Abstract
Purpose
The grape and wine polyphenol resveratrol exerts cardiovascular benefits but evidence from randomized human clinical trials is very limited. We investigated dose-depending effects of a resveratrol-containing grape supplement on stable patients with coronary artery disease (CAD) treated according to currently accepted guidelines for secondary prevention of cardiovascular disease.
Methods
In a triple-blind, randomized, placebo-controlled, one-year follow-up, 3-arm pilot clinical trial, 75 stable-CAD patients received 350 mg/day of placebo, resveratrol-containing grape extract (grape phenolics plus 8 mg resveratrol) or conventional grape extract lacking resveratrol during 6 months, and a double dose for the following 6 months. Changes in circulating inflammatory and fibrinolytic biomarkers were analyzed. Moreover, the transcriptional profiling of inflammatory genes in peripheral blood mononuclear cells (PBMCs) was explored using microarrays and functional gene expression analysis.
Results
After 1 year, in contrast to the placebo and conventional grape extract groups, the resveratrol-containing grape extract group showed an increase of the anti-inflammatory serum adiponectin (9.6 %, p = 0.01) and a decrease of the thrombogenic plasminogen activator inhibitor type 1 (PAI-1) (−18.6 %, p = 0.05). In addition, 6 key inflammation-related transcription factors were predicted to be significantly activated or inhibited, with 27 extracellular-space acting genes involved in inflammation, cell migration and T-cell interaction signals presenting downregulation (p < 0.05) in PBMCs. No adverse effects were detected in relation to the study products.
Conclusions
Chronic daily consumption of a resveratrol-containing grape nutraceutical could exert cardiovascular benefits in stable-CAD patients treated according to current evidence-based standards, by increasing serum adiponectin, preventing PAI-1 increase and inhibiting atherothrombotic signals in PBMCs.
Electronic supplementary material
The online version of this article (doi:10.1007/s10557-012-6427-8) contains supplementary material, which is available to authorized users.
Keywords: Resveratrol, Coronary artery disease, Adiponectin, Plasminogen activator inhibitor type 1 (PAI-1), Cardiovascular, Clinical trial
Introduction
Patients with established coronary artery disease (CAD) are at very high risk of recurrent cardiovascular events [1]. Atherothrombosis results from complex interactions between modified lipoproteins, monocyte-derived macrophages, components of innate and adaptive immunity, and the normal cellular elements of the arterial wall [2]. The activation of inflammatory pathways in atherothrombosis is not confined to coronary lesions but involves the participation of peripheral blood mononuclear cells (PBMCs) that are important actors in atherosclerosis and in acute manifestation of plaque destabilization [3]. In the whole process, some nuclear receptors respond to oxidative stress and inflammatory stimuli by regulating the expression of genes involved in inflammation and lipid metabolism [4]. Rising data suggest that integrated research work, approaching different pathways, is needed for better understanding the molecular basis of cardiovascular disease (CVD) [5].
Current guidelines in secondary prevention of CVD deal not only with clinical areas of intervention but also with the implementation of healthy lifestyles, including a healthy diet [6]. Some specific foods such as olive oil, chocolate, walnuts and red wine exert cardiovascular benefits due to the presence of specific compounds such as omega-3-polyunsaturated fatty acids [7] and polyphenols [8]. In this regard, the grape and wine polyphenol resveratrol has shown cardiovascular benefits through many animal studies [9–11]. However, the evidence in humans is particularly limited [12].
Our aims were to investigate the effects of a 1 year dietary intervention with a resveratrol-containing grape extract on the inflammatory and fibrinolytic status of stable CAD patients and treated according to current guidelines. In addition, we aimed to explore whether inflammatory pathways were affected in PBMCs from these patients in response to the dietary intervention, through a microarray approach focussed on several inflammation-related transcription factors as potential regulation targets.
Methods
Patients and Study Design
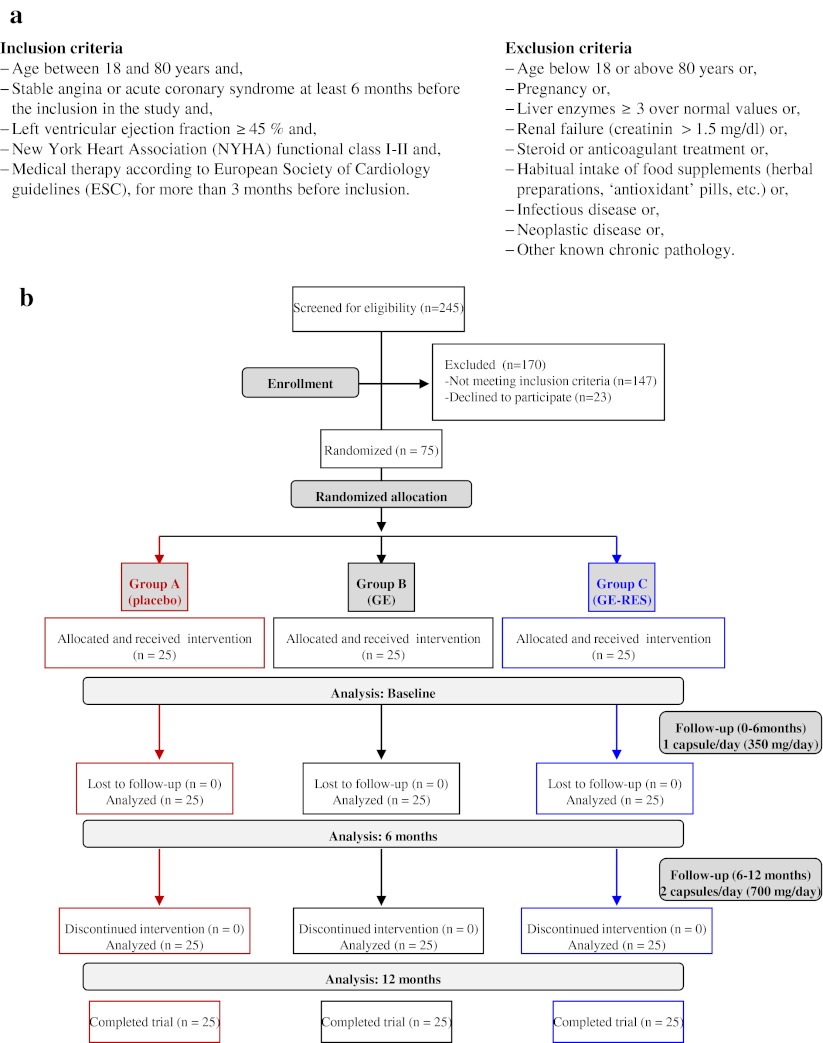
Stable CAD patients, treated according to current accepted guidelines for secondary prevention of CVD, were recruited at the Morales Meseguer University Hospital (MMUH) (Murcia, Spain). The study was included in the Spanish National Research Project BFU2007-60576 and conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its amendments. The design was approved by the Clinical Ethics Committee from the MMUH (reference 02/07) and by the CSIC’s Bioethics Committee (Madrid, Spain). Inclusion and exclusion criteria are indicated in Fig. 1a. A placebo-controlled, monocenter, triple-blind, dose–response, 1-year follow-up, randomized 3-arm pilot trial was conducted (Fig. 1b). Seventy-five out of 245 eligible patients were randomly divided in 3 equal numbered groups using a computer-generated random number sequence. The groups were coded as A (placebo, maltodextrin), B (GE, conventional grape extract) and C (GE-RES, resveratrol-containing grape extract). The primary end point was the change in high-sensitivity C-reactive protein (hsCRP) level from baseline to 12 months. Sample size was calculated to detect a difference of 1 mg/L on hsCRP, with a power of 80 % and a 2-sided test with an α of 0.05. Secondary outcomes were plasminogen activator inhibitor type 1 (PAI-1), adiponectin, interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor alpha (TNFα), soluble CD40 ligand (sCD40L) and soluble vascular adhesion molecule (sVCAM). Participants were given detailed trial information and required to provide written informed consent. The trial was registered at clinicaltrials.gov as NCT01449110.
