation, methylation or ubiquitination that are under the control of enzymes comprising histone acetyltransferases, histone deacetylases (HDACs), histone methylases and histone demethylases.
Another epigenetic mechanism of controlling gene transcription is ncRNAs [41]. As the name suggests, ncRNAs are not translated into protein and have been subdivided into several categories such as miRNAs, siRNAs, piRNAs and snoRNAs [41]. ncRNAs can regulate gene expression via various mechanisms such as transcriptional and post-transcriptional gene silencing or mRNA degradation.
The suggestion of an epigenetic regulation of brain development came from the observation that elements of the epigenetic apparatus, such as DNMTs, are expressed at various developmental time windows in the brain [45]. The human brain undergoes epigenetic changes throughout life, and the increasing number of epigenetic studies of the human brain is uncovering associations between alterations in epigenetic determinants and neurodevelopmental disorders [46,47]. It has been observed that altered expression and subsequent deregulation of methyl-CpG-binding proteins is associated with a variety of autism spectrum diseases, such as Rett syndrome, and DNA hypermethylation of the FMR1 gene promoter is associated with Fragile X syndrome, the most common form of mental retardation [46,47]. In humans, the brain starts forming approximately 3 weeks after conception and continues to develop and mature well into juvenile life. Early brain development is an especially sensitive time, during which changes in epigenetic marks can affect subsequent brain development and maturation. A critical player in embryonic brain development is the neural stem cell, which can self-renew and differentiate into the three major neural cell types: neurons, astrocytes and oligodendrocytes [47]. Our understanding of the involvement of epigenetic mechanisms in brain development has mostly emerged from animal model studies and shown that all three epigenetic mechanisms outlined above are critical players in the development of the healthy brain. The importance of DNA methylation in neural development came to light with the demonstration that null mutations of DMNTs resulted in decreased levels of DNA 5mCs, abnormal brain development and subsequent lethality in mouse embryos [48]. In addition, disruption of MeCP2 and MBD1 expression resulted in alterations of neuronal survival, differentiation and function in postnatal mouse brain [47]. Phosphorylation of MeCP2 was recently shown to be involved in synaptogenesis and spatial memory in a mouse model [49]. Several in vitro studies have also identified a role for DNA methylation in downregulating the pluripotency genes in embryonic stem cells during neural induction [50]. Interestingly, a recent study shows that the higher conversion rate from 5mC to 5hmC occurring postnatally in the mouse hippocampus and cerebellum is regulated by the binding of the MecP2 protein to 5mC, suggesting that hydroxymethylation of 5mC targets may be critical for brain development [51]. Indeed, 5hmC levels, which are intriguingly abundant in the brain compared with other tissues, were shown to increase from postnatal day 7 to 6 weeks of age (maturity) in the mouse cerebellum and hippocampus [51]. Also, a cerebellum-specific enrichment in 5hmC was observed from 6 weeks to 1 year of age. The observation that immunostaining for 5hmC associates with mature rather than immature neurons, suggests that 5hmC may be involved in neuronal differentiation. Gene profiling of mouse cerebellar tissue revealed that 5hmCs are enriched in gene bodies and upstream of transcriptional start sites, which was also observed in the adult human cortex [51]. Gene ontology analysis of genes exhibiting an age-related enrichment of stable or dynamic 5hmC sites identified genes involved in critical neurodevelopmental processes such as neuronal differentiation and synaptic function [51]. Taken together, these data highlight the importance of 5-hydroxymethylation of cytosine in shaping the brain.
Given its essential role in the regulation of gene expression, histone modifications have also been shown to regulate developing brain processes. For example, the transcription factor REST (also known as NRSF) blocks the neuronal phenotype in non-neuronal cells by recruiting a complex containing HDACs and other corepressors to the promoter of neuronal genes [50,52]. Furthermore, the HAT p300 is implicated in neuronal and astrocytic differentiation from neural stem cells [53], and HDACs play a role in oligodendrocyte differentiation [54]. Specifically, the inhibition of HDAC activity was shown to result in increased neuronal differentiation in the hippocampal neurogenic niche [55].
