Abstract
Expression of most plastid genes involves multiple post-transcriptional processing events, such as splicing, editing, and intercistronic processing. The latter involves the formation of mono-, di-, and multicistronic transcripts, which can further be regulated by differential stability and expression. The plastid pentacistronic psbB transcription unit has been well characterized in vascular plants. It encodes the subunits CP47 (psbB), T (psbT), and H (psbH) of photosystem II as well as cytochrome b 6 (petB) and subunit IV (petD) of the cytochrome b 6 f complex. Each of the petB and petD genes contains a group II intron, which is spliced during post-transcriptional modification. The small subunit of photosystem II, PsbN, is encoded in the intercistronic region between psbH and psbT but is transcribed in the opposite direction. Expression of the psbB gene cluster necessitates different processing events along with numerous newly evolved specificity factors conferring stability to many of the processed RNA transcripts, and thus exemplarily shows the complexity of RNA metabolism in the chloroplast.
Keywords: Arabidopsis, Editing, Processing, Splicing, Stability
Introduction
The chloroplast evolved as a result of an endosymbiotic event in which a cyanobacterial ancestor was taken over by a eukaryotic cell. Though main parts of the original plastid genes were transferred into the nucleus, chloroplasts still have retained a separate genome. Chloroplast genes are embedded in the regulatory network of the cell enabling an adaptive and developmentally regulated chloroplast biogenesis, which is mainly controlled by nuclear factors (Stern et al. 2010). A highly sophisticated system of transcript maturation including endo- and exonucleolytic activities, splicing, editing, and modulation of RNA stability has been developed which is not exploited to the same extent in the free-living cyanobacterial ancestor. Various mechanisms can determine the stability of chloroplast mRNAs, including protection of RNA termini by proteins or RNA secondary structures. Since untranslated regions are not protected by ribosomes they are typical sites of rather unspecific endonucleolytic cleavage. Newly formed RNA termini are subject to fast digestion by exonucleases making accessibility to such sequences a key determinant of mRNA stability (Stoppel and Meurer 2012). Gene-specific transacting factors encoded in the nucleus can bind the 5′ UTR of mRNAs to protect them against 5′ → 3′ exonucleases (Drager et al. 1998), while the transcript 3′-end can in turn be stabilized by stable stem-loop structures or proteins, protecting from digestion by 3′ → 5′ exonucleases (Barkan 2011).
Numerous nuclear-encoded factors have been acquired for processing and other post-transcriptional modifications of plastid transcripts (Stern et al. 2010; Barkan 2011). Most if not all protein-coding genes on vascular plant chloroplasts are found in polycistronic transcription units. Their intercistronic processing can differ between plant species and results in complex transcript pattern creating mono-, di-, and multicistronic transcripts which can further be regulated by differential stability. The psbB-psbT-psbH-petB-petD gene cluster has a promoter for the plastid-encoded RNA polymerase (PEP) and is highly conserved among vascular plants (Fig. 1). Each of the petB and petD genes contains a group II intron, which is spliced during post-transcriptional modification. Splicing along with intercistronic processing generates about 20 different mono-, di-, and oligocistronic transcripts (Barkan 1988; Westhoff and Herrmann 1988). Newly evolved specificity factors confer stability to many of these RNA transcripts by binding to their termini and blocking exoribonucleases. The evolution of the psbB cluster genes (Fig. 1), along with functions of the encoded proteins and known factors for transcript processing and stability events (Fig. 2) will be elaborated in this review.
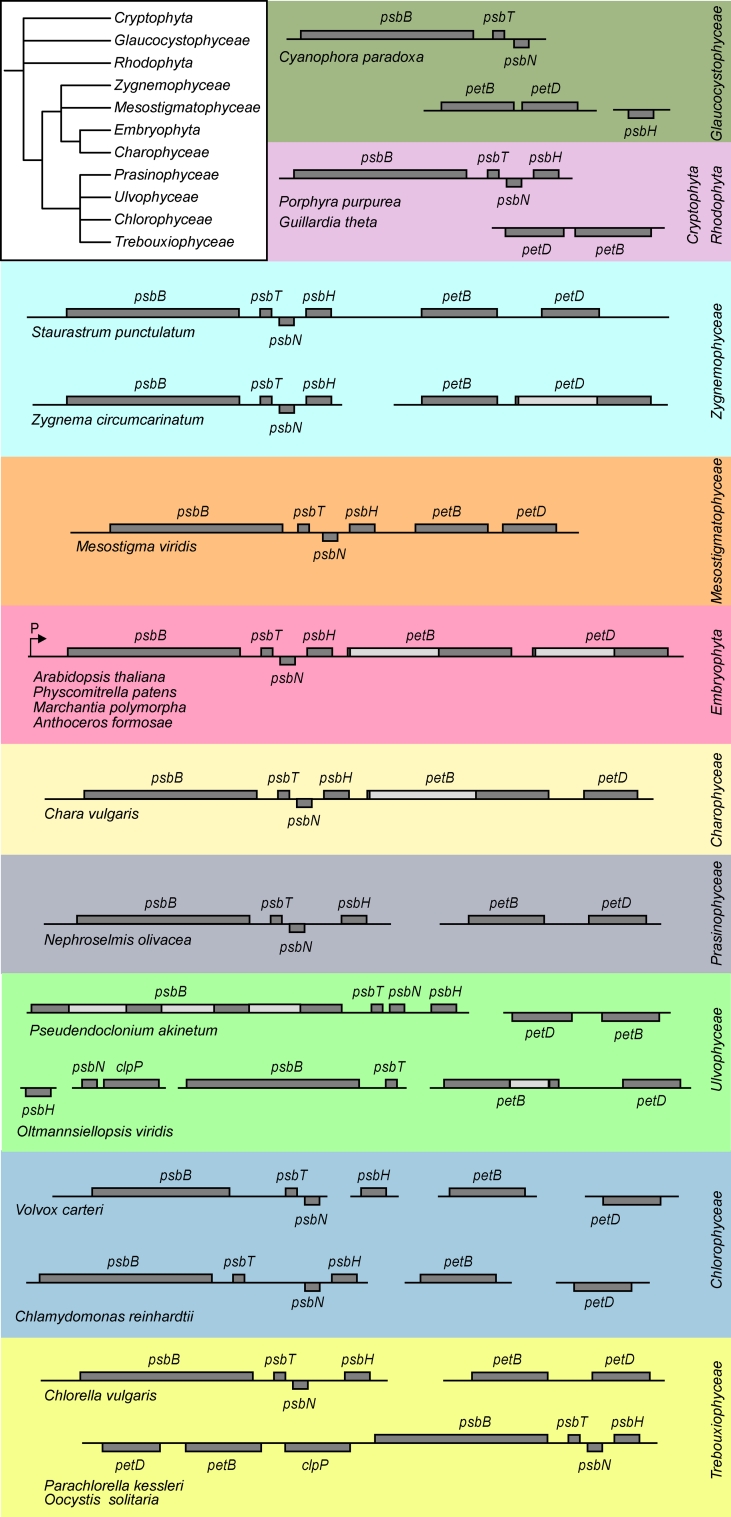
Fig. 1.
