Abstract
Objective
Transforming growth factor-1 (TGF-β1), vascular endothelial growth factor (VEGF), and interleukin-10 (IL-10) may be critical cytokines in the microenvironment of a tumor, playing roles in immune suppression. This study was conducted to elucidate the roles and immunosuppressive functions of these cytokines in epithelial ovarian cancer (EOC).
Methods
The expression levels of TGF-β1, VEGF and IL-10 in malignant tissue were evaluated by immune- histochemistry and compared with corresponding borderline, benign, and tumor-free tissues. Moreover, relationships among the levels of these cytokines and correlations between expression and the prognosis of EOC were analyzed by Pearson rank correlations and multi-factor Logistic regression. The roles of TGF-β1, VEGF, and IL-10 in the immunosuppressive microenvironment of ovarian cancer were studied through dendritic cell (DC) maturation and CD4+CD25+FoxP3+ Treg generation in vitro experiments.
Results
TGF-β1, VEGF, and IL-10 were expressed in 100%, 74.69%, and 54.96% of EOC patients, respectively. TGF-β1 was an independent prognostic factor for EOC. IL-10 was significantly co-expressed with VEGF. In vitro, VEGF and TGF-β1 strongly interfered with DC maturation and consequently led to immature DCs, which secreted high levels of IL-10 that accumulated around the tumor site. TGF-β1 and IL-10 induced Treg generation without antigen presentation in DCs.
Conclusions
TGF-β1, VEGF and IL-10 play important roles in EOC and can lead to frequent immune evasion events.
Key words: Epithelial ovarian cancer, Tumor microenvironment, Immunosuppression, Cytokines
INTRODUCTION
Ovarian cancer is the most lethal disease among gynecological malignancies. In 2008, it accounted for around 225,500 new cases and 140,200 deaths worldwide[1]. Despite improvements in the past decade in treatment strategies such as surgery, combination chemotherapy, and targeted molecular therapy, approximately 80% of patients after primary therapy develop recurrence and metastasis. Ovarian cancer has the ability to escape the immune monitoring, like many aggressive malignancies, by creating a highly suppressive environment around the tumor. Yet, ovarian cancer differs from other malignancies in its specific dissemination pattern. Ovarian cancer typically spreads in a diffuse intra-abdominal fashion; even after recurrence, it is mostly confined to the peritoneal cavity where immunosuppressive activities can be carried out. Therefore, the tumor microenvironment plays a critical role in modulating the process of progression, invasion, and metastasis of ovarian cancer. This tumor microenvironment largely includes cell-cell and cell- extracellular component cross-talk interactions that regulate cell activities such as proliferation, differenti- ation, and apoptosis, as well as secretion and activation of soluble cytokines.
Transforming growth factor (TGF-β) is an important soluble cytokine with various cell functions, including cell proliferation, differentiation, and extracellular matrix remodeling[2]. TGF-β, especially TGF-β1, has a profound inhibiting effect on the immune system by blocking major histocompatibility complex (MHC) class II expression on tumor cells and deactivating tumor infiltrating lymphocytes (TILs) and natural killer (NK) cells[3-5]. The overexpression of TGF-β affects the surrounding stromal cells, immune cells, and endothelial cells, leading to immuno- suppression, angiogenesis, and tumor invasion[6]. Vascular endothelial growth factor (VEGF), originally thought to be solely involved in vascular growth and permeability, has emerged as a multifunctional cytokine that stimulates angiogenesis, cell proliferation, and migration. In addition, it is believed that VEGF has an important immunosuppressive function[7]. VEGF overexpression has been reported in various cancers, including lung, colorectal, gastric, esophageal, and ovarian carcinomas[8-13]; yet, the latest report found high VEGF expression occurs in a small proportion (about 7%) of ovarian cancer patients[14]. Interleukin-10 (IL-10), an immunoregulatory cytokine that targets antigen- presenting cells (APCs), T cells, and other immune cells, may play an immunosuppressive role in the tumor micro-environment[15]. Some studies found IL-10 performs immunostimulatory functions and seems to either favor or inhibit tumor progression[16]. In ovarian cancer cells, different views exist about the expression of IL-10[17,18]. This study was conducted to analyze the expression of TGF-β1, VEGF and IL-10 in different ovarian tissues [including epithelial ovarian cancer (EOC), borderline ovarian tumor, benign ovarian tumor, and normal ovarian tissue], correlations between expression and the clinicopathologic features, and possible roles of these cytokines in the immunosuppressive micro- environment of ovarian cancer.