Fig. 1.
Design of the trial. (a) Inclusion/Exclusion criteria. (b) Flow of participants through the trial. A, placebo group; B, conventional grape extract group (GE); C, resveratrol-containing grape extract group (GE-RES); RAS-blockers, renin-angiotensin system blockers; ESC, European Society of Cardiology
Study Products and Intervention
Study products were provided to patients in identical code labeled (A, B or C) capsules, containing 350 mg of either maltodextrin (placebo), GE or GE-RES plus 20 mg of excipients (magnesium stearate and SiO2) and packed in same-code labeled blank boxes. The polyphenolic composition of both GE and GE-RES was very similar as previously reported [13] (~25 mg anthocyanins, ~1 mg flavonols, ~40 mg procyanidins, and ~0.8 mg hydroxycinnamic acids), but GE-RES also contained 8.1 ± 0.5 mg resveratrol per capsule. Resveratrol is a phytoalexin and its content is very low in grapes and derived products. The GE-RES extract used in the present trial was obtained from grapes following a patented procedure that uses ultraviolet light to increase the resveratrol content [14]. These products (including GE-RES) were kindly provided by Actafarma S.L. (Pozuelo de Alarcón, Madrid, Spain). Patients were instructed to take 1 capsule/day in the morning for the first 6 months and 2 capsules/day for the following 6 months and to return the remaining capsules at trial conclusion. Moreover, participants were requested to keep their medication (Table 1), customary lifestyle and diet throughout the trial and to minimize the intake of grape-derived products, including red wine. Dietary habit changes, treatment interruptions and adverse effects (digestive discomforts, allergic reactions, etc.) were monitored through questionnaires and phone calls along the study. In addition, patients noted down their dietary intake in the 3 days prior to blood withdrawal and were instructed to fast overnight before blood collections.
Table 1.
Baseline characteristics of patients (n = 75) with coronary artery disease (CAD) and treated according to currently accepted guidelines for secondary prevention of cardiovascular disease
| Groups | |||
|---|---|---|---|
| A | B | C | |
| (n = 25) | (n = 25) | (n = 25) | |
| Age, years | 58 ± 9 | 59 ± 10 | 60 ± 12 |
| Men, n (%) | 21 (84) | 19 (76) | 24 (96) |
| Body mass index, kg/m2 | 30.6 ± 4.3 | 30.8 ± 4.6 | 29.7 ± 5.1 |
| Systolic blood pressure, mmHg | 125 ± 18 | 124 ± 18 | 126 ± 16 |
| Diastolic blood pressure, mmHg | 72 ± 11 | 73 ± 10 | 71 ± 9 |
| Heart rate, beats/min | 61 ± 9 | 66 ± 9 | 63 ± 11 |
| Left ventricular ejection fraction, % | 54 ± 5 | 55 ± 5 | 54 ± 4 |
| Stable angina, n (%) | 3 (12) | 4 (16) | 2 (8) |
| ST-segment elevation myocardial infarction, n (%) | 7 (28) | 10 (40) | 10 (40) |
| non-ST-segment elevation acute coronary syndrome, n (%) | 15 (60) | 11 (44) | 13 (52) |
| Myocardial revascularization, n (%) | 19 (76) | 21 (84) | 21 (84) |
| Coronary stenting | 14 (56) | 17 (68) | 19 (76) |
| Coronary artery bypass grafting | 5 (20) | 4 (16) | 2 (8) |
| Ischemic ictus, n (%) | 1 (4) | 1 (4) | 2 (8) |
| Intermittent claudication, n (%) | 4 (16) | 5 (20) | 5 (20) |
| Family history of premature ischemic heart disease, n (%) | 10 (40) | 8 (32) | 10 (40) |
| Diabetes mellitus, n (%) | 14 (56) | 17 (68) | 14 (56) |
| Hypertension, n (%) | 17 (68) | 16 (64) | 19 (76) |
| Active smokers, n (%) | 11 (44) | 6 (24) | 4 (16) |
| Antiplatelet therapy, n (%) | 25 (100) | 25 (100) | 25 (100) |
| Acetyl salicylic acid | 12 (48) | 13 (52) | 13 (52) |
| Clopidogrel | 3 (12) | 3 (12) | 1 (4) |
| Both | 10 (40) | 9 (36) | 11 (44) |
| Statins, n (%) | 25 (100) | 25 (100) | 25 (100) |
| Atorvastatin | 16 (64) | 16 (64) | 17 (68) |
| Rosuvastatin | 2 (8) | 4 (16) | 3 (12) |
| Pravastatin | 3 (12) | 2 (8) | 1 (4) |
| Fluvastatin | 4 (16) | 3 (12) | 4 (16) |
| β Blockers, n (%) | 20 (80) | 22 (88) | 22 (88) |
| Renin-angiotensin system blockers, n (%) | 22 (88) | 22 (88) | 24 (96) |
Values are expressed as mean ± SD. No significant (p < 0.05) baseline inter-group differences were found. A placebo group, B grape extract group (GE), C resveratrol-containing grape extract group (GE-RES)
Sampling Procedure
Blood samples were collected between 8 and 10 AM at baseline, 6 and 12 months and the corresponding serum or plasma samples were kept at −80 °C until analysis. Serum levels of inflammation-related markers and PAI-1were measured, at least in duplicate, by enzyme-linked immunosorbent assays (ELISA) as previously described [15]. Those determinations that yielded either borderline or statistically significant changes were further repeated to confirm the results.
Microarrays, Bioinformatic Tools and Functional Analysis
For the analysis of peripheral blood mononuclear cells (PBMCs), a subpopulation of diabetic and hypertensive male patients was selected from the total population of participants. PMBCs were isolated from EDTA-treated blood at baseline, 6 and 12 months and their RNA was extracted [16]. Afterwards, 18 patients included in this subpopulation were randomly chosen (6 from each group) for microarray analysis. Microarrays (Affymetrix) were performed on samples from individual patients (not pooled) at baseline, 6 and 12 months for a total number of 54 microarray analyses. Differentially expressed genes were analyzed and uploaded into Ingenuity Pathway Analysis (IPA) software (Ingenuity® Systems, Redwood City, CA). Significant changes were defined as those with an adjusted p-value < 0.05 (FDR, false discovery rate: 5 %) and with ratios >1.2 (up-regulation) and < −1.2 (down-regulation). Minimum Information About a Microarray Experiment (MIAME) compliant data were submitted to the Gene Expression Omnibus database (GEO; accession number GSE36930). Detailed methodology regarding PBMCs isolation, RNA extraction, microarray procedures and functional analysis can be found in Supplementary Methods.