The psbB operon structure during evolution of the plant kingdom. The order of mono-, di-, and polycistrons of genes that are part of the psbB operon is shown for different organisms. A phylogenetic tree in the upper left corner shows the relationship among these organisms based on the nuclear genomes of the NCBI taxonomy tree (iTOL.embl.de). For details see text
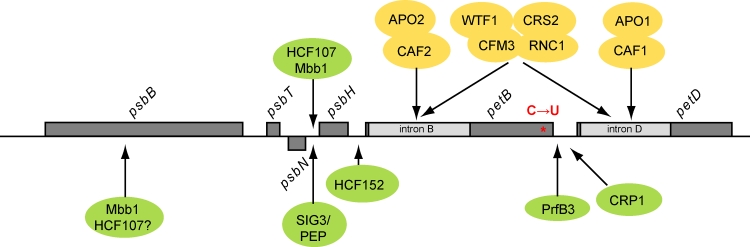
Fig. 2.
Complex RNA metabolism in the psbB operon. The structure of the psbB operon from Embryophyta is depicted in grey. The transcript stability factors HCF107, Mbb1, HCF152, PrfB3, CRP1 as well as the SIG3/PEP holoenzyme together with their corresponding/predicted binding sites are shown in green. Factors involved in splicing of the petB and petD introns are colored yellow. The petB editing site from maize and tobacco is drawn in red. The chronology of different events is still not clarified
Evolution of the psbB operon
We analyzed the evolution of the psbB operon structure in representative sequenced plastid genomes of different plant species (Fig. 1). The conserved organization of the chloroplast psbB operon can be found in all vascular plants, the liverwort Marchantia polymorpha, the moss Physcomitrella patens, and the hornwort Anthoceros formosae. The zygnemophycean green algae Staurastrum and Zygnema have a similar organization of the psbB operon like the Embryophyta with only slight differences. Both belong to the Charophyta that are assumed to have given rise to land plants (Turmel et al. 2002). In Staurastrum punctulatum the genes are clustered in the same way as in vascular plants, however, both petB and petD do not carry introns, whereas in Zygnema circumcarinatum parts of the operon encoding the proteins of PSII and cytochrome b 6 f complex are separated, and only petD contains an intron (Turmel et al. 2005). Mesostigma viride, also suggested to be a close relative of land plants (Lemieux et al. 2000; Karol et al. 2001), possesses complete psbB operons but is generally lacking introns. The charophycean green algae Chara vulgaris displays an almost typical psbB operon structure but lacks an intron in the petD gene.
In Chlorophyta the gene organization is different. Chlorella vulgaris and Nephroselmis olivacea, similar to Zygnema, have psbB/T/N/H and separate, uninterrupted petB/D clusters. However, it has to be noted that Nephroselmis similar to Mesostigma is lacking introns in its plastid genome. The other trebouxiophyceaen green algae Parachlorella and Oocystis have retained the conserved psbB/T/N/H organization but in contrast to Chlorella have the petB and petD genes moved 5′ of clpP, which in plastomes of vascular plants is located immediately upstream of psbB. Chlamydomonas reinhardtii and Volvox carteri have the vascular plant gene organization but petB and petD are not clustered and petD is transcribed on the opposite strand. V. carteri in addition has a separated psbH.
A quite different situation is found in two ulvophyceaen genomes. In Pseudendoclonium akinetum the psbN gene—with still unknown function—is on the same DNA strand as psbB, psbT, and psbH without changing the gene order, and psbB possesses three group I introns; the petB/D genes are clustered on the opposite strand and do not have introns. In Oltmannsiellopsis viridis the psbB cluster is completely fractured. While the dicistronic psbN/clpP and psbB/T are transcribed on the same strand followed by petB and petD, the psbH gene stands alone preceding all the other genes on the opposite strand. Again petB and petD are clustered and petB possesses a group IB intron, whereas petD has no intron (Pombert et al. 2006).
In Rhodophyta like f.e. Porphyra purpurea as well as in plastids of the secondary endosymbiont Guillardia theta, the psbB/T/N/H gene organization is conserved and petB and petD are clustered and intron-less, since plastomes of these species do not possess introns. In cyanelles of Cyanophora paradoxa in addition psbH is separated from psbB/T/N.