MATERIALS AND METHODS
Clinical Specimens
One hundred and twenty-five patients (age 22–78 years) with histologically confirmed primary ovarian tumor were classified into the following histological groups: malignant (n=79), borderline (n=16), and benign (n=30). The malignant and the borderline specimens were obtained from patients undergoing primary debulking surgery at the Gynecology Oncology Center, Peking University People’s Hospital, Beijing. Benign specimens were obtained from Department of Gynecology and Obstetrics, Haidian Hospital, Beijing. The malignant group contained serous adenocarcinoma (n=48), endometrioid adenocarcinoma (n=12), mucinous adenocarcinoma (n=5), clear cell carcinomas (n=4), malignant mixed mesodermal tumors (n=5), malignant Brenner tumor (n=2) and non-Brenner type transitional cell carcinoma (n=3), wherein the histological grade of malignant group including I: well differentiation (n=9), II: moderate differentiation (n=30), and III: poor differentiation (n=40); Federation International of Gynecology and Obstetrics (FIGO) stage of malignant group including I (n=11), II (n=13), III (n=50), and IV (n=5); lymphatic metastasis (n=30). The borderline group consisted of serous (n=6), and mucinous (n=10). The benign group contained serous cystadenoma (n=7), mucinous cystadenoma (n=9), endometriosis cyst (n=3), simple cyst (n=5), fibroma (n=4), cystic follicle (n=1), and serous cysts (n=1). Moreover, 20 cases of normal ovarian tissue beside endometriosis cyst were obtained from Department of Gynecology and Obstetrics, Haidian Hospital, Beijing.
Immunohistochemistry
Tissue samples were fixed in sodium phosphate- buffered neutral 10% formalin. After 1–2 d of fixation, selected tissue blocks were routinely processed and paraffin-embedded. Five micrometers thick sections were mounted on poly-L-lysine-coated slides (Sigma, St. Louis, MO, USA). The sections were deparaffinized, rehydrated and incubated with 3% H2O2 in methanol for 30 min to quench endogenous peroxidase activity. After a short rinse, the sections were boiled in water-bath for 15−20 min in citrate buffer. Following cooling and rinsing, normal goat serum was applied on the sections for 20 min in order to block non-specific binding. The sections were then incubated overnight at 4°C with specific antibodies directed against TGF-β1, VEGF (Santa Cruz, Sweden), and IL-10 (Biolegend, US). Localization of antigen-antibody complexes was performed with the streptavidin-perosidase (SP) technique using SP Kits (ZYMED, USA). At last, the sections were counterstained with Mayer’s hematoxylin.
TGF-β1 expression was evaluated by computer assessment method[19]. The immunostained sections were examined using light microscopy (BX40, OLYMPUS, Japan) to assess the prevalence of positive cases and the localization of immmunostaining within the tissues. Tumor cells with unequivocal staining of the granular cytoplasm were considered positive. In each case, at least five microscopic fields were captured using a 200× magnification. Images were then digitized and saved as image files (1,024×903 pixels) in the PC computer and optical density (A) was obtained from the image analysis software system (Leica-Qwin, UK).
For the semi-quantitative (PI value) evaluation of VEGF and IL-10, every section was judged by two observers under a standard light microscope (BX40, OLYMPUS, Japan). Each sample was evaluated regarding staining of epithelial cells while staining of stromal tumor cells was thus not registered. In normal ovaries, only ovarian surface epithelial cells were evaluated. The mean percentage of positive cells was determined in at least five areas at ×200 magnification and assigned to one of the following categories (P value): 0=<5%; 1=5%–25%; 3=50%–75%; 4=>75%. The immunostaining intensity (I value) was scored as 1 (yellow), 2 (buffy) or 3 (brown). The PI value was determined by the cross product of P value and I value: 0 (negative), 1–2 (weak), 3–4 (moderate), and 6–9 (intense).
