Abstract
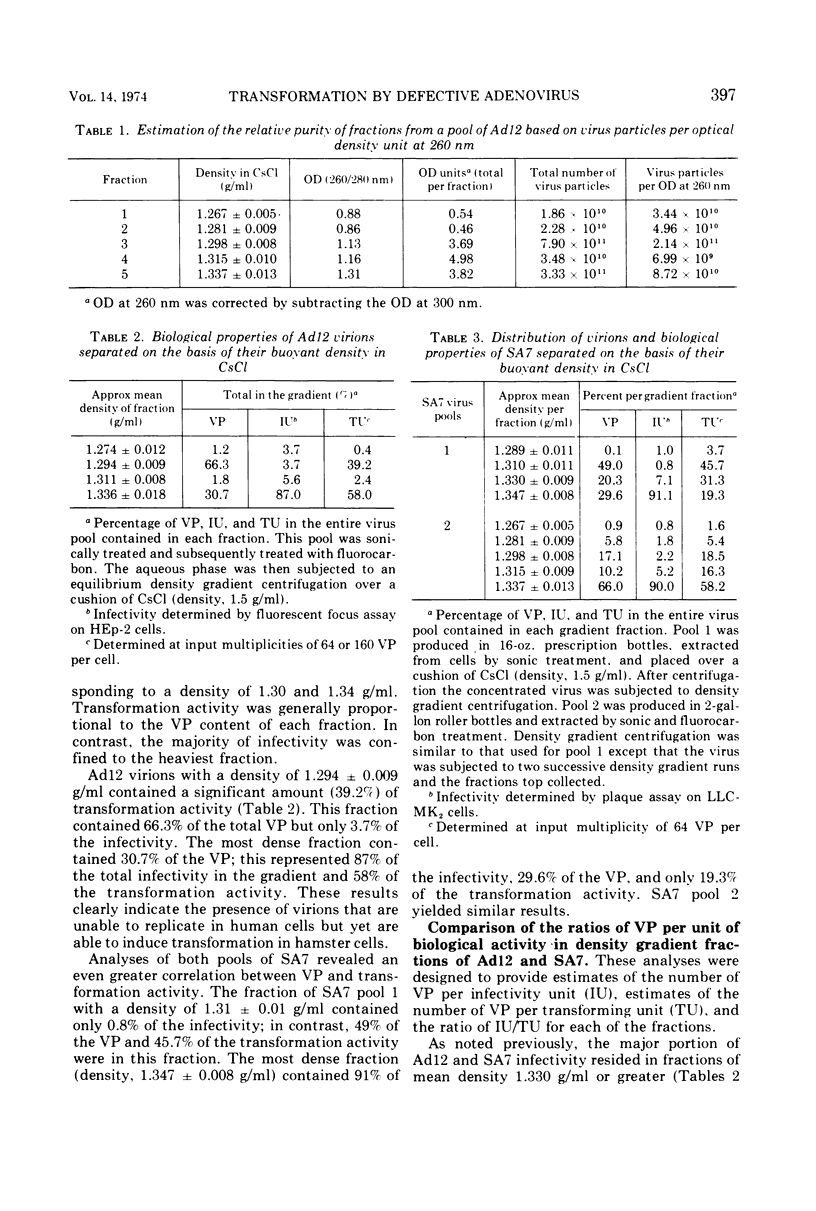
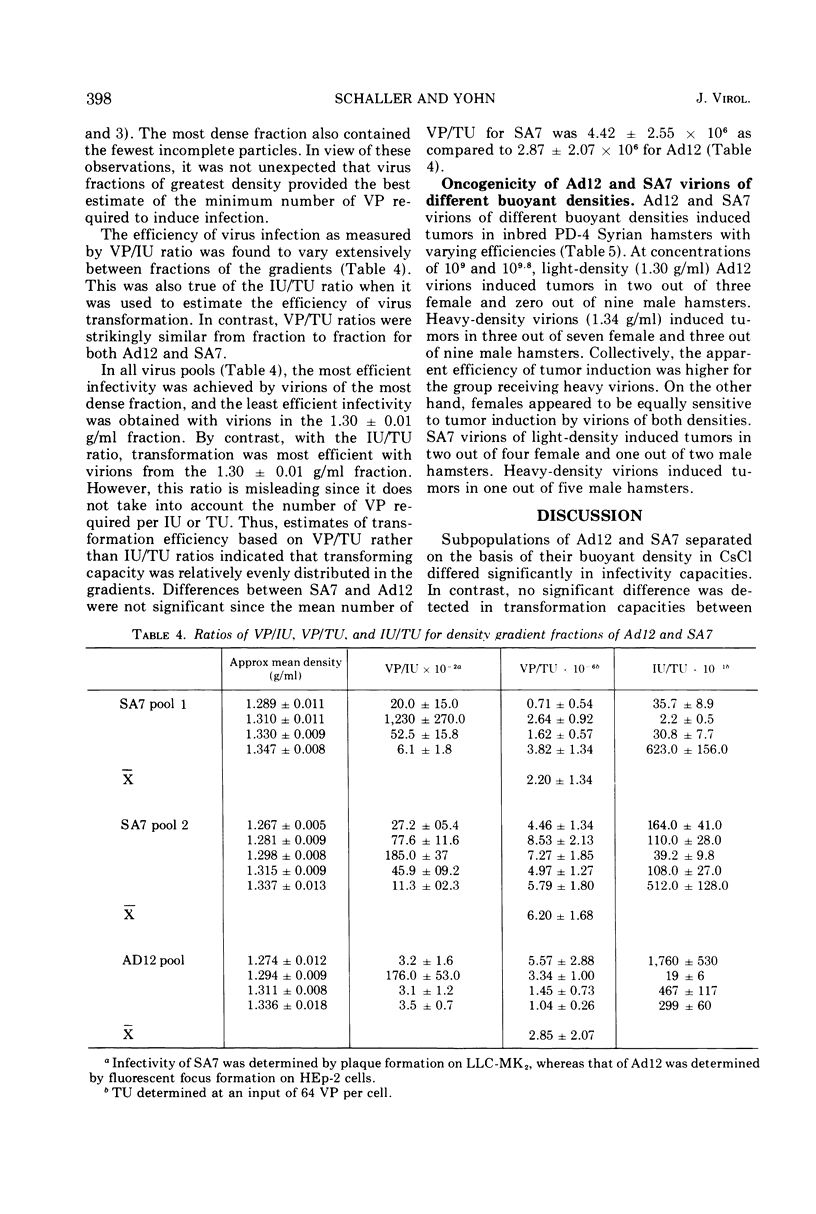
Pools of adenovirus 12 and simian adenovirus 7 were separated into four or five fractions by density gradient centrifugation in cesium chloride. Each fraction was analyzed for total in vitro infectivity units, total transformation activity, and for total virus particle (VP) content. Two major subpopulations were separated with mean densities of 1.30 ± 0.02 and 1.34 ± 0.02 g/ml, respectively. Virions in the 1.34 g/ml range were highly infectious (102 to 103 VP per infectivity unit) in contrast to virions at 1.30 g/ml density (104 to 105 VP per infectivity units). Transformation capacity was evenly distributed throughout fractions of both viruses, indicating that genetically incomplete or defective virus particles were not deficient in their ability to induce transformation. The average VP per transformation unit for adenovirus 12 (2.85 × 106) and for simian adenovirus 7 (4.00 × 106) did not vary significantly from fraction to fraction. These values were obtained with optimal input multiplicities of 16 to 64 VP per cell. At higher multiplicities the apparent increase in VP per transformation unit was attributable to the viral cytocidal effect on hamster cells. These studies revealed that quantitation of in vitro transformation based on VP multiplicities was more reliable than on the basis of infectious units. These estimates were independent of method of virus production, extraction, and purification.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAKKE M. K. Photometric scanning of centrifuged density gradient columns. Anal Biochem. 1963 Apr;5:271–283. doi: 10.1016/0003-2697(63)90079-4. [DOI] [PubMed] [Google Scholar]