Therefore, it appears that during evolution, the pentacistronic psbB-psbT-psbH-petB-petD transcript with the psbN gene on the opposite DNA strand evolved as a result of fusing psbB/T/N/H and uninterrupted petB/D clusters after divergence of Streptophyta including Charophyta and land plants. The group II introns of petB and petD found in vascular plants might have been gained during evolution of the Charophyta. The diversity of the gene organization in Chlorophyta is consistent with the ability of introns to behave as mobile elements leading to intron gain and loss (Lambowitz and Belfort 1993).
The high degree of divergence as well as fluctuation of gene and intron composition of the psbB operon also attest the fast evolving operon organization accompanied by the recent acquisition of factors involved in processing of the primary transcript. In summary, the data indicate that the ontogenetic and phylogenetic integration of the chloroplast into the eukaryotic cell was predominantly established through controlling and functional clustering of plastid gene expression.
Functions of psbB gene cluster encoded proteins
The psbB gene encodes the photosystem II (PSII) chlorophyll-binding protein of 47 kDa (CP47). Together with the chlorophyll-binding protein of 43 kDa (CP43) it builds up the inner light-harvesting complex (Barber et al. 1997). CP47 is closely attached to the PsbA/PsbD heterodimer and transfers excitation energy from the outer light-harvesting complexes onto them (Lucinski and Jackowski 2006).
Two small peptides, both associated with PSII, were originally designated PsbT: a 4 kDa protein encoded in the chloroplast (PsbTc) and an unrelated 11 kDa protein of nuclear origin (PsbTn) (Shi and Schröder 2004; Müh et al. 2008). PsbTc stabilizes the Q B binding site in vivo that is essential for oxidation of reduced plastoquinone in darkness in an oxygen-dependent manner, possibly to keep the PSII acceptor site oxidized (Umate et al. 2008).
The PSII subunit H protein (PsbH) is important for PSII activity and was originally identified as an 8 kDa phosphoprotein in higher plant chloroplasts. The phosphorylation sites are thought to account for a regulatory role (Michel and Bennett 1987; Vener et al. 2001). Furthermore, PsbH might play a role in regulating PSII assembly/stability and repair of photodamaged PSII (Bennett 1977; Shi and Schröder 2004).
The small PSII subunit N (PsbN) is encoded on the opposite strand between psbT and psbH. Synechocystis mutants lacking both psbH and psbN showed no additional defects to psbH mutants alone, indicating that psbN is rather not essential for photoautotrophic growth (Mayes et al. 1993). In fact, the localization of PsbN as a PSII subunit has been a subject of a long debate that has not yet been satisfactorily solved. The gene product originally identified and named PsbN turned out to be PsbTc according to re-examinations of the PSII core oxygen-evolving complex (Kashino et al. 2002a). In addition, recent proteomics studies could not identify any PsbN associated to PSII (Gomez et al. 2002; Kashino et al. 2002b).
The last two genes of the psbB operon encode two proteins of the cytochrome b 6 f complex. Having an oxidoreductase activity, this complex is one of the central points of electron transport through the thylakoid membrane (Allen 2002). In addition to cytochrome b 6 (petB) and subunit IV (petD), this complex consists of cytochrome f (petA), the Rieske protein (petC), and the four small polypeptides PetG, PetL, PetM, and PetN (Schwenkert et al. 2007). Apart from its function in linear electron transport from PSII to PSI, the cytochrome b 6 f complex is also involved in cyclic electron transport around PSI, regulation of gene expression, and reversible phosphorylation of plastid proteins (Joliot and Joliot 2006).
Transcript specificity factors
The sigma-like transcription factor SIG3
The activity of the PEP is regulated by sigma-like transcription initiation factors (SIG) that share a widely conserved C-terminal RNA polymerase sigma-70 factor domain. One of six SIG factors encoded in the Arabidopsis nuclear genome and with homologs only among Embryophyta is SIG3. The functionality of SIG3 is not essential for plastid functions and was proposed to depend on its attachment to thylakoid membranes (Privat et al. 2003). The strong reduction of psbN mRNA in sig3 mutants as revealed by microarray and RNA gel blot analysis was proven to result from tight regulation of psbN gene expression by the SIG3-PEP holoenzyme binding to a promoter region upstream of psbN (Fig. 2) (Zghidi et al. 2007). Furthermore, psbN read-through transcription produces antisense RNA to psbT mRNA (Zghidi et al. 2007; Zghidi-Abouzid et al. 2011). It was shown that during photooxidative stress conditions the presence of this psbT antisense RNA leads to the formation of RNA double-strand hybrids and accordingly to translational inactivation of psbT (Zghidi-Abouzid et al. 2011). Thus, psbT mRNA can be protected from nucleolytic degradation by single-strand specific nucleases. Besides its function in transcription of psbN mRNA and psbT antisense RNA, SIG3 was recently shown to participate in transcription initiation of genes atpI/H/F/A of the large atp operon by specifically recognizing an internal promoter between atpI and atpH (Zghidi et al. 2007; Malik Ghulam et al. 2012).