The expression of small nonprotein-coding miRNAs that bind to target mRNAs and inhibit their translation is also dynamically regulated during brain development [56]. Deletion of DICER, a disruptor of miRNA production, is known to affect neurogenesis, gliogenesis and neuroplasticity [56].
Prenatal or postnatal experiences can readily affect the brain epigenome and such changes may be transient or last a lifetime. For example, rat studies showed that quality of maternal care modified the epigenetic profile of a glucocorticoid receptor in the hippocampus of offspring, thereby influencing their response to stress as adults [57]. By contrast, short-lasting epigenetic changes were shown to be key to the establishment of learning and memory processes in fear conditioning experiments [58]. Taken together, these examples emphasize how important epigenetic mechanisms are in moderating every step of the brain developmental process, and how slight changes in these pathways caused by environmental exposures could trigger a cascade of neuro logical damage and dysfunction that may have immediate effects or manifest only later in life.
Early-life exposure to Pb causes changes in epigenetic marks
The nacent field of neuroepigenetics has already produced a small number of reports of great importance to elucidating the epigenetic basis of heavy metal neurotoxicity. The growing body of literature suggests that epigenetic determinants may indeed be strategic intermediaries between environmental exposures and brain development, and that such epigenetic targets can be altered by exposure to heavy metals. Thus, arsenic, nickel, chromium, cadmium and methylmercury have been shown to modify the DNA methylation status of a variety of genes [59–62]. Furthermore, changes in histone modifications have been reported for arsenic, nickel, chromium, methylmercury and copper, while arsenic, aluminum and cadmium have been identified as impacting ncRNA expression [59–62].
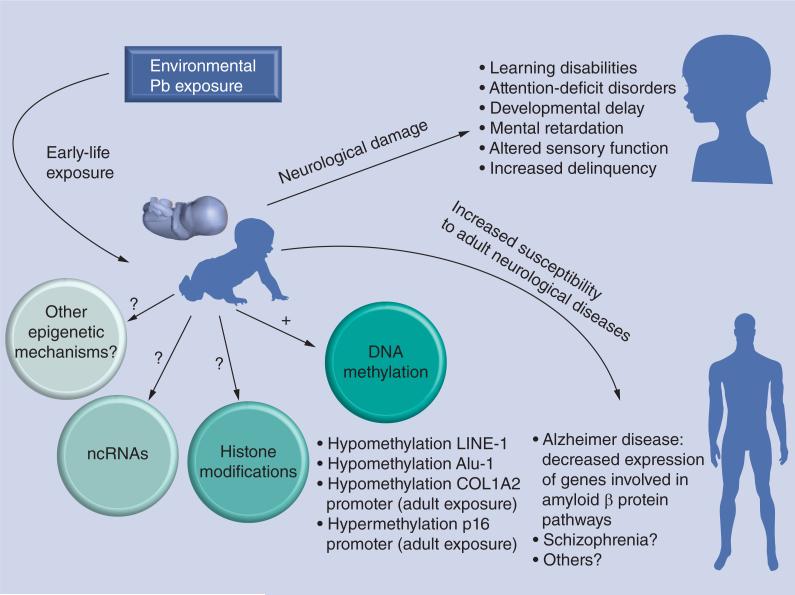
Although epidemiological studies of the effects of early-life Pb exposure on the human epigenome are still sparse, the first evidence that prenatal exposure to Pb can also influence the fetal epigenome in humans came from the work of Pilsner and coworkers [63]. This group analyzed the impact of maternal-prenatal Pb exposure on the DNA methylation profile of umbilical cord blood. Using pyrosequencing, they showed that maternal patella and tibia Pb levels, reflective of cumulative exposure, were inversely associated with cord blood methylation levels in LINE-1 and Alu1, respectively, two elements frequently analyzed in DNA methylation studies. Conversely, no association between cord blood Pb levels and DNA methylation changes was found, suggesting that Pb levels in bone (chronic exposure) rather than blood (recent exposure) are good biomarkers for Pb neurodevelopmental toxicity. The authors speculate that Pb-induced modifications in either oxidative stress or homocysteine levels may have destabilized epigenetic processes. These data, combined with previous studies showing an association between Pb maternal levels and IQ deficits in children [64,65], suggest that epigenetic mechanisms may be in part responsible for the cognitive impairments in Pb-exposed children. Further indication of Pb influence on methylation marks comes from studies performed in adults where an association between bone Pb levels and reduced LINE-1 DNA methylation was also found in elderly men [66]. In adult women undergoing in vitro fertilization, a statistically significant negative correlation was recently found between BLLs and promoter methylation of the COL1A2, a gene associated with preterm birth in humans [67]. In addition to hypomethylation, a study reporting a positive correlation between BLLs of Pb-exposed adult men and methylation of the p16 gene, a cell cycle regulator upregulated during neurodegeneration [68], showed that Pb exposure could also induce DNA hypermethylation.