Statistical Analysis
All statistical analyses were performed with SPSS v. 19.0 (SPSS Inc, Chicago, USA). Quantitative data were expressed as mean ± SD and qualitative data were expressed as proportions. Patient baseline characteristics were analyzed by Chi–square test or one-way analysis of variance (ANOVA). Parameters with skewed distribution were logarithm transformed for analysis. Intra and inter-group comparisons were made using one-way analysis of covariance (ANCOVA) for repeated measurements followed by post-hoc for multiple comparisons. The covariates used for adjustment were age, gender, body mass index, arterial hypertension, smoking, diabetes mellitus, diagnosed hypercholesterolemia, antiplatelet medication, β-blockers, renin-angiontensin system blockers, type of statin and dietary habits (including red wine or grape-derived products). Accepted significance level was p < 0.05 with 95 % coefficient interval (CI). Borderline statistical differences (p = 0.05) and tendencies (p > 0.05–0.1) were also acknowledged.
Results
Patients’ Characteristics, Serobiochemical Variables and Trial Compliance
All patients completed the study and none experienced adverse effects (intolerance, dyspepsia, allergic reactions, etc.) regarding the intake of the supplied products during the trial period. As expected, questionnaires indicated quite uniform dietary habits in this cohort undergoing secondary prevention of cardiovascular risk (results not shown). Tables 1 and 2 show patients’ baseline characteristics and laboratory values at the inclusion. No clinically relevant changes were observed in the routine serobiochemical variables analyzed after 12 months (Supplementary Table 1). The atherogenic blood lipid load, measured as the non-high density lipoprotein-cholesterol fraction (non-HDLc), decreased by 10.2 % in group B (p = 0.01) and by 13.4 % in group C (p = 0.03) (Supplementary Table 1). No changes were observed in routine hematological parameters (results not shown). Patients from all groups consumed more than 95 % of the expected capsules (compliance levels >95 %). Thirteen cardiac events occurred in the follow-up, i.e. 5 (3 acute coronary syndromes-ACS, 1 elective coronary revascularization-ECR, 1 stroke), 6 (3 ACS, 2 ECR, 1 stroke) and 2 ACS in groups A, B and C, respectively. These patients continued taking the capsules. All events occurred at least 2 months prior to blood collections and resultant biomarker alterations were expected to be stabilized by then. There were no fatalities during the one-year follow-up.
Table 2.
PAI-1, inflammatory markers and serobiochemical variables of patients with coronary artery disease (n = 75) in secondary prevention of cardiovascular disease at the inclusion
| Groups | |||
|---|---|---|---|
| A | B | C | |
| (n = 25) | (n = 25) | (n = 25) | |
| Plasminogen activator inhibitor type, ng/mL | 18.7 ± 14.4 | 19.8 ± 14.9 | 17.2 ± 10.3 |
| High-sensitivity C-reactive protein, mg/L | 3.3 ± 2.2 | 3.5 ± 2.3 | 3.9 ± 4.1 |
| Adiponectin, μg/mL | 11.0 ± 5.8 | 11.2 ± 4.5 | 12.4 ± 5.6 |
| Tumor necrosis factor-α, pg/mL | 13.7 ± 8.6 | 14.2 ± 8.7 | 14.9 ± 8.6 |
| Interleukin-6, pg/mL | 2.6 ± 1.7 | 2.2 ± 1.7 | 2.3 ± 1.8 |
| Interleukin-10, pg/mL | 19.6 ± 12.4 | 22.7 ± 16.1 | 23.05 ± 16.7 |
| Interleukin-6/Interleukin-10 | 0.16 ± 0.14 | 0.12 ± 0.12 | 0.12 ± 0.1 |
| Soluble CD40 ligand, ng/mL | 7.5 ± 3.9 | 6.5 ± 4.4 | 6.1 ± 3.6 |
| Soluble vascular adhesion molecule type 1, ng/mL | 1063 ± 253 | 1033 ± 368 | 1030 ± 463 |
| Total cholesterol, mg/dL | 162.4 ± 36.5 | 156.7 ± 30.9 | 165.6 ± 35.2 |
| Low-density lipoprotein cholesterol, mg/dL | 89.1 ± 30.7 | 81.4 ± 26.4 | 92.4 ± 31.5 |
| High-density lipoprotein cholesterol, mg/dL | 39.5 ± 8.7 | 43.2 ± 10.2 | 44.8 ± 7.2 |
| LDLc/HDLc, mg/dL | 2.3 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.1 |
| non-HDLc, mg/dL | 140 ± 10 | 127 ± 7 | 135 ± 11 |
| Triglycerides, mg/dL | 139 ± 61 | 138 ± 49 | 123 ± 48 |
| Fibrinogen, g/L | 3.3 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 |
| D-Dimer, mg/L | 0.13 ± 0.07 | 0.12 ± 0.06 | 0.13 ± 0.05 |
| Aspartate transaminase, U/L | 24 ± 10 | 24 ± 6 | 30 ± 11 |
| Alanine transaminase, U/L | 27 ± 15 | 33 ± 17 | 33 ± 17 |
| Alkaline phosphatase, U/L | 184 ± 60 | 190 ± 57 | 180 ± 45 |
| Creatine phosphokinase, U/L | 112 ± 69 | 114 ± 52 | 154 ± 67 |