The high-chlorophyll-fluorescence phenotype protein HCF107
HCF107 is a tetratricopeptide repeat (TPR)-like protein with 11 half-a-TPR (HAT) helical repeats arranged in tandem (Sane et al. 2005; Hammani et al. 2012). Mutants of this gene are seedling lethal and therefore have to be maintained on sucrose-supplemented medium. Their inability to accumulate 5′-end processed psbH transcripts results in the loss of PsbH and consequently in the disruption of PSII activity (Felder et al. 2001; Sane et al. 2005). Accordingly, HCF107 was proposed to function in intercistronic processing or stabilization of the psbH 5′ UTR (Felder et al. 2001). It was suggested, that only those psbH-containing transcripts can be translated that have 5′ processed ends at position −45 with respect to the ATG start codon (Felder et al. 2001). Similar to the molecular function of the well-described PPR10 protein (Pfalz et al. 2009), processing at the −45 site would lead to unfolding of stable stem loops which otherwise would prevent translation. The HCF107 binding site was postulated to be at the psbH 5′-end as indicated by RNA footprint analysis with a small RNA defining the position of the processed psbH 5′-terminus by blocking 5′ → 3′ degradation (Zhelyazkova et al. 2012). This hypothesis was recently confirmed by RNA-binding studies, revealing that the sequence-specific RNA-binding properties of HCF107 come from the HAT motif (Fig. 2) (Hammani et al. 2012). Upon binding to its native RNA ligand in the psbH 5′ UTR, the local RNA structure undergoes conformational changes, which in turn protect the adjacent RNA from a 5′ → 3′ exonuclease in vitro, thus defining the 5′-end of processed psbH transcripts and stabilizing the downstream transcript. The psbH 5′ UTR and the translation initiation region are predicted to form stable duplexes if HCF107 is absent. Upon binding of HCF107, these inhibitory duplexes dissociate and expose the sequence so that ribosomes can easily bind, resulting in increased psbH translation efficiency (Hammani et al. 2012).
In a similar manner, HCF107 could be involved in translation of the psbB gene, since along with PsbH also the CP47 protein (encoded by the psbB gene) was reported to be missing in hcf107 mutants. On the other hand, there are no sequence similarities between the 5′ psbH and psbB sequences. Since it was reported that hcf107 mutants grown under very low light are able to accumulate slight amounts of CP47 (Plücken et al. 2002), the translational deficiencies are likely to represent a secondary effect of the missing PsbH rather than representing a dual function of HCF107.
The psbB mRNA maturation factor Mbb1
The well-characterized Chlamydomonas protein Mbb1 is sharing a sequence identity of about 40 % to the Arabidopsis HCF107 and similar proteins also occur in other Chlorophyta species. Knockout mutants of mbb1 are affected in psbB 5′-end processing and psbH processing/stability and predominantly fail to accumulate the psbB encoded CP47 (Fig. 2) (Vaistij et al. 2000a). This is inconsistent with the Arabidopsis hcf107 mutation, that is only affecting psbH accumulation. Chlamydomonas mbb1 mutants consequently display broader defects in PSII complex assembly (Monod et al. 1992). Furthermore, Mbb1 is a stromal protein compared to HCF107 being a membrane bound protein, most likely because the similarities between both proteins are spanning only the TPR region. Despite these differences phylogenetic analysis has clearly shown that both proteins are evolutionary orthologs (Felder et al. 2001). Similar to HCF107, Mbb1 has ten HAT motifs arranged in tandem. These motifs most likely mediate protein–protein interaction, supported by the fact that Mbb1 has been identified as part of a 300 kDa complex (Vaistij et al. 2000b).
The high-chlorophyll-fluorescence phenotype protein HCF152
The Arabidopsis protein HCF152 is a member of the pentatricopeptide repeat (PPR) protein family and forms homodimers via its C-terminal non-PPR regions (Nakamura et al. 2003). It is required for the accumulation of 5′ or 3′ processed RNA termini mapping in the intercistronic region of psbH-petB in Arabidopsis chloroplasts and accordingly hcf152 mutants are lacking the cytochrome b 6 f complex (Meierhoff et al. 2003; Nakamura et al. 2003). It has been shown that the psbH 3′-end maps downstream of the petB 5′-end with an overlap of about 25-nt (Pfalz et al. 2009). After initial uncertainties about the HCF152 binding site, it has now been clearly shown, that this 25-nt overlap in the psbH–petB intergenic region constitutes the binding site for HCF152 in analogy to the recently characterized PPR10 and HCF107 proteins (Fig. 2). Thus, HCF152 is an another example for a transcript specificity factor, that defines processed transcript termini and protects upstream and downstream RNA transcripts from digestion by 5′ → 3′ or 3′ → 5′-exonucleases (Zhelyazkova et al. 2012). Similar to HCF107 homologies to proteins from other organisms are restricted to Embryophyta and Chlorophyta, clearly showing the recent evolvement of these stability factors.
The ribosomal peptide chain release factor B (PrfB)-like protein PrfB3
The protein PrfB3 is localized in the chloroplast stroma in a petB RNA-containing complex (Stoppel et al. 2011). Absence of the PrfB3 gene in sequenced genomes of cyanobacteria, red, green, and diatom algae suggests that PrfB3 evolved after the divergence of vascular plants, probably as a result of a duplication of the ancestral PrfB gene and subsequent loss of the peptide chain release function, followed by loss of the two conserved motifs, harboring the sites for UGA stop-codon recognition and peptidyl-tRNA hydrolysis. It is tempting to suggest that PrfB3 might have arisen after the appearance of the typical psbB operon organization for higher plants. This hypothesis can be further substantiated by absence of PrfB3 in C. reinhardtii which does not involve petB and petD as part of the psbB transcription unit. PrfB3 is essentially required for photoautotrophic growth and mutations in this gene lead to a specific deficiency of the cytochrome b 6 f complex (Stoppel et al. 2011). PrfB3 has been shown to bind specifically to the 3′ region of processed petB transcripts, stabilizing, and protecting them from digestion by exonucleases (Fig. 2). Furthermore, the stability of these transcripts is regulated in a light- and stress-dependent manner, to adjust cytochrome b 6 levels (Stoppel et al. 2011). Thereby, overall photosynthesis rates can be controlled according to the plants’ needs. Interestingly, no RNA footprint has been identified for the 3′ petB region, indicating that non-PPR proteins like PrfB3 underlie a different mechanism of RNA stabilization than PPR proteins, possibly by binding to the transcript in a less strong manner. This would also facilitate faster and more sensitive regulation of the transcript’s RNA stability.
