Abstract
Objective
To investigate the expressions of caveolin-1, E-cadherin and β-catenin in gastric carcinoma, precancerous gastric and chronic non-atrophic gastritis tissues, and evaluate the correlation of these expressions with the development of gastric cancer.
Methods
The expressions of caveolin-1, E-cadherin and β-catenin were detected by biotin-streptavidin- peroxidase (SP) immunohistochemistry on 58 gastric cancer tissues, 40 precancerous gastric tissues and 42 chronic non-atrophic gastritis tissues. The correlation between the expressions of caveolin-1, E-cadherin and β-catenin, and the clinicopathologic parameters of gastric cancer was analyzed retrospectively.
Results
The positive rates of caveolin-1 and E-cadherin expressions in gastric carcinoma were significantly lower than precancerous gastric and chronic non-atrophic gastritis tissues (P<0.01). An abnormal rate of β-catenin expression in gastric carcinoma was higher than precancerous gastric and chronic non-atrophic gastritis tissues (P<0.01). Moreover, low expressions of caveolin-1, E-cadherin and β-catenin correlated with tumor size, depth of invasion, lymph node metastasis and TNM stage (P<0.05). The positive rates of caveolin-1 and E-cadherin expressions decreased (P<0.01), while an abnormal rate of β-catenin expression increased inversely, with the degree of atypical hyperplasia (P<0.01). Caveolin-1 expression correlated positively with E-cadherin (r=0.41, P<0.05). Caveolin-1 (r=-0.36, P<0.05) and E-cadherin (r=-0.45, P<0.05) expressions negatively correlated with abnormal β-catenin expression.
Conclusion
These results suggested that dysregulated expressions of caveolin-1, E-cadherin and β-catenin correlated with the development of gastric cancer and its biological behavior.
Key words: Gastric carcinoma, Caveolin-1, E-cadherin, β-catenin, Immunohistochemistry
INTRODUCTION
Cancer biology researches have demonstrated that a multi-step process and a lot of genetic alterations are involved in the development of malignant tumors. The loss of cell-cell adhesion is associated with tumoral progression, invasion and metastasis. A number of membrane molecules and genes, including caveolin-1, E-cadherin and β-catenin, are involved[1-8]. Caveolin-1 is an essential structural protein that acts as a tumor modulator regulating cells proliferation, different- tiation, apoptosis, adhesion, and invasion by interacting with cell adhesion molecules and signaling receptors[3-4,8-11]. E-cadherin and β-catenin as a complex are specifically involved in the regulation of epithelial cell-to-cell adhesion and they have a close relationship with the invasion and metastasis ability of cancer[6,7,12-15]. In many malignant tumors, down- expression or destruction of E-cadherin and β-catenin is one of the changes that characterize an invasive phenotype. These genes are considered to be invasion/tumor suppressor genes, and partial or complete loss of E-cadherin and β-catenin expression is reported to be associated with poor prognosis in malignant tumors[11, 14-17].
The aim of this study was to investigate the changes of caveolin-1, E-cadherin and β-catenin expressions in gastric carcinoma, precancerous, and chronic non-atrophic gastritis tissues by using immunohistochemical staining, and to explore their roles in gastric carcinogenesis and development.
MATERIALS AND METHODS
Patients and Specimens
Fifty-eight patients with gastric carcinoma underwent surgical resection at the First Affiliated Hospital of Wenzhou Medical College from January 2007 to October 2009. There were 49 males and 9 females. The median age was 59.6±10.4 years (range 29 to 78 years). The histopathological diagnosis was well differentiated adenocarcinoma in 14 patients, poorly differentiated adenocarcinoma in 42, and undifferentiated carcinoma in 2. The TNM staging based on International Union Against Cancer (UICC) classification (6th edition)[18] was stage I in 9 patients, stage II in 7, stage III in 17 and stage IV in 25. During the same study period, precancerous gastric tissues from 40 patients with a median age of 60.8±12.6 years (range 28.6 to 87 years) were collected. These included 19 patients with intestinal metaplasia and 21 with dysplasia. Also, chronic non-atrophic gastritis tissues were collected from 42 patients with a median age of 46.3±10.7 years (range 21 to 70 years). This study was approved by the Ethics Committee of the Hospital (No. 2006-06).