In vitro cellular models have also been extremely useful to examine the epigenetic effects of Pb exposure. For example, Pb exposure inhibits the activity of IGF1-stimulated methionine synthase, an enzyme involved in the regulation of DNA methylation in cultured human neuroblastoma cells [69]. By contrast, exposure of human embryonic stem cells to Pb concentrations similar to those found in maternal and cord blood, had no effect on heterochromatin formation [70]. We have indicated earlier, that the synapse is a target of Pb toxicity. MeCP2, a crucial epigenetic regulator that binds methylated cytosines at the CpG sites on DNA, regulates the expression of factors key to synapse development, such as BDNF. Exposure to Pb impedes BDNF transcription and translation in rat embryonic hippocampal neurons [25]. By contrast, although Pb had no observable effect on the methylation status of the BDNF gene promoter, it induced a decrease in the levels of phosphorylated MeCP2 at S421, total MeCP2 and ratio of S421MeCP2:total MeCP2, thereby maintaining the repressor function of MeCP2 and preventing BDNF transcription [25]. Taken together, these studies provide compelling evidence that changes in the DNA methylation status of genes crucial to brain development may mediate some of the neurotoxic effects observed following early-life exposure to Pb (Figure 1). Whether early exposure to Pb impacts other epigenetic mechanisms, such as histone modifications or ncRNA expression, has yet to be determined.
Figure 1.
Epigenetic and neurological effects of environmental lead exposure identified in human epidemiological studies.
Pb: Lead.
Early Pb exposure, epigenetics & susceptibility to adult diseases
By modifying the global DNA methylation landscape, early-life Pb exposure may not only have immediate dire consequences for brain development, but may also have effects that persist after the initial exposure defining the ‘fetal origin of adult diseases’ or ‘Barker hypothesis’ [71]. Gestational exposure to Pb may increase the susceptibility to diseases later in life, as recently shown for obesity in a mouse model [31]. The idea of an association between developmental Pb exposure and adult-onset neurological diseases came from rodent studies showing that postnatal exposure to Pb induces an increase in the neonatal expression of the amyloid precursor protein (APP), a key protein in Alzheimer's disease. While this increase abated in adulthood, it resumed 20 months after the termination of Pb exposure. The increase in APP expression was observed to parallel an increase in expression and DNA-binding of the APP-regulating transcription factor SP1 [8]. Consequently, enhanced APP expression resulted into an increase in β-amyloid protein, which in turn promoted oxidative stress and enhanced the potential susceptibility to neurodegeneration. Not only were these observations confirmed in a primate model of developmental Pb exposure, but the possible involvement of epigenetic mechanisms was also put forward by Wu and collaborators [5]. In this study, it was shown that female Macaca fascicularis monkeys exposed as infants to Pb displayed a 50–100% increase in APP mRNA expression and β-amyloid protein levels in late adulthood. Furthermore, intracellular and extracellular deposits of β-amyloid protein, a pathological hallmark of Alzheimer's disease, were also noted in the cortex of Pb-exposed animals. Interestingly, a 20% decrease in the activity of the methylating enzyme DNMT1 was observed in adult brain tissues from developmentally Pb-exposed animals, suggesting that epigenetic mechanisms may have been mediating the neurotoxic effects of Pb. In addition, microarray analysis of brain-related genes identified 22 genes altered by Pb infantile exposure, 20 of which had enriched CpG sequences, making them vulnerable to Pb-induced changes in DNMT1 expression [5]. Another recent study indicated that infantile Pb exposure may also reduce the expression levels of several acetylated and methylated histones [72]. These data are of great potential importance given that an inverse relationship between prenatal BLLs and adult expression of genes involved in amyloid protein biology was recently reported in humans [6]. Another study has recently shown that aging