The chloroplast RNA processing 1 (CRP1) protein
Originally, the PPR protein CRP1 had been described to be required for the accumulation of processed 5′- and 3′-termini in the maize petB–petD intergenic region (Fig. 2) (Barkan et al. 1994; Fisk et al. 1999). crp1 mutants lack both monocistronic petB and petD, but in contrast to prfB3 are able to accumulate cytochrome b 6 protein to normal levels (Barkan et al. 1994). Accordingly, the 3′ petB and 5′ petD transcript ends of Arabidopsis do not overlap—whereas in maize they do—and independent intercistronic processing events produce the respective ends (Barkan et al. 1994; Stoppel et al. 2011). Thus, similar to prfB3, the lack of a monocistronic transcript can lead to severe defects, indicating that normal levels of a polycistronic precursor transcript are not always sufficient for translation of a protein. A reason for the inability to translate polycistronic transcripts can be the formation of stable hairpins that inhibit the start codon from being recognized by the translation machinery (Barkan et al. 1994). The necessity of such a regulatory mechanism is evolutionary young demonstrated by the fact that again homologs to CRP1 can only be found among Embryophyta and some Chlorophyta. In addition to the lack of monocistronic petB and petD, crp1 mutants display defects in translation of petA, another subunit of the cytochrome b 6 f complex, and psaC, a PSI subunit (Fisk et al. 1999). However, it seems that both defects occur independent from each other and binding affinity of a recombinant CRP1 protein has been shown only for petA transcripts (Williams-Carrier et al. 2008).
Splice factors of the psbB operon
The psbB operon of higher plants has two group II intron-containing genes, petB and petD. Though being derived from ‘self-splicing’ ribozymes, introns of higher plant chloroplasts depend on specific splice-factors for proper intron folding into catalytically active structures (Barkan 2011). The complexity of protein association to group II introns involves for example six proteins being required for splicing of petB and petD, respectively. This includes genes of the APO domain family (APO1 and APO2), the CRM domain family (CAF1, CAF2, CRS2, CFM3), and the proteins WTF1 and RNC1 (Fig. 2). Except CRS2 that also has homologs among Chlorophyta all other splice factors can only be found in Embryophyta, consistent with the sole presence of both introns in this clade. Furthermore, this is an evidence that both intron splice sites and corresponding factors depend at least in part on each other.
The mutant apo1 was originally described as being affected in PSI assembly (Amann et al. 2004) and APO1 protein was later found to be part of ribonucleoprotein particles involved in splicing of group II intron transcripts. While the major function of APO1 is in splicing of the second intron of ycf3, apo1 mutants also fail to properly splice petD and clpP-intron 1 (Watkins et al. 2011). Similarly, APO2 seems to account for splicing of the petB intron (Barkan 2011). CAF1, CAF2, CRS2, and CFM3 are all members of the chloroplast RNA splicing and maturation (CRM) domain family (Asakura et al. 2008). The paralogs CAF1 and CAF2 build a heterodimeric complex that functions together with CRS2, a peptidyl-tRNA hydrolase homolog (Ostheimer et al. 2003, 2006). CFM3 associates with the CRS2/CAF complex to promote splicing of a certain set of group II introns, including those of petB and petD (Asakura et al. 2008). Knockout mutants of either of these genes exhibit strong splicing defects, indicating the non-redundancy of the respective proteins. Other members of the CRM domain family have been shown to enhance intron folding and this was also suggested to be the function of CFM3 (Ostersetzer et al. 2005; Asakura et al. 2008). In contrast, the role of CAF1 and CAF2 seems to be restricted to the recruitment of the splicing factor CRS2 to specific introns. The exact role of CRS2 however still has to be elucidated. The protein ‘What’s This Factor 1’ (WTF1) and an RNase III domain protein (RNC1), that does not exhibit endonucleolytic activity, were independently discovered through co-immunoprecipitation analyses with proteins of the CRM domain family (Watkins et al. 2007; Kroeger et al. 2009). In a similar manner to the CRM proteins, WTF1 and RNC1 build a heterodimer that binds RNA and associates with several group II introns, among them the petB and petD introns (Kroeger et al. 2009). The reason for this highly complex RNA splicing machinery for group II introns is still more than cryptic and future studies will have to show specific functions for each of the proteins described.
Editing in the psbB operon
During post-transcriptional RNA editing events the exchange of individual nucleotides in transcripts, altering amino acid identity or creating new translation initiation codons or stop codons, is often essentially required for the production of functional proteins. While editing in land plants is usually a cytidine-to-uridine (C-to-U) change some moss and fern organelles have additional U-to-C editing reactions (Castandet and Araya 2011). A recent study of the moss Takakia lepidozioides identified 116 C-to-U conversions in transcripts of the psbB operon (Sugita et al. 2006).
One of these editing sites at position 204 within the petB coding region is conserved in tobacco and maize (Fig. 2) but not in Arabidopsis or Chlamydomonas (Freyer et al. 1993; Tillich et al. 2005; Zito et al. 1997). This editing event changes the amino acid proline at this position into leucine and already occurs before splicing and processing of the psbB operon primary transcript and is therefore an independent processing step in the maturation of the psbB transcription unit. All factors involved in editing identified so far are members of the PPR protein family (Bentolila et al. 2012; Fujii and Small 2011). One possibility to identify the factor for this specific editing site could be to look for PPR proteins present in tobacco and maize but not in species where this editing site is not found.