Moderately-differentiated tubular adenocarcinoma, and mucinous carcinoma, were regarded histo- logically as differentiated tumors. Solid poorly differentiated adenocarcinoma, non-solid poorly differentiated adenocarcinoma, and signet-ring cell carcinoma were regarded as undifferentiated tumors. The depth of invasion was classified as mucosal, submucosal, muscularis and subserosal involvement.
Immunohistochemistry
Immunohistochemistry was performed by the standard biotin-streptavidin-peroxidase (SP) method on 4 μm thick formalin-fixed, paraffin-embedded tissue sections. After deparaffinization in xylene and rehydration in descending concentrations of alcohol, an antigen retrieval step was performed by microwaving in 10 mmol/L citrate buffer (pH 6) for 20 min at boiling temperature. Endogenous peroxidase was blocked by 3% hydrogen peroxide. After blocking with 3% bovine serum albumin for 1 h, the sections were incubated with diluted primary antibody for an appropriate time as listed in Table 1. The 3, 3′-diaminobenzidine (DAB) solution was used as a chromogen. The sections were then lightly counterstained with hematoxylin. The sections without primary antibody served as negative controls.
Table 1. Primary antibodies used for immunohistochemical analysis.
| Protein | Company | Antibody dilution/incubation time |
|---|---|---|
| Caveolin-1 | Santa Cruz Biotechnology, Inc., USA | 1:100/1 h |
| β-catenin | Santa Cruz Biotechnology, Inc., USA | 1:50/1 h |
| E-cadherin | ZSGB-BIO, Beijing, China | 1:100/1 h |
Incubation was at room temperature.
Evaluation of Immunohistochemical Staining
The immunostaining was independently evaluated semiquantitatively by two pathologists who were unaware of the clinical data. Caveolin-1 expression was based on the presence of cytoplasmic and/or membranous staining. On the basis of the percentage of immunopositive cells, the data were subdivided into four categories: 0, no positive cell; 1, <25%; 2, 25%-50%; and 3, >50% positive cells. E-cadherin expression was based on the presence of membranous staining. The expression of E-cadherin was classified as more than 90% of cell membranes stained as positive, otherwise as reduced expression. The expression of β-catenin was classified as more than 70% of cell membranes stained as positive, otherwise as negative, and more than 10% of cells with cytosol or nuclei stained as ectopic expression. Ectopic expression and negative expression were considered to reflect abnormal expression.
Statistical Analysis
The chi-square and Fisher’s exact tests were used to analyze the statistical significance of the relationship between caveolin-1, E-cadherin, β-catenin expressions and the clinicopathological variables. SPSS 11.5 program package (SPSS Inc., IL, USA) was used for statistical analysis. A P value of less than 0.05 was regarded as statistically significant.
RESULTS
Caveolin-1 Protein Expression Correlated with Clinicopathological Variables
Caveolin-1 protein appeared at cell cytoplasm and/or membrane, and it was located at the base and neck of gastric pits within all fundic mucosal fragments in gastric intestinal metaplasia as well as the cells. The positive rates of caveolin-1 expression in chronic non-atrophic gastritis was 100% (42/42), but it was decreased in precancerous gastric tissues (80.0%, 32/40) and in gastric carcinoma (48.3%, 28/58) (Figure 1A-C). The difference between these groups was significant (χ2=34.40, P<0.01, Table 2). The positive rates of caveolin-1 expression decreased with the degree of atypical hyperplasia (χ2=39.08, P<0.01). Moreover, the rate of positive caveolin-1 staining of smaller (<5 cm) tumors (66.7%, 14/21) was significantly higher than larger (≥5 cm in diameter) tumors (37.8%, 14/37) (χ2=4.46, P<0.05), and the expression in T3+T4 lesions (35.6%, 16/45) was significantly lower than that in T1+T2 lesions (92.3%, 12/13) (χ2=13.01, P<0.01). The difference in caveolin-1 expression between tumors with lymph node metastases (38.1%, 16/42) and those without (75.0%, 12/16) was significant (χ2=6.32, P<0.05). Significant differences in caveolin-1 expression were found in patients with stage I+II diseases (93.8%, 15/16) than stage III+IV diseases (31.0%, 13/42) (χ2=18.30, P<0.01) (Table 3).