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- Casto B. C. Adenovirus transformation of hamster embryo cells. J Virol. 1968 Apr;2(4):376–383. doi: 10.1128/jvi.2.4.376-383.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. E., Stich H. F., Yohn D. S. Viruses and mammalian chromosomes. 8. Dose response studies with human adenoviruses types 18 and 4. Virology. 1967 Nov;33(3):533–541. doi: 10.1016/0042-6822(67)90130-4. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Quantitative characteristics of the transformation of hamster cells by PARA (defective simian virus 40)-adenovirus 7. J Virol. 1970 May;5(5):568–577. doi: 10.1128/jvi.5.5.568-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., CHANOCK R. M., RUBIN B. A., CASEY M. J. INDUCTION BY ADENOVIRUS TYPE 7 OF TUMORS IN HAMSTERS HAVING THE ANTIGENIC CHARACTERISTICS OF SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1333–1340. doi: 10.1073/pnas.52.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Stimulation of DNA synthesis and thymidine kinase activity in human embryonic kidney cells infected by Adenovirus 2 or 12. Cancer Res. 1967 Aug;27(8):1459–1469. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- Mak S. Defective virions in human adenovirus type 12. J Virol. 1971 Apr;7(4):426–433. doi: 10.1128/jvi.7.4.426-433.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Macpherson I. Transformation of a hamster cell line by adenovirus type 12. J Gen Virol. 1968 Jan;2(1):99–106. doi: 10.1099/0022-1317-2-1-99. [DOI] [PubMed] [Google Scholar]
- Olsen R. G., McCammon J. R., Yohn D. S. Simplified procedure for preparation of specific antibodies to gamma globulins. Appl Microbiol. 1970 Jul;20(1):75–77. doi: 10.1128/am.20.1.75-77.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow A. J., Mak S. Functional heterogeneity of virions in human adenovirus types 2 and 12. J Virol. 1970 Feb;5(2):188–193. doi: 10.1128/jvi.5.2.188-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O. STUDIES ON ADENOVIRUS-12. I. QUANTITATIVE CORRELATIONS BETWEEN SOME PHYSICAL, ANTIGENIC AND INFECTIOUS PROPERTIES. J Immunol. 1965 Jun;94:976–989. [PubMed] [Google Scholar]