Conclusions/perspectives
It appears that plastid gene expression is mainly regulated and controlled by products of newly evolved nuclear genes or by conserved proteins, which often acquired new functions and/or new domains. Extensive endonucleolytic cleavage events were important to extract individual gene segments from the polycistronic context and to independently regulate both stability and translation of each gene, irrespective whether they are co-transcribed or not. The specificity by which the expression of plastid genes is regulated is evident by the variety of observed mutant phenotypes. Another example is the higher divergence of target sequence elements in UTRs and intergenic regions as compared to conserved coding regions even between closely related species (Greiner et al. 2008a, b). Coding regions are assumed not to be significantly subjected to the control of gene expression. This is also consistent with the fact that different genome-plastome incompatibilities are based on malfunction of plastid gene expression. Importantly, the frequent occurrence of novel plant-specific genes required for the chloroplast mRNA homeostasis demonstrates that transcript regulation represents a fast evolving process during evolution. In contrast, nuclear-encoded factors such as HCF136, ALB3, VIPP1, YCF3, PSB27, and HCF101 required for assembly of conserved structures like the photosynthetic complexes mainly remained conserved and have already been established in cyanobacteria.
Acknowledgments
The authors wish to thank the Deutsche Forschungsgemeinschaft for funding (SFB TR1 project B2).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Abbreviations
- PSII
Photosystem II
- PEP
Plastid-encoded RNA polymerase
- PPR
Pentatricopeptide repeat
- TPR
Tetratricopeptide repeat
- HAT
Half-a-TPR
Footnotes
A contribution to the Special Issue on Evolution and Biogenesis of Chloroplasts and Mitochondria.
References
- Allen J. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell. 2002;110:273–276. doi: 10.1016/S0092-8674(02)00870-X. [DOI] [PubMed] [Google Scholar]
- Amann K, Lezhneva L, Wanner G, Herrmann RG, Meurer J. ACCUMULATION OF PHOTOSYSTEM ONE1, a member of a novel gene family, is required for accumulation of [4Fe-4S] cluster-containing chloroplast complexes and antenna proteins. Plant Cell. 2004;16:3084–3097. doi: 10.1105/tpc.104.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura Y, Bayraktar OA, Barkan A. Two CRM protein subfamilies cooperate in the splicing of group IIB introns in chloroplasts. RNA. 2008;14:2319–2332. doi: 10.1261/rna.1223708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B. The structure, function and dynamics of photosystem two. Physiol Plant. 1997;100:817–827. doi: 10.1111/j.1399-3054.1997.tb00008.x. [DOI] [Google Scholar]
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988;7:2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Expression of plastid genes: organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155:1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Walker M, Nolasco M, Johnson D. A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 1994;13:3170–3181. doi: 10.1002/j.1460-2075.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Phosphorylation of chloroplast membrane polypeptides. Nature. 1977;269:344–346. doi: 10.1038/269344a0. [DOI] [Google Scholar]
- Bentolila S, Heller WP, Sun T, Babina AM, Friso G, van Wijk KJ, Hanson MR. RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc Natl Acad Sci USA. 2012;109:E1453–E1461. doi: 10.1073/pnas.1121465109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castandet B, Araya A. RNA editing in plant organelles. Why make it easy? Biochemistry. 2011;76:924–931. doi: 10.1134/S0006297911080086. [DOI] [PubMed] [Google Scholar]
- Drager RG, Girard-Bascou J, Choquet Y, Kindle KL, Stern DB. In vivo evidence for 5′ → 3′ exoribonuclease degradation of an unstable chloroplast mRNA. Plant J. 1998;13:85–96. doi: 10.1046/j.1365-313X.1998.00016.x. [DOI] [PubMed] [Google Scholar]
- Felder S, Meierhoff K, Sane AP, Meurer J, Driemel C, Plucken H, Klaff P, Stein B, Bechtold N, Westhoff P. The nucleus-encoded HCF107 gene of Arabidopsis provides a link between intercistronic RNA processing and the accumulation of translation-competent psbH transcripts in chloroplasts. Plant Cell. 2001;13:2127–2141. doi: 10.1105/TPC.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk DG, Walker MB, Barkan A. Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J. 1999;18:2621–2630. doi: 10.1093/emboj/18.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer R, Hoch B, Neckermann K, Maier RM, Kossel H. RNA editing in maize chloroplasts is a processing step independent of splicing and cleavage to monocistronic mRNAs. Plant J. 1993;4:621–629. doi: 10.1046/j.1365-313X.1993.04040621.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 2011;191:37–47. doi: 10.1111/j.1469-8137.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- Gomez SM, Nishio JN, Faull KF, Whitelegge JP. The chloroplast grana proteome defined by intact mass measurements from liquid chromatography mass spectrometry. Mol Cell Proteomics. 2002;1:46–59. doi: 10.1074/mcp.M100007-MCP200. [DOI] [PubMed] [Google Scholar]