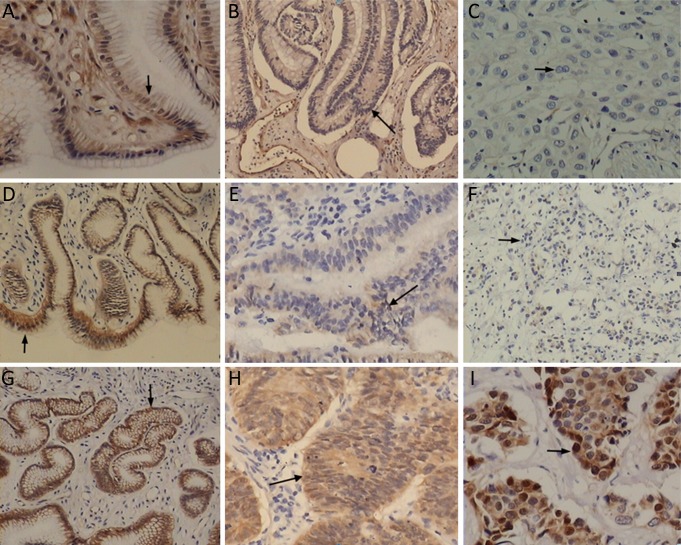
Figure 1.
Immunohistochemical staining for caveolin-1, E-cadherin and β-catenin. A. The expression of caveolin-1 (arrow) in chronic non-atrophic gastritis (SP method, ×200); B. The expression of caveolin-1 (arrow) in precancerous lesion (dysplasia, ×100); C. The expression of caveolin-1 (arrow) in gastric carcinoma (×200); D. The expression of E-cadherin (arrow) in chronic non-atrophic gastritis (×100); E. The expression of E-cadherin (arrow) in precancerous lesion (dysplasia, ×100); F. The expression of E-cadherin in (arrow) in gastric carcinoma (×100); G. The expression of β-catenin (arrow) in chronic non-atrophic gastritis (×200); H. The expression of β-catenin in precancerous lesion (dysplasia, ×200); I. The expression of β-catenin (arrow) in gastric carcinoma (×200).
Table 2. Expressions of caveolin-1, E-cadherin and β-catenin in gastric cancer tissues.
| Groups | Caveolin-1 positive expression (n, %) |
E-cadherin positive expression (n, %) |
β-catenin abnormal expression (n, %) |
|---|---|---|---|
| Chronic non-atrophic gastritis (n=42) | 42 (100)a | 42 (100)b | 0c |
| Precancerous lesion (n=40) | 32 (80.0) | 25 (62.5) | 13 (32.5) |
| IM (n=19) | 19 (100) | 15 (78.9) | 2 (10.5) |
| Dysplasia (n=21) | 13 (61.9) | 10 (47.6) | 11 (52.4) |
| Gastric carcinoma (n=58) | 28 (48.3)d | 16 (27.6)e | 40 (69.0)f |
aχ2=34.40, P=0.000, vs. precancerous lesion and gastric carcinoma; bχ2=53.16, P=0.000, vs. precancerous lesion and gastric carcinoma; cχ2=49.93, P=0.000, vs. precancerous lesion and gastric carcinoma; dχ2(trend of proportion)=39.08, P=0.000, vs. dysplasia, intestinal metaplasia (IM) and chronic non-atrophic gastritis; eχ2(trend of proportion)=57.00, P=0.000, vs. dysplasia, IM and chronic non-atrophic gastritis; fχ2(trend of proportion)=55.85, P=0.00, vs. dysplasia, IM and chronic non-atrophic gastritis.
Table 3. Relationship between the expressions of caveolin-1, E-cadherin and β-catenin and pathological variables in gastric carcinoma.
| Clinical variables | Caveolin-1 positive expression (n, %) |
E-cadherin positive expression (n, %) |
β-catenin abnormal expression (n, %) |
|---|---|---|---|
| Age | |||
| <60 (n=25) | 11 (44.0) | 8 (32.0) | 18 (72.0) |
| ≥60 (n=33) | 17 (51.5) | 8 (24.2) | 23 (69.7) |
| Gender | |||
| Male (n=49) | 23 (46.9) | 13 (26.5) | 33 (67.3) |
| Female (n=9) | 5 (55.6) | 3 (33.3) | 8 (88.9) |
| Tumor size | |||
| <5 cm (n=21) | 14 (66.7)* | 9 (42.9)** | 11 (52.4)* |
| ≥5 cm (n=37) | 14 (37.8) | 7 (18.9) | 30 (81.1) |
| Histopathological differentiation | |||
| Differentiated Ad (n=14) | 9 (64.3) | 5 (35.7) | 7 (50.0) |
| Undifferentiated Ad (n=44) | 19 (43.2) | 11 (25.0) | 35 (79.5) |
| Depth of invasion# | |||
| T1+T2 (n=13) | 12 (92.3)* | 9 (69.2)* | 3 (23.1)* |
| T3+T4 (n=45) | 16 (35.6) | 7 (15.6) | 38 (84.4) |
| Lymph node metastasis# | |||
| No (n=16) | 12 (75.0)* | 12 (75.0)* | 4 (25.0)* |
| Yes (n=42) | 16 (38.1) | 4 (9.5) | 37 (88.1) |
| TNM stage# | |||
| I+II (n=16) | 15 (93.8)* | 12 (75.0)* | 4 (25.0)* |
| III+IV (n=42) | 13 (31.0) | 4 (9.5) | 37 (88.1) |
Statistical analysis was performed by chi-square test (*P<0.05; **P=0.05); Ad, adenocarcinoma. #TNM classification of malignant tumors (6th edition)[18].