- STOKER M., ABEL P. Conditions affecting transformation by polyoma virus. Cold Spring Harb Symp Quant Biol. 1962;27:375–386. doi: 10.1101/sqb.1962.027.001.035. [DOI] [PubMed] [Google Scholar]
- Sauer G., Kidwai J. R. The transcription of the SV40 genome in productively infected and transformed cells. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1256–1263. doi: 10.1073/pnas.61.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Koprowski H., Defendi V. The genetic heterogeneity of simian virus 40. Proc Natl Acad Sci U S A. 1967 Aug;58(2):599–606. doi: 10.1073/pnas.58.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. 1. Cell killing and T antigen synthesis as correlated viral genome functions. Virology. 1969 Dec;39(4):642–652. doi: 10.1016/0042-6822(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Van Hoosier G. L., Jr, Trentin J. J. Stimulation of DNA synthesis in human and hamster cells by human adenovirus types 12 and 5. Proc Soc Exp Biol Med. 1966 Jul;122(3):740–746. doi: 10.3181/00379727-122-31241. [DOI] [PubMed] [Google Scholar]
- Thiel J. F., Smith K. O. Fluorescent focus assay of viruses on cell monolayers in plastic Petri plates. Proc Soc Exp Biol Med. 1967 Jul;125(3):892–895. doi: 10.3181/00379727-125-32232. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H. High frequency of SV40 transformation of mouse cell line 3T3. Virology. 1966 Apr;28(4):756–759. doi: 10.1016/0042-6822(66)90261-3. [DOI] [PubMed] [Google Scholar]
- Yohn D. S. Sex-related resistance in hamsters to adenovirus oncogenesis. Prog Exp Tumor Res. 1973;18:138–165. doi: 10.1159/000393166. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Sokol F. Fate of adenovirus type 12 genomes in nonpermissive cells. J Virol. 1969 Sep;4(3):256–263. doi: 10.1128/jvi.4.3.256-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]