- Greiner S, Wang X, Herrmann RG, Rauwolf U, Mayer K, Haberer G, Meurer J. The complete nucleotide sequences of the 5 genetically distinct plastid genomes of Oenothera, subsection Oenothera: II. A microevolutionary view using bioinformatics and formal genetic data. Mol Biol Evol. 2008;25:2019–2030. doi: 10.1093/molbev/msn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Wang X, Rauwolf U, Silber MV, Mayer K, Meurer J, Haberer G, Herrmann RG. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. sequence evaluation and plastome evolution. Nucleic Acids Res. 2008;36:2366–2378. doi: 10.1093/nar/gkn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K, Cook WB, Barkan A. RNA binding and RNA remodeling activities of the half-a-tetratricopeptide (HAT) protein HCF107 underlie its effects on gene expression. Proc Natl Acad Sci USA. 2012;109:5651–5656. doi: 10.1073/pnas.1200318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P, Joliot A. Cyclic electron flow in C3 plants. Biochim Biophys Acta. 2006;1757:362–368. doi: 10.1016/j.bbabio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Karol KG, McCourt RM, Cimino MT, Delwiche CF. The closest living relatives of land plants. Science. 2001;294:2351–2353. doi: 10.1126/science.1065156. [DOI] [PubMed] [Google Scholar]
- Kashino Y, Koike H, Yoshio M, Egashira H, Ikeuchi M, Pakrasi HB, Satoh K. Low-molecular-mass polypeptide components of a photosystem II preparation from the thermophilic cyanobacterium Thermosynechococcus vulcanus. Plant Cell Physiol. 2002;43:1366–1373. doi: 10.1093/pcp/pcf168. [DOI] [PubMed] [Google Scholar]
- Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry. 2002;41:8004–8012. doi: 10.1021/bi026012+. [DOI] [PubMed] [Google Scholar]
- Kroeger TS, Watkins KP, Friso G, van Wijk KJ, Barkan A. A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing. Proc Natl Acad Sci USA. 2009;106:4537–4542. doi: 10.1073/pnas.0812503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz AM, Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Lemieux C, Otis C, Turmel M. Ancestral chloroplast genome in Mesostigma viride reveals an early branch of green plant evolution. Nature. 2000;403:649–652. doi: 10.1038/35001059. [DOI] [PubMed] [Google Scholar]
- Lucinski R, Jackowski G. The structure, functions and degradation of pigment-binding proteins of photosystem II. Acta Biochim Pol. 2006;53:693–708. [PubMed] [Google Scholar]
- Malik Ghulam M, Zghidi-Abouzid O, Lambert E, Lerbs-Mache S, Merendino L. Transcriptional organization of the large and the small ATP synthase operons, atpI/H/F/A and atpB/E, in Arabidopsis thaliana chloroplasts. Plant Mol Biol. 2012;79:259–272. doi: 10.1007/s11103-012-9910-5. [DOI] [PubMed] [Google Scholar]
- Mayes SR, Dubbs JM, Vass I, Hideg E, Nagy L, Barber J. Further characterization of the psbH locus of Synechocystis sp. PCC 6803: inactivation of psbH impairs QA to QB electron transport in photosystem 2. Biochemistry. 1993;32:1454–1465. doi: 10.1021/bi00057a008. [DOI] [PubMed] [Google Scholar]
- Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell. 2003;15:1480–1495. doi: 10.1105/tpc.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel HP, Bennett J. Identification of the phosphorylation site of an 8.3 kDa protein from photosystem II of spinach. FEBS Lett. 1987;212:103–108. doi: 10.1016/0014-5793(87)81565-X. [DOI] [Google Scholar]
- Monod C, Goldschmidt-Clermont M, Rochaix JD. Accumulation of chloroplast psbB RNA requires a nuclear factor in Chlamydomonas reinhardtii. Mol Gen Genet. 1992;231:449–459. doi: 10.1007/BF00292715. [DOI] [PubMed] [Google Scholar]
- Müh F, Renger T, Zouni A. Crystal structure of cyanobacterial photosystem II at 3.0 A resolution: a closer look at the antenna system and the small membrane-intrinsic subunits. Plant Physiol Biochem. 2008;46:238–264. doi: 10.1016/j.plaphy.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Meierhoff K, Westhoff P, Schuster G. RNA-binding properties of HCF152, an Arabidopsis PPR protein involved in the processing of chloroplast RNA. Eur J Biochem. 2003;270:4070–4081. doi: 10.1046/j.1432-1033.2003.03796.x. [DOI] [PubMed] [Google Scholar]
- Ostersetzer O, Cooke AM, Watkins KP, Barkan A. CRS1, a chloroplast group II intron splicing factor, promotes intron folding through specific interactions with two intron domains. Plant Cell. 2005;17:241–255. doi: 10.1105/tpc.104.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostheimer GJ, Williams-Carrier R, Belcher S, Osborne E, Gierke J, Barkan A. Group II intron splicing factors derived by diversification of an ancient RNA-binding domain. EMBO J. 2003;22:3919–3929. doi: 10.1093/emboj/cdg372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostheimer GJ, Rojas M, Hadjivassiliou H, Barkan A. Formation of the CRS2-CAF2 group II intron splicing complex is mediated by a 22-amino acid motif in the COOH-terminal region of CAF2. J Biol Chem. 2006;281:4732–4738. doi: 10.1074/jbc.M508921200. [DOI] [PubMed] [Google Scholar]
- Pfalz J, Bayraktar OA, Prikryl J, Barkan A. Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 2009;28:2042–2052. doi: 10.1038/emboj.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plücken H, Müller B, Grohmann D, Westhoff P, Eichacker LA. The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 2002;532:85–90. doi: 10.1016/S0014-5793(02)03634-7. [DOI] [PubMed] [Google Scholar]
- Pombert JF, Lemieux C, Turmel M. The complete chloroplast DNA sequence of the green alga Oltmannsiellopsis viridis reveals a distinctive quadripartite architecture in the chloroplast genome of early diverging ulvophytes. BMC Biol. 2006;4:3. doi: 10.1186/1741-7007-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privat I, Hakimi MA, Buhot L, Favory JJ, Mache-Lerbs S. Characterization of Arabidopsis plastid sigma-like transcription factors SIG1, SIG2 and SIG3. Plant Mol Biol. 2003;51:385–399. doi: 10.1023/A:1022095017355. [DOI] [PubMed] [Google Scholar]
- Sane AP, Stein B, Westhoff P. The nuclear gene HCF107 encodes a membrane-associated R-TPR (RNA tetratricopeptide repeat)-containing protein involved in expression of the plastidial psbH gene in Arabidopsis. Plant J. 2005;42:720–730. doi: 10.1111/j.1365-313X.2005.02409.x. [DOI] [PubMed] [Google Scholar]