mice developmentally exposed to Pb from gestational day 13 to postnatal day 20 exhibited changes in the DNA methylation profiles of many neural genes [73]. Four gene categories were identified: metal binding, metabolic enzymes, transduction/transcription and immune response. Upregulation of these genes, which normally occurs in aging, was suppressed by developmental Pb exposure, rendering the aging brain more susceptible to degeneration [73]. Interestingly, expression status of these genes correlated with their DNA methylation profiles. Although needing further confirmation in human epidemiological studies, these data strongly support an epi-genetic basis for the effects of developmental Pb exposure on the late-onset of neurological diseases (Figure 1).
Conclusion & future perspective
Untangling the complex epigenetics of early-life Pb exposure will undoubtedly shed new light on the mechanisms of developmental Pb neurotoxicity. Importantly, it will help us identify epigenetic biomarkers of early-life Pb exposure, which will be essential for early diagnosis, disease prevention and design of novel therapeutic strategies. Because Pb is known to cause oxidative stress, and TET proteins that convert 5mC into 5hmC are sensitive to oxidation, we speculate that Pb-induced changes in methylation patterns could be partly mediated through Pb oxidative stress and metabolism alterations [74]. This possibility, added to the fact that 5hmC marks are important for brain development, makes the analysis of Pb-induced hydroxylation of 5mC a new research avenue to improve our understanding of the epigenetic basis of Pb-induced brain dysfunction. Much research also remains to be carried out to define the role of early Pb exposure on other epigenetic processes known to affect brain development, such as his-tone modifications and expression of ncRNAs. Although long believed to be restricted to an individual or an individual generation, it now appears that some changes in epigenetic determinants can extend to the germline [75], raising the possibility that Pb-induced alterations could be propagated transgenerationally. Identifying the epigenetic basis of neurological effects of early-life Pb exposure will however be a complicated path, hampered by multiple challenges. First, although early-life Pb exposure is known to cause neurological problems in children, it is not yet understood why some children appear more susceptible than others to the Pb-triggered neurotoxic effects. It is likely that such differences in sensitivity will be partly due to the combination of individual epigenetic variability and interplay between Pb exposure, epigenetics and genetic background, which will need to be elucidated. Second, because sex differences in the neurodevelopmental effects of early Pb exposure have been reported in humans, future research should focus on how Pb exposure modulates gene imprinting and chromosome X inactivation, two processes crucial to normal development. Finally, given our natural environment, it is likely that coexposure to toxicants other than Pb may further complicate the interpretation of epigenetic data collected from Pb-related epidemiological studies [76]. Other factors, such as diet or stress [77], may predispose some individuals more than others to Pb-induced epigenetic changes and will have to be identified. Overcoming these difficulties and understanding how the DNA methylome and other epigenetic determinants act together to influence the neurodevelopmental effects of exposure to Pb and other heavy metals would have immediate implications for the prevention and treatment of heavy metal exposure and should be a central objective of the expanding field of toxicogenomics in years to come.
Executive summary.
Background
■ Lead (Pb) is a pervasive environmental threat.
■ Pb is a ubiquitous pollutant of our environment.
■ Developing embryos and children are especially sensitive to the health impact of Pb.
■ The developing brain is particularly susceptible to Pb toxicity.
Prenatal exposure to Pb affects brain development
■ Chronic exposure during prenatal life or early childhood can have dramatic effects on cognitive ability and neuropsychiatric function.
■ Severity of Pb-induced neurotoxicity is affected by sex.