E-cadherin Protein Expression Correlates With Clinicopathological Variables
E-cadherin immunohistochemical staining was located at cell membrane. The positive rates of E-cadherin expression in gastric carcinoma (27.6%, 16/58) and in precancerous gastric tissues (62.5%, 25/40) were significantly less than in chronic non-atrophic gastritis (100%, 42/42) (χ2=53.16, P<0.01) (Figure 1D-F, Table 2). The positive rates of E-cadherin expression decreased with worsening degrees of atypical hyperplasia (χ2=57.00, P<0.01). Reduced E-cadherin expression correlated with tumor size 18.9% (7/37) vs. 42.9% (9/21) (χ2=3.84, P<0.05), depth of invasion 15.6% (7/45) vs. 69.2% (9/13) (χ2=14.55, P<0.01), lymph node metastasis 9.5% (4/42) vs. 75.0% (12/16) (χ2=24.87, P<0.01), and TNM stage 9.5% (4/42) vs. 75.0% (12/16) (χ2=24.87, P<0.01), but not with histopathological differentiation, age or gender (P>0.05) (Table 3).
β-catenin Protein Expression Correlated with Clinicopathological Variables
In this study, β-catenin protein was expressed in cytosol or nuclei, and no immunoreactivity of β-catenin was found in normal gastric mucosal tissues. The positive expression of β-catenin protein was 69.0% (40/58) in patients with gastric carcinoma and 32.5% (13/40) in precancerous gastric tissues. The difference was significant (χ2=49.93, P<0.01) (Figure 1G-I, Table 2). The positive rates of abnormal rate of β-catenin expression increased inversely with worsening degrees of atypical hyperplasia (χ2=39.90, P<0.01). β-catenin expression correlated with tumor size 81.1% (30/37) vs. 52.4%(11/21) (χ2=5.33, P<0.05), depth of invasion 84.4% (38/45) vs. 23.1% (3/13) (χ2=18.33, P<0.01), lymph node metastasis 88.1% (37/42) vs. 25.0% (4/16) (χ2=24.26, P<0.01) and TNM stage 88.1% (37/42) vs. 25.0% (4/16) (χ2=22.26, P<0.01), but not with histopathological differentiation, age or gender (P>0.05) (Table 3).
Correlation among Expressions of Caveolin-1, E-cadherin and β-catenin
Positive correlation (r=0.41, P<0.05) was observed by a Spearman correlation analysis between the expressions of caveolin-1 and E-cadherin, but they were negatively correlated with the expression of β-catenin (r=-0.36 and r=-0.45, respectively, P<0.05) (Table 4).
Table 4. Correlation among expressions of caveolin-1, E-cadherin and β-catenin.
| Protein | E-cadherin |
r value | β-catenin |
r values | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Caveolin-1 | 0.407 | -0.363 | ||||
| Positive | 13* | 15 | 15* | 13 | ||
| Negative | 3 | 27 | 26 | 4 | ||
| E-cadherin | -0.450 | |||||
| Positive | 6* | 10 | ||||
| Negative | 35 | 7 | ||||
Statistical analysis was performed by Spearman rank correlation test (*P<0.05).