| γ-Glutamyl transferase, U/L | 39 ± 21 | 40 ± 27 | 34 ± 17 |
| Lactate dehydrogenase, U/L | 305 ± 46 | 317 ± 59 | 361 ± 72 |
| Glucose, mg/dL | 121 ± 51 | 122 ± 20 | 127 ± 45 |
| Glycated hemoglobin, % | 6.3 ± 1.3 | 6.4 ± 0.4 | 6.6 ± 1.3 |
| Tyroid stimulating hormone, mU/L | 2.4 ± 1.2 | 1.7 ± 0.8 | 1.7 ± 1.2 |
| Thyroxine, ng/dL | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| Bilirubin, mg/dL | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.2 |
| Creatinin, mg/dL | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.2 |
| Urate, mg/dL | 6.5 ± 1.6 | 6.2 ± 1.5 | 5.8 ± 1.5 |
| Albumin, g/L | 45.2 ± 2.4 | 44.4 ± 2.6 | 44.6 ± 2.1 |
Values are expressed as mean ± SD. A placebo group, B grape extract group (GE); C resveratrol-containing grape extract group (GE-RES). No inter-group differences (p < 0.05) were found at baseline
Effect of Dietary Interventions on Inflammatory and Fibrinolytic Markers
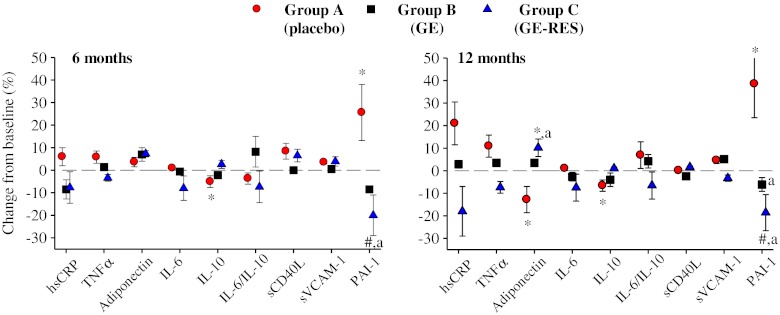
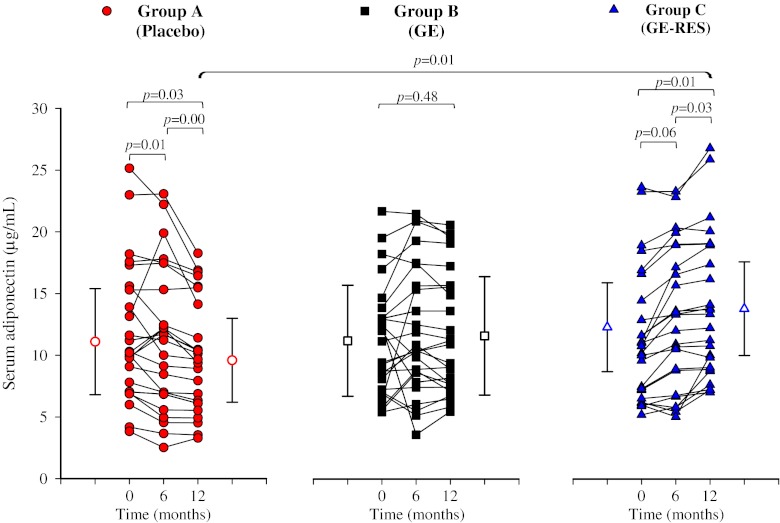
Figure 2 shows the overall impact of each dietary intervention on circulating biomarkers after 6 and 12 months with respect to their baseline values. In the placebo group (A), the anti-inflammatory interleukin-10 (IL-10) and adiponectin significantly decreased by 6.6 % (95 % CI −3.7 to −0.2, p = 0.03), and 12.7 % (95 % CI −2.4 to 0.3, p = 0.01), respectively, whereas PAI-1 significantly increased by 38.5 % (95 % CI 3.5 to 10.9, p = 0.00) after 12 months (Table 3, Fig. 2). In group B (GE, conventional grape extract) no marker was significantly affected (Table 3, Fig. 2). In group C (GE-RES, resveratrol-containing grape extract) a non-statistically significant dose-dependent decrease of 8 % (p = 0.09) and 18 % (p = 0.17) was observed for high-sensitivity C-reactive protein (hsCRP) after 6 and 12 months, respectively. Even though hsCRP decreased 38 % versus placebo after 12 months, no significant inter-group differences were found (Table 3, Fig. 3). However, serum adiponectin increased in this group (10 %; 1.2 μg/mL, 95 % CI 0.2 to 2.3, p = 0.01), with 80 % of patients showing a rise in this marker’s level (Fig. 3). Interestingly, adiponectin values were higher (23 %) in the GE-RES group than in placebo after 12 months (p < 0.05) (Table 3, Figs. 2 and 3). A statistically significant decrease (p < 0.05) of adiponectin was seen after 12 months for the diabetic subgroup (n = 12) of placebo group. Inversely to adiponectin, glycated haemoglobin levels presented a significant increase for the same subgroup of patients at this time point which was also correlated with a significant increase of glucose levels (results not shown). An inverse correlation was found between adiponectin vs. HbAc1 and adiponectin vs. glucose levels although statistical significance was not reached probably due to the small sample size.
Fig. 2.
Changes of plasminogen activator inhibitor type 1 (PAI-1) and inflammatory-related markers from baseline after 6 and 12 months. Patients consumed 1 capsule/day for the first 6 months (350 mg/day) and 2 capsules/day (700 mg/day) for the following 6 months. Percent changes are expressed as mean ± SD. Intra-group changes over time: *p < 0.05; # p = 0.05. Differences versus group A (placebo): a p < 0.05
Table 3.
Plasminogen activator inhibitor type 1 and inflammatory markers of patients with coronary heart disease (n = 75) after consumption of placebo (group A, n = 25), grape extract (GE, group B, n = 25) or resveratrol-containing grape extract (GE-RES, group C, n = 25) for 12 months
| Experimental values after 6 months | Experimental values after 12 months | |||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| High-sensitivity C-reactive protein, mg/L | 3.5 ± 2.1 | 3.2 ± 1.6 | 3.6 ± 3.7 | 4.0 ± 1.8 | 3.6 ± 2.2 | 3.2 ± 2.1 |
| p-value 0–6 months | 0.21 | 0.67 | 0.09‡, ↓8 % | |||
| p-value 6–12 months | 0.13 | 0.56 | 0.40 | |||
| p-value 0–12 months | 0.66 | 0.95 | 0.17 | |||
| Tumor necrosis factor-α, pg/mL | 14.5 ± 9.2 | 14.4 ± 8.6 | 14.4 ± 8.6 | 15.2 ± 7.0 | 14.7 ± 9.9 | 13.8 ± 8.2 |
| p-value 0–6 months | 0.63 | 0.93 | 0.56 | |||
| p-value 6–12 months | 0.38 | 0.75 | 0.30 | |||
| p-value 0–12 months | 0.49 | 0.76 | 0.15 | |||
| Adiponectin, μg/mL | 11.4 ± 6.8 | 12.0 ± 5.2 | 13.3 ± 5.6 | 9.6 ± 4.4 | 11.6 ± 4.8 | 13.6 ± 5.2 |
| p-value 0–6 months | 0.55 | 0.25 | 0.06‡, ↑7% | |||