■ Experimental studies have shown that the synapse is a major target of Pb toxicity.
■ Recent advances in the analysis of human neurons in vitro will accelerate our understanding of the molecular and cellular effects of Pb and other toxicants.
Epigenetic mechanisms regulate brain development
■ The human brain undergoes transient and stable epigenetic changes throughout its life.
■ Alterations of epigenetic determinants have been associated with neurodevelopmental disorders.
■ Researchers are trying to better understand how epigenetic determinants regulate brain development. In particular, emphasis has been put on the role of 5-hydroxymethylcytosines that are abundant in specific regions of the developing brain.
■ Multiple epigenetic regulating systems are involved in neurodevelopmental processes such as neural stem cell proliferation and neuronal/glial differentiation.
Early-life exposure to Pb causes changes in epigenetic determinants
■ Research to elucidate the epigenetic basis of Pb neurotoxicity is in its infancy.
■ Gestational Pb exposure results in changes of the methylation status of cord blood LINE-1 and Alu1. Hypo-/hyper-methylation in COL1A2 (a gene associated with preterm birth in humans), and p16 (a gene involved in neurodegeneration) have been reported.
■ Early-life exposure to Pb induces a decrease in phosphorylation of MeCp2 inhibiting transcription of BDNF, a gene key to synapse development and function.
Early Pb exposure, epigenetics & susceptibility to disease
■ Recent research raised the idea that early-life Pb exposure may play a role in the adult onset of diseases, a so-called ‘fetal origin of adult diseases’.
■ Animal model studies associate developmental exposure to Pb to increased risk of Alzheimer's disease, a neurodegenerative disease that generally affects older adults.
Future perspective
■ Recent research has shed light on the involvement of epigenetic mechanisms in neurotoxicity resulting from early-life exposure to Pb.
■ Challenges to be met are the elucidation of the relationships between Pb exposure, epigenetics, genetics, sex and coexposure to other toxicants.
■ Identification of the cellular systems downstream of the epigenome that are altered by Pb will reveal targets amenable to the design of actionable therapeutic intervention.
Acknowledgements
The authors wish to thank the members of J Cibelli's Laboratory (Michigan State University, MI, USA) for helpful discussions. We apologize to all our colleagues whose important work could not be directly cited.
This work was supported by ES012933 to H Hirsch and D Ruden.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References/Websites
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Kuehn BM. Panel advises tougher limits on lead exposure. JAMA. 2012;307:445. doi: 10.1001/jama.2012.50. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC. Teratogen update: lead and pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:409–420. doi: 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- 3.Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev. Environ. Health. 2009;24:15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LD, Cory-Slechta DA, Gilbert ME, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5■.Wu J, Basha MR, Brock B, et al. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [Demonstrated that epigenetic mechanisms may mediate the increased susceptibility to Alzheimer's disease-like pathology following developmental exposure to lead (Pb).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazumdar M, Xia W, Hofmann O, et al. Prenatal lead levels, plasma amyloid β levels, and gene expression in young adulthood. Environ. Health Perspect. 2012;120:702–707. doi: 10.1289/ehp.1104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2011;33:560–574. doi: 10.1016/j.neuro.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8■.Basha MR, Wei W, Bakheet SA, et al. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and β-amyloid in the aging brain. J. Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [Established that developmental exposure to Pb is associated with a delayed overexpression of the amyloid precursor protein, a protein involved in Alzheimer's disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernberg S. Lead poisoning in a historical perspective. Am. J. Ind. Med. 2000;38:244–254. doi: 10.1002/1097-0274(200009)38:3<244::aid-ajim3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC. The protean toxicities of lead: new chapters in a familiar story. Int. J. Environ. Res. Public Health. 2011;8:2593–2628. doi: 10.3390/ijerph8072593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyer RA. Transplacental transport of lead. Environ. Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 microg/dl. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan W, Holland SK, Cecil KM, et al. The impact of early childhood lead exposure on brain organization: a functional magnetic resonance imaging study of language function. Pediatrics. 2006;118:971–977. doi: 10.1542/peds.2006-0467. [DOI] [PubMed] [Google Scholar]