DISCUSSION
In our study, we found (1) the positive rates of caveolin-1 and E-cadherin expressions in gastric carcinoma were significantly lower than those in precancerous gastric and chronic non-atrophic gastritis tissues; (2) the positive rate of β-catenin expression in gastric carcinoma was significantly higher than that in precancerous gastric and chronic non-atrophic gastritis tissues; (3) the positive rates of caveolin-1 and E-cadherin expression decreased with the degrees of atypical hyperplasia; and (4) the positive rates of abnormal rate of β-catenin expression increased inversely with the degrees of atypical hyperplasia. All these suggested that a reduction or loss of expression of caveolin-1 and E-cadherin, and an over-expression of β-catenin were implicated in the pathogenesis of oncogenic cell transformation and promoted the occurrence of gastric cancer. Williams et al.[19] found disruption of the caveolin-1 gene in transgenic mice promoted mammary tumorigenesis. Burgermeister, et al.[9] reported that the overall caveolin-1 mRNA and protein were decreased in 74% and 54% gastric carcinoma patients, respectively. Furthermore, recent evidences showed that caveolin-1 was implicated in the pathogenesis of oncogenic cell transformation and tumorigenesis through Wnt signaling pathway by regulating β-catenin location[9-12]. In this study, we have also found that gastric intestinal metaplasia as well as the cells at the base and neck of gastric pits within all fundic mucosal fragments showed caveolin-1 immuno-staining, suggesting a histogenetic derivation of these lesions from the trans- differentiation of chief cells or from a cryptic progenitor population at the base of fundic glands.
Chu, et al.[16] discovered a decreased E-cadherin expression was related to invasion and metastasis of gastric carcinoma. Utsunomiya, et al.[15] also discovered that a decrease of E-cadherin or β-catenin or both could weaken cell-cell adhesion and resulted in cell dissipation that made cancer cell infiltration and metastasis easier. Our study showed that down-regulated expressions of caveolin-1 and E-cadherin were related to the depth of invasion, lymph node metastasis and TNM stages, and up-regulated expression of β-catenin was correlated to the depth of invasion, lymph node metastasis and TNM stage. These suggested that a reduction or loss of expression of caveolin-1 and E-cadherin and an over-expression of β-catenin promoted the development of gastric cancer and its malignant biological behaviors such as invasion and metastasis.
A report from Zhou, et al[7], suggested that the abnormal expressions of β-catenin and E-cadherin were 43.2% and 44.6% in gastric cancer, respectively. Abnormal β-catenin and E-cadherin staining occurred more frequently in poorly differentiated tumors than in well differentiated tumors (P<0.005, respectively). However, in our study, the difference in the expression of β-catenin and E-cadherin between the differentiated and undifferentiated groups was not significant (P>0.05). The possible reason may be related to our small sample size.
Our study also showed that (1) the expressions of caveolin-1 and E-cadherin in the β-catenin (+) group were significantly lower than those in the β-catenin (-) group in gastric cancer tissues; (2) the expressions of caveolin-1 and E-cadherin were negatively correlated with the expression of β-catenin, respectively; (3) the expression of caveolin-1 in the E-cadherin (+) group was significantly higher than that in the E-cadherin (-) gastric cancer tissues; (4) the expression of caveolin-1 was positively correlated with the expression of E-cadherin; (5) the expression of β-catenin in the gastric cancer patients with lymph node metastasis was significantly higher than that in patients without lymph node metastasis; and (6) the expressions of caveolin-1 and E-cadherin were negatively correlated. These suggested that a reduction or loss of the expressions of caveolin-1 and E-cadherin worked in coordination with an over-expression of β-catenin to promote the development of gastric cancer and its malignant biological behaviors. Recent studies revealed that the cytoplasmic domain of E-cadherin is associated with catenins, and β-catenin directly connects the E-cadherin to β-catenin, which in turn links up with the actin-based cytoskeleton[20,21]. These associations are essential for the intercellular adhesive function of E-cadherin. Any significant change in the expression or structure of one of these components may lead to an adhere junction disassembly, which has been implicated in the loss of tumor differentiation and the development of invasive phenotype of tumor cells[22-24]. Taking these into account, we can say that there may be a close link among these three indicators during the occurrence and development of gastric cancer.
Utsunomiya, et al.[15] reported that a disordered β-catenin expression pattern was significantly correlated with diffuse type adenocarcinoma and deep tumor infiltration (P<0.05), and its expression was significantly associated with reduced E-cadherin expression (P<0.01). Joo, et al.[14] reported that abnormal expressions of E-cadherin and β-catenin were correlated with poorly differentiated and diffuse-type carcinoma and poor survival. However, no significant difference was found between poorly differentiated and well differentiated adenocarcinoma in our study.