| p-value 6–12 months | 0.00*, ↓16 % | 0.13 | 0.03*, ↑2% | |||
| p-value 0–12 months | 0.01*, ↓13 % | 0.48 | 0.01*, ↑10% | |||
| Interleukin-6, pg/mL | 2.6 ± 1.8 | 2.2 ± 1.6 | 2.1 ± 1.7 | 2.6 ± 1.8 | 2.1 ± 1.6 | 2.1 ± 1.7 |
| p-value 0–6 months | 0.99 | 0.24 | 0.83 | |||
| p-value 6–12 months | 0.56 | 0.51 | 0.88 | |||
| p-value 0–12 months | 0.80 | 0.10‡, ↓5 % | 0.84 | |||
| Interleukin-10, pg/mL | 18.6 ± 13.3 | 22.2 ± 17.2 | 23.6 ± 17.0 | 18.3 ± 12.2 | 21.8 ± 16.9 | 23.3 ± 16.9 |
| p-value 0–6 months | 0.01*, ↓5 % | 0.40 | 0.42 | |||
| p-value 6–12 months | 0.55 | 0.33 | 0.93 | |||
| p-value 0–12 months | 0.03*, ↓7 % | 0.14 | 0.45 | |||
| Interleukin-6/Interleukin-10 | 0.15 ± 0.12 | 0.13 ± 0.11 | 0.11 ± 0.10 | 0.17 ± 0.14 | 0.13 ± 0.11 | 0.11 ± 0.10 |
| p-value 0–6 months | 0.64 | 0.24 | 0.24 | |||
| p-value 6–12 months | 0.10‡, ↑13% | 0.50 | 0.66 | |||
| p-value 0–12 months | 0.19 | 0.48 | 0.27 | |||
| Soluble CD40 ligand, ng/mL | 8.1 ± 3.4 | 6.5 ± 3.8 | 6.5 ± 2.8 | 7.5 ± 2.5 | 6.4 ± 3.3 | 6.2 ± 2.1 |
| p-value 0–6 months | 0.54 | 0.97 | 0.61 | |||
| p-value 6–12 months | 0.18 | 0.69 | 0.55 | |||
| p-value 0–12 months | 0.30 | 0.78 | 0.83 | |||
| Soluble adhesion vascular molecule type 1, ng/mL | 1101 ± 251 | 1039 ± 330 | 1071 ± 561 | 1111 ± 347 | 1087 ± 332 | 996 ± 443 |
| p-value 0–6 months | 0.07‡, ↑4% | 0.91 | 0.36 | |||
| p-value 6–12 months | 0.82 | 0.31 | 0.09‡, ↓7 % | |||
| p-value 0–12 months | 0.28 | 0.39 | 0.26 | |||
| Plasminogen activator inhibitor type 1, ng/mL | 23.5 ± 15.5 | 18.1 ± 10.2 | 13.6 ± 7.2 | 25.9 ± 15.0 | 18.6 ± 9.5 | 14.0 ± 7.0 |
| p-value 0–6 months | 0.02*, ↑26% | 0.55 | 0.05†, ↓21 % | |||
| p-value 6–12 months | 0.26 | 0.52 | 0.50 | |||
| p-value 0–12 months | 0.00*, ↑38% | 0.62 | 0.05†, ↓19 % | |||
Values are expressed as mean ± SD. Daily doses were 1 capsule (350 mg) from 0 to 6 months and 2 capsules (700 mg) from 6 to 12 months. A placebo group, B grape extract group (GE), C resveratrol-containing grape extract group (GE-RES). *p < 0.05; † p = 0.05. Tendencies (‡ p > 0.05–0.1) are also indicated. Inter-group differences (p < 0.05) were found in adiponectin after 12 months (A vs. C); and in plasminogen activator inhibitor type 1 after 6 (A vs. C) and 12 months (A vs. B; A vs. C)
Fig. 3.
Effect on serum adiponectin concentration. Filled symbols and lines designate individual subjects. Open symbols designate mean ± SD concentration at baseline and 12 months
Regarding PAI-1, a clear decrease was observed in the GE-RES group after 12 months (−18.6 %, p = 0.05) (Table 3, Fig. 2). Significant inter-group differences were observed between groups A and C at 6 and 12 months, as well as between groups A and B after 12 months (Table 3, Fig. 2). In addition, even though statistical significance was not reached, Fig. 2 shows clear inter-group differences for tumor necrosis factor alpha (TNFα) and IL-6/IL-10 after 12 months.
GE-RES Downregulates Pro-Inflammatory Gene Expression in PBMCs
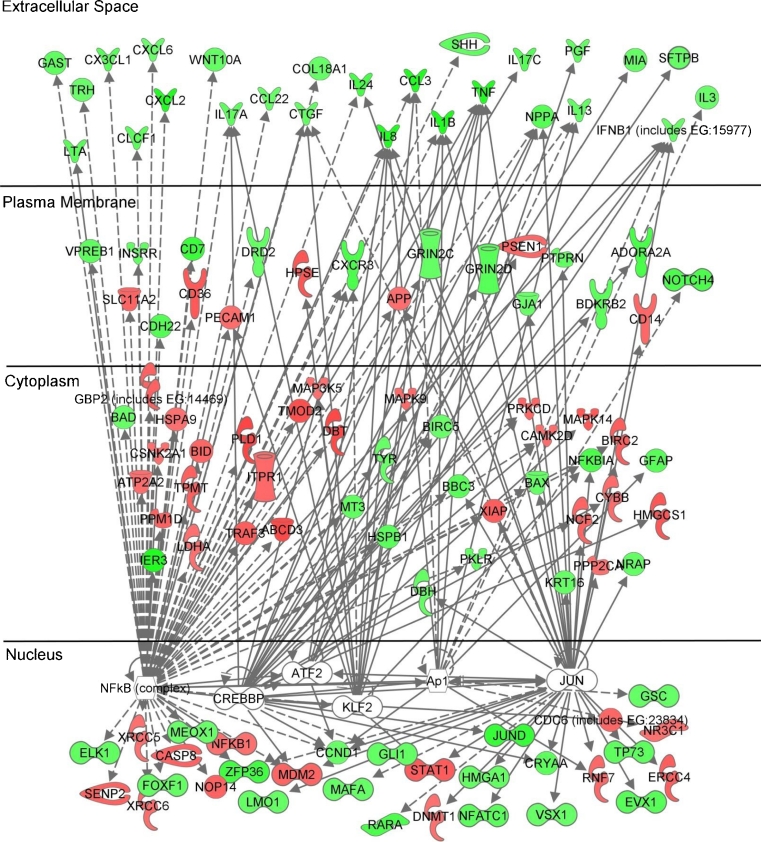
Differentially expressed genes in PBMCs (at 6 and 12 months vs. baseline; p < 0.05) were analyzed for placebo, GE and GE-RES groups. After 12 months, 6 key inflammation-related transcription factors (TFs) were predicted to be significantly activated or inhibited only in the GE-RES group (Table 4): Kruppel-like factor 2 (KLF2), nuclear factor kappa-B (NF-κB), activator protein 1 (Ap-1), proto-oncogen c-JUN (JUN), activating transcription factor 2 (ATF-2) and CREB-binding protein (CREBBP). According to the functions of these transcription factors (Table 4), these results suggest a general inhibition of the lymphocytes/monocytes-mediated inflammatory response following the consumption of GE-RES. This specific subset of transcription factors together with their target genes were used to create a pathway using the Ingenuity Pathway Analysis (IPA) software (Fig. 4). Accordingly, this pathway called attention to a significantly downregulated subset of 27 extracellular-space acting genes in group C (GE-RES, Supplementary Table 2). Functional analysis revealed their involvement in processes such as cell movement, immune cell trafficking, cellular growth and proliferation, cell-to-cell signaling and interaction and inflammatory response. Supplementary Table 3 includes a detailed list of these genes and their functions.
Table 4.