- Schwenkert S, Legen J, Takami T, Shikanai T, Herrmann RG, Meurer J. Role of the low-molecular-weight subunits PetL, PetG, and PetN in assembly, stability, and dimerization of the cytochrome b6f complex in tobacco. Plant Physiol. 2007;144:1924–1935. doi: 10.1104/pp.107.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LX, Schröder WP. The low molecular mass subunits of the photosynthetic supracomplex, photosystem II. Biochim Biophys Acta. 2004;1608:75–96. doi: 10.1016/j.bbabio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Stern DB, Goldschmidt-Clermont M, Hanson MR. Chloroplast RNA metabolism. Annu Rev Plant Biol. 2010;61:125–155. doi: 10.1146/annurev-arplant-042809-112242. [DOI] [PubMed] [Google Scholar]
- Stoppel R, Meurer J. The cutting crew–ribonucleases are key players in the control of plastid gene expression. J Exp Bot. 2012;63:1663–1673. doi: 10.1093/jxb/err401. [DOI] [PubMed] [Google Scholar]
- Stoppel R, Lezhneva L, Schwenkert S, Torabi S, Felder S, Meierhoff K, Westhoff P, Meurer J. Recruitment of a ribosomal release factor for light- and stress-dependent regulation of petB transcript stability in Arabidopsis chloroplasts. Plant Cell. 2011;23:2680–2695. doi: 10.1105/tpc.111.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M, Miyata Y, Maruyama K, Sugiura C, Arikawa T, Higuchi M. Extensive RNA editing in transcripts from the PsbB operon and RpoA gene of plastids from the enigmatic moss Takakia lepidozioides. Biosci Biotechnol Biochem. 2006;70:2268–2274. doi: 10.1271/bbb.60204. [DOI] [PubMed] [Google Scholar]
- Tillich M, Funk HT, Schmitz-Linneweber C, Poltnigg P, Sabater B, Martin M, Maier RM. Editing of plastid RNA in Arabidopsis thaliana ecotypes. Plant J. 2005;43:708–715. doi: 10.1111/j.1365-313X.2005.02484.x. [DOI] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C. The chloroplast and mitochondrial genome sequences of the charophyte Chaetosphaeridium globosum: insights into the timing of the events that restructured organelle DNAs within the green algal lineage that led to land plants. Proc Natl Acad Sci USA. 2002;99:11275–11280. doi: 10.1073/pnas.162203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C. The complete chloroplast DNA sequences of the charophycean green algae Staurastrum and Zygnema reveal that the chloroplast genome underwent extensive changes during the evolution of the Zygnematales. BMC Biol. 2005;3:22. doi: 10.1186/1741-7007-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umate P, Fellerer C, Schwenkert S, Zoryan M, Eichacker LA, Sadanandam A, Ohad I, Herrmann RG, Meurer J. Impact of PsbTc on forward and back electron flow, assembly, and phosphorylation patterns of photosystem II in tobacco. Plant Physiol. 2008;148:1342–1353. doi: 10.1104/pp.108.126060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij FE, Goldschmidt-Clermont M, Wostrikoff K, Rochaix JD. Stability determinants in the chloroplast psbB/T/H mRNAs of Chlamydomonas reinhardtii. Plant J. 2000;21:469–482. doi: 10.1046/j.1365-313x.2000.00700.x. [DOI] [PubMed] [Google Scholar]
- Vaistij FE, Boudreau E, Lemaire SD, Goldschmidt-Clermont M, Rochaix JD. Characterization of Mbb1, a nucleus-encoded tetratricopeptide-like repeat protein required for expression of the chloroplast psbB/psbT/psbH gene cluster in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2000;97:14813–14818. doi: 10.1073/pnas.97.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener AV, Harms A, Sussman MR, Vierstra RD. Mass spectrometric resolution of reversible protein phosphorylation in photosynthetic membranes of Arabidopsis thaliana. J Biol Chem. 2001;276:6959–6966. doi: 10.1074/jbc.M009394200. [DOI] [PubMed] [Google Scholar]
- Watkins KP, Kroeger TS, Cooke AM, Williams-Carrier RE, Friso G, Belcher SE, van Wijk KJ, Barkan A. A ribonuclease III domain protein functions in group II intron splicing in maize chloroplasts. Plant Cell. 2007;19:2606–2623. doi: 10.1105/tpc.107.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KP, Rojas M, Friso G, van Wijk KJ, Meurer J, Barkan A. APO1 promotes the splicing of chloroplast group II introns and harbors a plant-specific zinc-dependent RNA binding domain. Plant Cell. 2011;23:1082–1092. doi: 10.1105/tpc.111.084335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P, Herrmann RG. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988;171:551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]
- Williams-Carrier R, Kroeger T, Barkan A. Sequence-specific binding of a chloroplast pentatricopeptide repeat protein to its native group II intron ligand. RNA. 2008;14:1930–1941. doi: 10.1261/rna.1077708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghidi W, Merendino L, Cottet A, Mache R, Lerbs-Mache S. Nucleus-encoded plastid sigma factor SIG3 transcribes specifically the psbN gene in plastids. Nucleic Acids Res. 2007;35:455–464. doi: 10.1093/nar/gkl1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghidi-Abouzid O, Merendino L, Buhr F, Malik Ghulam M, Lerbs-Mache S. Characterization of plastid psbT sense and antisense RNAs. Nucleic Acids Res. 2011;39:5379–5387. doi: 10.1093/nar/gkr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelyazkova P, Hammani K, Rojas M, Voelker R, Vargas-Suarez M, Borner T, Barkan A. Protein-mediated protection as the predominant mechanism for defining processed mRNA termini in land plant chloroplasts. Nucleic Acids Res. 2012;40:3092–3105. doi: 10.1093/nar/gkr1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito F, Kuras R, Choquet Y, Kossel H, Wollman FA. Mutations of cytochrome b6 in Chlamydomonas reinhardtii disclose the functional significance for a proline to leucine conversion by petB editing in maize and tobacco. Plant Mol Biol. 1997;33:79–86. doi: 10.1023/A:1005734809834. [DOI] [PubMed] [Google Scholar]