- 14.Wright JP, Dietrich KN, Ris MD, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J. Child Psychol. Psychiatry. 2010;51:58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ. Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brubaker CJ, Schmithorst VJ, Haynes EM, et al. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology. 2009;30:867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokes L, Letz R, Gerr F, et al. Neurotoxicity in young adults 20 years after childhood exposure to lead: the Bunker Hill experience. Occup. Environ. Med. 1998;55:507–516. doi: 10.1136/oem.55.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman A, Brew BJ, Guillemin GJ. Lead dysregulates serine/threonine protein phosphatases in human neurons. Neurochem. Res. 2011;36:195–204. doi: 10.1007/s11064-010-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellinger DC. Lead. Pediatrics. 2004;113:1016–1022. [PubMed] [Google Scholar]
- 22.Jedrychowski W, Perera F, Jankowski J, et al. Gender specific differences in neurodevelopmental effects of prenatal exposure to very low-lead levels: the prospective cohort study in three-year olds. Early Hum. Dev. 2009;85:503–510. doi: 10.1016/j.earlhumdev.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verina T, Rohde CA, Guilarte TR. Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience. 2007;145:1037–1047. doi: 10.1016/j.neuroscience.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmer B, Kuegler PB, Baudis B, et al. Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. Cell Death Differ. 2011;18:383–395. doi: 10.1038/cdd.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.Stansfield KH, Pilsner JR, Lu Q, Wright RO, Guilarte TR. Dysregulation of BDNF–TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders. Toxicol. Sci. 2012;127:277–295. doi: 10.1093/toxsci/kfs090. [Shows the role of Pb exposure on the phosphorylation and expression of MeCP2 in embryonic hippocampal neurons during synaptogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider J, Anderson D, Talsania K, Mettil W, Vadigepalli R. Effects of developmental lead exposure on the hippocampal transcriptome: influences of sex, developmental period, and lead exposure level. Toxicol. Sci. 2012;129(1):108–125. doi: 10.1093/toxsci/kfs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal AP, Guilarte TR. Molecular neurobiology of lead (Pb(2+)): effects on synaptic function. Mol. Neurobiol. 2010;42:151–160. doi: 10.1007/s12035-010-8146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He T, Hirsch HV, Ruden DM, Lnenicka GA. Chronic lead exposure alters presynaptic calcium regulation and synaptic facilitation in Drosophila larvae. Neurotoxicology. 2009;30:777–784. doi: 10.1016/j.neuro.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altmann L, Gutowski M, Wiegand H. Effects of maternal lead exposure on functional plasticity in the visual cortex and hippocampus of immature rats. Brain Res. Dev. Brain Res. 1994;81:50–56. doi: 10.1016/0165-3806(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JS, Anderson DW, Sonnenahalli H, Vadigepalli R. Sex-based differences in gene expression in hippocampus following postnatal lead exposure. Toxicol. Appl. Pharmacol. 2011;256:179–190. doi: 10.1016/j.taap.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leasure JL, Giddabasappa A, Chaney S, et al. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective effect of 17-β-estradiol in human neurocellular models of lead exposure. Neurotoxicology. 2007;28:396–401. doi: 10.1016/j.neuro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 36.Marchetto MC, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum. Mol. Genet. 2010;19:R71–R76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl Acad. Sci. USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.112. doi:10.1038/npp.2012.112 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 42.Barlow DP. Genomic imprinting: a mammalian epigenetic discovery model. Annu. Rev. Genet. 2011;45:379–403. doi: 10.1146/annurev-genet-110410-132459. [DOI] [PubMed] [Google Scholar]
- 43.Brockdorff N. Chromosome silencing mechanisms in X-chromosome inactivation: unknown unknowns. Development. 2011;138:5057–5065. doi: 10.1242/dev.065276. [DOI] [PubMed] [Google Scholar]