Predicted activation status of inflammation-related transcription factors of differentially expressed genes in peripheral blood mononuclear cells (PBMCs) from patient with coronary artery disease (CAD) after consumption of resveratrol-rich grape extract (GE-RES) for 12 months
| Transcription factor | Function | Predicted status | z-score | References |
|---|---|---|---|---|
| Kruppel-like factor 2 (KLF2) | Negative regulator of pro-inflammatory monocyte activation. Its effect is mediated through inhibition of nuclear factor-KB (NF-κB) and activator protein 1 (AP-1) pathways. Activation of KLF2 strongly induces thrombomodulin and endothelial nitric oxide synthase expression and reduces plasminogen activator inhibitor type 1 (PAI-1) expression. | Activated | 2.546 | 4 |
| Activator protein 1 (Ap-1) | Together with NF-κB is one major inflammation regulator. NF-κB and AP-1 coordinated actions propagate inflammation via promoting transcription of cytokines, chemokines, and other proinflammatory genes. | Inhibited | 3.423 | 40 |
| Proto-oncogen c-JUN (JUN) | Major component of the heterodimeric transcription factor AP-1; plays an important role in regulating cell growth, apoptosis, differentiation, and transformation. | Inhibited | 2.702 | 40 |
| Activating transcription factor 2 (ATF-2) | Component of the heterodimeric of AP-1; regulates the transcription of genes involved in in the stress response, cell growth and differentiation, and immune response. | Inhibited | 2.661 | 40 |
| CREB-binding protein (cAMP response element-binding) | Essential co-activator for nuclear receptors and several other classes of regulated transcription factors like NF-κB and AP-1. | Inhibited | 2.334 | 41 |
According to the Ingenuity Transcription Regulator Analysis (TRA). TRA examines the known targets of each transcription factor in the user’s dataset and compares their expression in the experimental samples relative to control to what is expected from the literature. TRA defines a quantity (z-score) that determines whether an upstream transcription factor has significantly more activated predictions than inhibited predictions (z > 0) or vice versa (z < 0), where significance means that the observed number of activated or inhibited predictions are unlikely relative to randomly chosen predictions (null hypothesis). In practice, the predicted activation status is only established when z-score >2 or < −2
Fig. 4.
Predicted pathways by the Ingenuity Pathway Analysis software (IPA) according to microarrays results in peripheral blood mononuclear cells (PBMCs). Green and red colors designate down- and upregulation of gene expression, respectively. Transcription factors involved in the predicted pathway are listed in Table 4. Expression changes in extracellular-space genes and their functions are listed in Supplementary Tables 2 and 3, respectively
Discussion
Resveratrol content in red wine has been recurrently used to justify red wine benefits, such as the so-called ‘French Paradox’, i.e. low mortality due to CVD in France compared to that in other developed countries in spite of sharing CVD risk factors such as obesity, fat intake, smoking, etc. [17]. However, the presence of resveratrol in the diet is almost negligible [18] because it is a minor polyphenol whose content in red wine is low and highly variable [19]. Resveratrol is a defensive compound (phytoalexin) that is synthesized by grapes to face adverse environmental conditions [14] and, by definition, its presence in grapes (and red wine) is low and unpredictable. The vast majority of studies carried out with resveratrol have used the synthetic molecule or non-dietary herbal preparations. Therefore, i) the consumption of wine does not ensure the intake of enough resveratrol to exert beneficial effects, ii) the conjecture that directly correlates the cardiovascular protection of red wine and its resveratrol content is not fully true and iii) the specific contribution of the minor resveratrol content against the rest of major phenolic compounds in red wine is not known. In this regard, we used a resveratrol-rich grape extract (GE-RES) obtained from ultraviolet-treated grapes that induced resveratrol to face such challenge. Therefore, this nutraceutical offers the possibility of including resveratrol in the human diet within its natural edible matrix, the grape. In addition, in order to evaluate the relevance of RES against the rest of the grape phenolics we included a conventional grape extract (GE) group (same grapes without UV treatment).
Adiponectin is an anti-inflammatory cytokine involved in the pathogenesis of vulnerability of coronary lesions by exerting protective pleiotropic effects on the vascular system [20]. Adiponectin release has been reported to be suppressed in the epicardial adipose tissue of patients with obesity and coronary artery disease (CAD) [21]. Despite its anti-inflammatory and protective role against cardiovascular disease (CVD), high adiponectin levels have been correlated with mortality in specific clinical conditions of CAD patients [22]. However, in this case, it has been suggested that higher adiponectin levels are a consequence rather than a cause, i.e. the attempt to protect, reduce or limit endothelial damage provoked by an inflammatory milieu in these patients [23, 24]. In fact, serum adiponectin values have been reported to be lower in CAD patients with vulnerable plaques [25], acute myocardial infarct (AMI) and unstable angina than in those patients with stable angina [26]. In this regard, methods for increasing adiponectin have been suggested as a promising therapy for the prevention and treatment of CAD [27]. Unlike adiponectin, PAI-1 is higher in CAD patients [28] and its reduction can decrease CVD risk because the impairment of fibrinolysis, due to high circulating PAI-1 levels, is associated with the development of AMI [29]. The inverse relation between adiponectin and PAI-1 has also been described in obese [30] and stable angina patients [31]. However, the evolution of these markers is not routinely assayed in the follow-up of CAD patients with optimized medication [32, 33]. In fact, in the present trial, even though patients were being treated according to current evidence-based standards (Table 1), both adiponectin and PAI-1 presented an unfavorable evolution along the trial in the placebo group. Remarkably, the outcomes of our study suggest that a daily, yearlong, dietary intervention with a resveratrol-rich grape nutraceutical increases adiponectin and decreases PAI-1 circulating levels in CAD patients, which is in accordance with the effects exerted by resveratrol in a number of in vitro and animal studies [34]. Other mechanisms related to the cardiovascular protection exerted by resveratrol include the decrease in endothelin-1, adhesion molecules to endothelium, platelet aggregation, and pro-inflammatory cytokines, the increase of NO synthesis and the activation of sirtuins, among others [35].
The specific mechanisms of action reported for GE-RES have been linked to the regulation of genes involved in lipid metabolism and metabolic disorders [36] and a reduction of vascular oxidative stress resulting in the prevention of early atherosclerotic lesions in the aorta of pigs fed with an atherogenic diet [15]. However, despite the abundant preclinical cardiovascular benefits described for resveratrol [35], human clinical trials, especially long-term interventions, are very scarce [11]. Resveratrol consumption has been reported to improve glycemic control in diabetics [37, 38] and endothelial function in CAD patients [39]. However, these studies involved a short follow-up (maximum 3 months) and the statistical analyses did not take into account possible covariates (medication, age, gender, etc.) that could influence the results. Recently, we have shown that a 6-months consumption of GE-RES decreased the concentration of oxidized low-density lipoprotein particles and apolipoprotein B in statin-treated subjects at high CVD risk (primary prevention of CVD) [13]. In the same cohort of subjects, we further reported the improvement of their inflammatory (mainly by decreasing hsCRP) and fibrinolytic (PAI-1) status after one year follow-up [14]. In the present trial, CAD patients were treated with higher statin doses than high-risk subjects in primary prevention (i.e. 20 mg rosuvastatin, 40–80 mg atorvastatin, 40 mg pravastatin or 80 mg fluvastatin). It is known that treatments with high statin doses are correlated with a reduction of CRP [40]. For example, 40 mg pravastatin decreased hsCRP by 13 % (median from 2.7 to 2.4 mg/L) [41] and 18 % (mean from 3.6 to 3.1 mg/L) [42] after 6 months and 5 years, respectively, in secondary prevention cohorts. Therefore, the high statin dose could be behind the low added-effect on hsCRP after consuming GE-RES in this study in comparison with our previous trial in primary prevention of CVD [14]. Nevertheless, the evolution of hsCRP levels in the present trial was clearly better in the GE-RES group than in the other groups (Fig. 2). In contrast to the moderate effect on hsCRP, the effects on PAI-1 and adiponectin upon consumption of GE-RES were clearer in CAD patients than in primary prevention of CVD [14].