In summary, our results suggested that dysregulated expressions of caveolin-1, E-cadherin and β-catenin may be correlated with the development of gastric cancer and its biological behaviors.
ACKNOWLEDGMENT
We thank W. Y. Joseph Lau (Department of General Surgery, Prince of Wales Hospital, the Chinese University of Hong Kong Faculty of Medicine) for revising this paper.
REFERENCES
- 1.Tamura G.Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol 2006;12:192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol 2003;36(5Suppl):S37-43 [DOI] [PubMed] [Google Scholar]
- 3.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 2005;288:C494-506 [DOI] [PubMed] [Google Scholar]
- 4.Lu Z, Ghosh S, Wang Z, et al. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 2003;4:499-515 [DOI] [PubMed] [Google Scholar]
- 5.Couffinhal T, Dufourcq P, Duplàa C.Beta-catenin nuclear activation: common pathway between Wnt and growth factor signaling in vascular smooth muscle cell proliferation? Circ Res 2006;99:1287-9 [DOI] [PubMed] [Google Scholar]
- 6.Zhou YN, Xu CP, Han B, et al. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol 2002;8:987-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou YN, Li M. Relationship between abnormal expression of beta-catenin in gastric carcinoma and survival. Ai Zheng (in Chinese)2002;21:536-40 [PubMed] [Google Scholar]
- 8.Huang MF, Zhu YQ, Chen ZF, et al. Syndecan-1 and E-cadherin expression in differentiated type of early gastric cancer. World J Gastroenterol 2005;11:2975-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgermeister E, Xing X, Röcken C, et al. Differential expression and function of caveolin-1 in human gastric cancer progression. Cancer Res 2007;67:8519-26 [DOI] [PubMed] [Google Scholar]
- 10.Torres VA, Tapia JC, Rodríguez DA, et al. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci 2006;119:1812-23 [DOI] [PubMed] [Google Scholar]
- 11.Galbiati F, Volonte D, Brown AM, et al. Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem 2000;275:23368-77 [DOI] [PubMed] [Google Scholar]
- 12.Chan AO. E-cadherin in gastric cancer. World J Gastroenterol 2006;12:199-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racine C, Belanger M, Hirabayashi H, et al. Reduction of caveolin 1 gene expression in lung carcinoma cell lines. Biochem Biophys Res Commun 1999;255:580-6 [DOI] [PubMed] [Google Scholar]
- 14.Joo YE, Park CS, Kim HS, et al. Prognostic significance of E-cadherin/catenin complex expression in gastric cancer. J Korean Med Sci 2000;15:655-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utsunomiya T, Doki Y, Takemoto H, et al. Clinical significance of disordered beta-catenin expression pattern in human gastric cancers. Gastric Cancer 2000;3:193-201 [DOI] [PubMed] [Google Scholar]
- 16.Chu YQ, Ye ZY, Tao HQ, et al. Relationship between cell adhering molecules expression and the biological behavior of gastric carcinoma. World J Gastroenterol 2008;14:1990-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Zhang F, Wu PP, et al. Disordered beta-catenin expression and E-cadherin/CDH1 promoter methylation in gastric carcinoma. World J Gastroenterol 2006;12:4228-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin LH, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 6th edition. New York: Wiley; 2002. [Google Scholar]
- 19.Williams TM, Medina F, Badano I, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. J Biol Chem 2004;279:51630-46 [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi Y, Ochiai A, Akimoto S, et al. Expression of E-cadherin, alpha-catenin, beta-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology 1997;54:158-65 [DOI] [PubMed] [Google Scholar]
- 21.Yoon CS, Hyung WJ, Lee JH, et al. Expression of S100A4, E-cadherin, alpha- and beta-catenin in gastric adenocarcinoma. Hepatogastroenterology 2008;55:1916-20 [PubMed] [Google Scholar]
- 22.Williams TM, Sotgia F, Lee H, et al. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol 2006;169:1784-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo HM, Tang SS, Liao DF, et al. Effect of caveolin-1 on growth of human gastric cancer cell line MGC803. Shi Jie Hua Ren Xiao Hua Za Zhi (in Chinese)2006;14:1448-52 [Google Scholar]
- 24.Luo HM, Zhang MS, Tang SS, et al. Study of the relationship between suspected anti-oncogene caveolin-1 expression and gastric carcinoma biological behavior. Zhong Liu Fang Zhi Za Zhi (in Chinese)2004;11:341-69 [Google Scholar]