- 44■.Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [Demonstrated the in vitro conversion of 5-methylcytosine to 5-hydroxymethylcytosine by TET1 in mammalian DNA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr. Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 46.Murgatroyd C, Spengler D. Genetic variation in the epigenetic machinery and mental health. Curr. Psychiatry Rep. 2012;14:138–149. doi: 10.1007/s11920-012-0255-1. [DOI] [PubMed] [Google Scholar]
- 47.Jobe EM, McQuate AL, Zhao X. Crosstalk among epigenetic pathways regulates neurogenesis. Front. Neurosci. 2012;6:59. doi: 10.3389/fnins.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48■■.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [Key study that demonstrated that DNA methylation is crucial for mammalian development.] [DOI] [PubMed] [Google Scholar]
- 49.Li H, Zhong X, Chau KF, Williams EC, Chang Q. Loss of activity-induced phosphorylation of MeCP2 enhances synaptogenesis, LTP and spatial memory. Nat. Neurosci. 2011;14:1001–1008. doi: 10.1038/nn.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nat. Rev. Neurosci. 2010;11:377–388. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- 51■.Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [Demonstrated the importance of 5-hydroxymethylcytosine in brain development and neurological disorders.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima K, Yanagisawa M, Arakawa H, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 54.Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl Acad. Sci. USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S, Kim SY, Artis S, et al. Histone methylation regulates memory formation. J. Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng TF, Choudhuri S, Muldoon-Jacobs K. Epigenetic targets of some toxicologically relevant metals: a review of the literature. J. Appl. Toxicol. 2012;32(9):643–653. doi: 10.1002/jat.2717. [DOI] [PubMed] [Google Scholar]
- 60.Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicol. Mech. Methods. 2011;21:343–352. doi: 10.3109/15376516.2011.557878. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6:820–827. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63■■.Pilsner JR, Hu H, Ettinger A, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ. Health Perspect. 2009;117:1466–1471. doi: 10.1289/ehp.0800497. [Provided the first demonstration that gestational exposure to maternal Pb can modify the fetal epigenome in humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ. Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright RO, Schwartz J, Wright RJ, et al. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ. Health Perspect. 2010;118:790–795. doi: 10.1289/ehp.0901429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanna CW, Bloom MS, Robinson WP, et al. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF. Hum. Reprod. 2012;27:1401–1410. doi: 10.1093/humrep/des038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovatsi L, Georgiou E, Ioannou A, et al. p16 promoter methylation in Pb2+-exposed individuals. Clin. Toxicol. 2010;48:124–128. doi: 10.3109/15563650903567091. [DOI] [PubMed] [Google Scholar]
- 69.Waly M, Olteanu H, Banerjee R, et al. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol. Psychiatry. 2004;9:358–370. doi: 10.1038/sj.mp.4001476. [DOI] [PubMed] [Google Scholar]
- 70.Arai Y, Ohgane J, Yagi S, et al. Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J. Reprod. Dev. 2011;57:507–517. doi: 10.1262/jrd.11-034a. [DOI] [PubMed] [Google Scholar]
- 71.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bihaqi SW, Huang H, Wu J, Zawia NH. Infant exposure to lead (Pb) and epigenetic modifications in the aging primate brain: implications for Alzheimer's disease. J. Alzheimers Dis. 2011;27:819–833. doi: 10.3233/JAD-2011-111013. [DOI] [PubMed] [Google Scholar]
- 73■.Dosunmu R, Alashwal H, Zawia NH. Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging. Mech. Ageing Dev. 2012;133:435–443. doi: 10.1016/j.mad.2012.05.003. [Shows that developmental exposure to Pb alters the expression of aging-related genes accordingly to their DNA methylation status.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chia N, Wang L, Lu X, Senut MC, Brenner C, Ruden DM. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6:853–856. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- 75.Daxinger L, Whitelaw E. Transgenerational epigenetic inheritance: more questions than answers. Genome Res. 2010;20:1623–1628. doi: 10.1101/gr.106138.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim Y, Kim BN, Hong YC, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30:564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicol Sci. 2011;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.CDC lead homepage www.cdc.gov/nceh/lead.
- 102.EPA lead homepage www.epa.gov/lead.