Many of the reported effects for adiponectin are related to the inhibition of pro-inflammatory signaling in PBMCs, monocyte adhesion to vascular endothelium and migration into tunica intima, macrophage activation and transformation to foam cell, smooth muscle cell proliferation and migration into the intima [25, 26]. Therefore, we next investigated, through a microarray analysis, whether this dietary intervention could affect inflammatory pathways in PBMCs. Gene expression response to inflammatory signals is mediated through the activation of transcription factors like NF-κB, which contributes to the pathophysiology of inflammatory disease and triggers monocyte recruitment into subendothelial space, a crucial milestone in the pathogenesis of atherosclerosis [43]. Another important transcription factor is AP-1, whose dimers such as JUN/ATF2 are involved in the regulation of diverse subsets of target genes whose products regulate the activation, proliferation, differentiation and apoptosis of leukocytes [44]. In addition, the activity of both NF-κB and AP-1 is regulated by CREBBP [45]. All these transcription factors were predicted to be inhibited following the yearlong consumption of resveratrol-rich grape extract (GE-RES). Furthermore, KLF2, which downregulates PAI-1 expression and negatively regulates pro-inflammatory activation of monocytes through inhibition of NF-κB and AP-1 [4], was predicted to be activated exclusively in the GE-RES group. After combining all the above transcription factors in a joint pathway, a subset of 27 significantly downregulated genes, whose products act in the extracellular space, stood out. The functional analysis of this orchestrated downregulation pointed towards the inhibition of clinically relevant key features in atherothrombosis such as inflammation, cell migration and T-cell interaction [5]. In this regard, Karastergiou et al. [17] reported that the adhesion of monocytes to human endothelial cells was increased by the release of epicardial adipose tissue cytokines in CAD patients. Interestingly, adiponectin reversed these atherogenic effects and this supports the link found in our trial between adiponectin increase and the inhibition of atherothrombotic signals in PBMCs from CAD patients belonging to the GE-RES group.
We next tried to correlate the effects of GE-RES with circulating resveratrol and/or other phenolic metabolites derived from the grape extract GE-RES. Plasma samples were routinely analyzed using state-of-the-art analytical techniques (UPLC-QqQ). However, we were not able to detect circulating resveratrol or other grape-derived metabolites in the GE-RES group. This was in accordance with our previous studies in both humans [13, 15] and pigs [16] using the same grape nutraceutical. At first, this could be explained by the low resveratrol dose assayed (8 mg for 6 months and 16 mg for the last 6 months), by the fast clearance of resveratrol and also because blood withdrawals were carried out after fasting overnight. In this regard, Azorín-Ortuño et al. [46] coined the expression ‘Resveratrol Paradox’ to illustrate the high activity exerted by resveratrol despite its low bioavailability. Circulating resveratrol metabolites were not found in pigs fed with this nutraceutical as stated above. However, a number of resveratrol metabolites were detected in the aortic tissue of these pigs [16]. Although the direct association between resveratrol and/or specific circulating resveratrol metabolites and the biological activity of resveratrol is far from being demonstrated in vivo, this suggests that repetitive, chronic exposure of low resveratrol doses such as those assayed in the present trial could be directly related to the beneficial effects observed. Overall, this implies that each specific product containing resveratrol should demonstrate both its safety and efficacy.
Limitations
This is the longest exploratory trial dealing with resveratrol in patients with CAD reported so far although we acknowledge that the small sample size (n = 75) and follow-up (1 year) impede definite conclusions on the clinical impact of these dietary interventions. There were 13 events during this trial, i.e. acute coronary syndrome (ACS) cases such as unstable angina, non-ST elevation myocardial infarction (NSTEMI) and stroke/transient ischemic attack were all included as events (there were no cases of STEMI or death). Overall, there was a 16 % rate of events during the trial in these very high risk patients. Data from stable patients undergoing secondary prevention is scarce, nevertheless, our rate of events is very similar to the one described in other observational reports such as the REACH registry [47], where a population with a similar profile to ours presented a 15.4 % rate of events in the first year of follow-up. Regarding Spanish registries, similar populations presented a rate of events between 14 and 44 % after 3 years of follow-up according to the cardiovascular risk factors [48]. Other registries like the CLARIFY registry [49] showed a year rate of events inferior to ours (around 7 %), however, stroke was not included in the rate calculations reported. In the present trial, in contrast to the 5 and 6 cardiac events occurred in the placebo and GE groups, respectively, only 2 ACS were recorded in the GE-RES group. However, a larger sample size and longer follow-up is needed to confirm this positive result. In addition, our microarrays results provide preliminary evidence regarding the possible role of the genes and pathways studied in this trial. However, our patient cohort was well characterized and the adjusted statistical analysis as well as the long-term follow up supports that this resveratrol-rich grape nutraceutical (GE-RES) could complement the optimized medication of these CAD patients. In our opinion, the combined evaluation of circulating markers related to cardiovascular disease risk and the gene expression profiling of PBMCs yields an attractive translational approach of molecular mechanisms to clinical applications in patients with CAD.
Conclusion
In stable CAD-patients, the daily consumption of this resveratrol-containing grape nutraceutical (GE-RES) for 1 year increased serum adiponectin, prevented plasminogen activator inhibitor type 1 increase and inhibited atherothrombotic signals in PBMCs from these patients. The presence of resveratrol in the grape nutraceutical appeared essential to exert these effects. Our data warrant further research on this nutraceutical as a possible safe coadjuvant food supplement in the follow-up of CAD patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 128 kb)
Acknowledgements
This study was supported by public funds: Projects CICYT-BFU2007-60576 and Consolider-Ingenio 2010 (CSD2007-00063, Fun-C-Food) from the Spanish Ministry of Science and Innovation (MICINN) and GERM-06-04486 (Fundación Séneca, Murcia, Spain). Dr. Tomé-Carneiro received a FPI grant from MICINN and Dr. Larrosa received a JAE-DOC contract from the Consejo Superior de Investigaciones Científicas (CSIC, Spain). Authors are grateful to Dr. M. Canteras for his support in the statistical analysis of results, to all the subjects who volunteered in the study and also to the nurses M.J. Gómez and J. Sánchez for their assistance in blood withdrawal at the MMUH.
Conflict of Interest
F.T-B and J.C.E. are co-inventors of the patent ES 2177465 that describes the process to obtain resveratrol-enriched grapes. The rest of authors declare no conflict of interest. The products used in this study were provided by Actafarma S.L. (Madrid, Spain). This company did not fund the study and had no role in trial design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization . Prevention of cardiovascular disease. Guidelines for assessment and management of cardiovascular risk. Geneva: WHO Press; 2007. [Google Scholar]
- 2.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 3.Benagiano M, Azzurri A, Ciervo A, et al. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci USA. 2003;100:6658–6663. doi: 10.1073/pnas.1135726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Crea F. Clinical implications of inflammation for cardiovascular primary prevention. Eur Heart J. 2010;31:777–783. doi: 10.1093/eurheartj/ehq022. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC, Jr, Benjamin EJ, Bonow RO, World Heart Federation and the Preventive Cardiovascular Nurses Association et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 7.Kazemian P, Kazemi-Bajestani SM, Alherbish A, Steed J, Oudit GY. The use of ω-3 poly-unsaturated fatty acids in heart failure: a preferential role in patients with diabetes. Cardiovasc Drugs Ther. 2012;26:311–320. doi: 10.1007/s10557-012-6397-x. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels EK. The protective effect of the mediterranean diet: focus on cancer and cardiovascular risk. Med Princ Pract. 2011;20:103–111. doi: 10.1159/000321197. [DOI] [PubMed] [Google Scholar]
- 9.Chen YR, Yi FF, Li XY, Wang CY, Chen L. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc Drugs Ther. 2009;23:449–458. doi: 10.1007/s10557-009-6198-z. [DOI] [PubMed] [Google Scholar]
- 10.Akar F, Pektas MB, Tufan C, et al. Resveratrol shows vasoprotective effect reducing oxidative stress without affecting metabolic disturbances in insulin-dependent diabetes of rabbits. Cardiovasc Drugs Ther. 2011;25:119–131. doi: 10.1007/s10557-010-6255-7. [DOI] [PubMed] [Google Scholar]
- 11.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann NY Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 13.Tomé-Carneiro J, Gonzálvez M, Larrosa M, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients from primary prevention of cardiovascular disease. A triple-blind, 6-months follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012;56:810–821. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 14.Cantos E, Espín JC, Tomás-Barberán FA. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: a new ‘functional’ fruit? J Agric Food Chem. 2001;49:5052–5058. doi: 10.1021/jf010366a. [DOI] [PubMed] [Google Scholar]
- 15.Tomé-Carneiro J, Gonzálvez M, Larrosa M, et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Azorín-Ortuño M, Yáñez-Gascón MJ, Pallarés FJ, et al. A dietary resveratrol-rich grape extract prevents the developing of atherosclerotic lesions in the aorta of pigs fed an atherogenic diet. J Agric Food Chem. 2012;60:5609–5620. doi: 10.1021/jf301154q. [DOI] [PubMed] [Google Scholar]
- 17.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 18.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br J Nutr. 2008;100:188–196. doi: 10.1017/S0007114507882997. [DOI] [PubMed] [Google Scholar]
- 19.Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007;101:449–457. doi: 10.1016/j.foodchem.2006.01.047. [DOI] [Google Scholar]
- 20.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci. 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 21.Karastergiou K, Evans I, Ogston N, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 22.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 23.Teoh H, Strauss MH, Szmitko PE, Verma S. Adiponectin and myocardial infarction: a paradox or a paradigm? Eur Heart J. 2006;27:2266–2268. doi: 10.1093/eurheartj/ehl248. [DOI] [PubMed] [Google Scholar]
- 24.Kunita E, Yamamoto H, Kitagawa T, et al. Association between plasma high-molecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687–1696. doi: 10.1253/circj.CJ-11-1442. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. J Am Coll Cardiol. 2011;57:761–770. doi: 10.1016/j.jacc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 28.Belalcazar LM, Ballantyne CM, Lang W, Look Action for Health in Diabetes Research Group et al. Look action for health in diabetes research group. Metabolic factors, adipose tissue, and plasminogen activator inhibitor-1 levels in type 2 diabetes: findings from the Look AHEAD study. Arterioscler Thromb Vasc Biol. 2011;31:1689–95. doi: 10.1161/ATVBAHA.111.224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens I, Ballaux D, Funahashi T, et al. Inverse relationship between plasminogen activator inhibitor-I activity and adiponectin in overweight and obese women. Interrelationship with visceral adipose tissue, insulin resistance, HDL-chol and inflammation. Thromb Haemost. 2005;94:1190–1195. [PubMed] [Google Scholar]
- 30.Maruyoshi H, Kojima S, Funahashi T, et al. Adiponectin is inversely related to plasminogen activator inhibitor type 1 in patients with stable exertional angina. Thromb Haemost. 2004;91:1026–1030. doi: 10.1160/TH03-12-0731. [DOI] [PubMed] [Google Scholar]
- 31.Chan KC, Chou HH, Huang CN, Chou MC. Atorvastatin administration after percutaneous coronary intervention in patients with coronary artery disease and normal lipid profiles: impact on plasma adiponectin level. Clin Cardiol. 2008;31:253–258. doi: 10.1002/clc.20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaba NK, Francis CW, Moss AJ, et al. Effects of lipids and lipid-lowering therapy on hemostatic factors in patients with myocardial infarction. J Thromb Haemost. 2004;2:718–725. doi: 10.1111/j.1538-7836.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 33.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes. 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- 35.Petrowski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann NY Acad Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 36.Azorín-Ortuño M, Yáñez-Gascón MJ, González-Sarrías A, et al. Effects of long-term consumption of low doses of resveratrol on diet-induced mild hypercholesterolemia in pigs: a transcriptomic approach to disease prevention. J Nutr Biochem. 2012;23:829–837. doi: 10.1016/j.jnutbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Brasnyó P, Molnár GA, Mohás M. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Magyar K, Halmosi R, Palfi A, et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 40.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Reversal of atherosclerosis with aggressive lipid lowering (REVERSAL) investigators. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 41.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.CIR.100.3.230. [DOI] [PubMed] [Google Scholar]
- 43.Kutuk O, Basaga H. Inflammation meets oxidation: NF-kappaB as a mediator of initial lesion development in atherosclerosis. Trends Mol Med. 2003;9:549–557. doi: 10.1016/j.molmed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Foletta VC, Segal DH, Cohen DR. Transcriptional regulation in the immune system: all roads lead to AP-1. J Leukoc Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 45.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 46.Azorín-Ortuño M, Yáñez-Gascón MJ, Vallejo F, et al. Metabolites and tissue distribution of resveratrol in the pig. Mol Nutr Food Res. 2011;55:1154–1168. doi: 10.1002/mnfr.201100140. [DOI] [PubMed] [Google Scholar]
- 47.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 48.Moreno-Palanco MA, Ibáñez-Sanz P, Ciria-de Pablo C, et al. Impact of comprehensive and intensive treatment of risk factors concerning cardiovascular mortality in secondary prevention: MIRVAS study. Rev Esp Cardiol. 2011;64:179–185. doi: 10.1016/j.recesp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Steg PG, Greenlaw N, Tardif JC, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. 2012;33:2831–2840. doi: 10.1093/eurheartj/ehs289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 128 kb)