Cell Culture
The EOC cell line SKOV3.ip was obtained from M.D. Anderson and cultured in RPMI 1640 (Gibco BRL, USA) with 15% calf serum (Gibco BRL), 100 U/ml penicillin, and 100 mg/ml streptomycin, and maintained at 37°C in a 5% CO2 humidified atmosphere. When the cells had adhered to the whole bottom of the bottle, the medium was changed to serum-free RPMI 1640. After incubated for 72 h, the supernatant was collected and concentrated by using Ultrafree MC 3,000 kDa centrifugal filter units (Millipore, USA), and then filtered to remove bacteria through a 0.22-μm filter. Finally, the concentrations of TGF-β1, VEGF and IL-10 in the supernatant were determined using a human enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, US). Concentrated serum-free RPMI 1640 was acted as negative control.
Peripheral blood mononuclear cells (PBMCs) from healthy adult donors who provided informed consent were separated by Ficoll-Hypaque (GE Healthcare Bio-Sciences, Little Chalfont, UK) density gradient centrifugation. The plastic adherence method was used to separate monocytes from lymphocytes. Lympho- cytes and monocytes were cultured in serum-free AIM-V medium at 37°C in a 5% CO2 humidified atmosphere for 6 d, 1,000 U/ml recombinant human (rh) granulocyte-macrophage colony stimulating factor (GM-CSF) and 1,000 U/ml rh IL-4 (Peprotech) were used to generate immature dendritic cells (imDCs).
After 6 d of incubation, SKOV3.ip supernatant or negative control medium was added to the culture medium of lymphocytes and imDCs. In addition, 1,000 U/ml rh TNF-α, rh interferon (IFN)-α, and rh IL-6 (Peprotech, Israel) and 1 mg/ml prostaglandin E2 (PGE2) (Sigma, USA) were used to induce imDC maturation. All of the cells were incubated for another 3 d. Human TGF-β1, VEGF and IL-10 (Peprotech) were added to lymphocytes and imDCs in different group.
Flow Cytometry Analysis
DCs were analyzed by extracellular immuno- labeling with fluorescein isothiocyanate (FITC)- conjugated anti-human CD83, phycoerythrin (PE)- conjugated anti-human CD1a (BD Biosciences, USA). Lymphocytes were first stained extracellularly with a monoclonal antibody mixture of peridinin chlorophyll protein (PerCP)-conjugated anti-human CD3, FITC- conjugated anti-human CD4, and PE-conjugated anti- human CD25 (BD Biosciences), then fixed and permeabilized with Perm/Fix solution (eBioscience). The cells were stained intracellularly with allophycocyanin (APC)-conjugated anti-human FoxP3 (eBioscience).
Meanwhile, FITC-conjugated normal mouse IgG1, PE-conjugated normal mouse IgG2, and APC- conjugated normal mouse IgG2 (BD Biosciences) were used as isotype controls. At least 50,000 cells per sample were processed with an LSR II cell analyzer (BD Biosciences), and the data were analyzed with the DIVA software (BD Biosciences).
Statistical Analysis
Data are expressed as‾x±s and were analyzed using the t-test when the distribution was normal. Kruskal-Wallis test was used to evaluate the expression of TGF-β1, VEGF and IL-10 in different ovarian tissues. Correlations between TGF-β1, VEGF and IL-10 were assessed by Pearson rank correlations. The probability of survival was estimated by Kaplan-Meier and log rank test. The prognostic factors were determined by multi-factor Logistic regression. P<0.05 was considered to be statistically significant. All of the statistical analyses were performed using GraphPad Prism 5.0 and the statistical package SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
TGF-β1, VEGF, and IL-10 Expression in Different Kinds of Ovarian Tissue and Correlation Analysis of These Three Cytokines
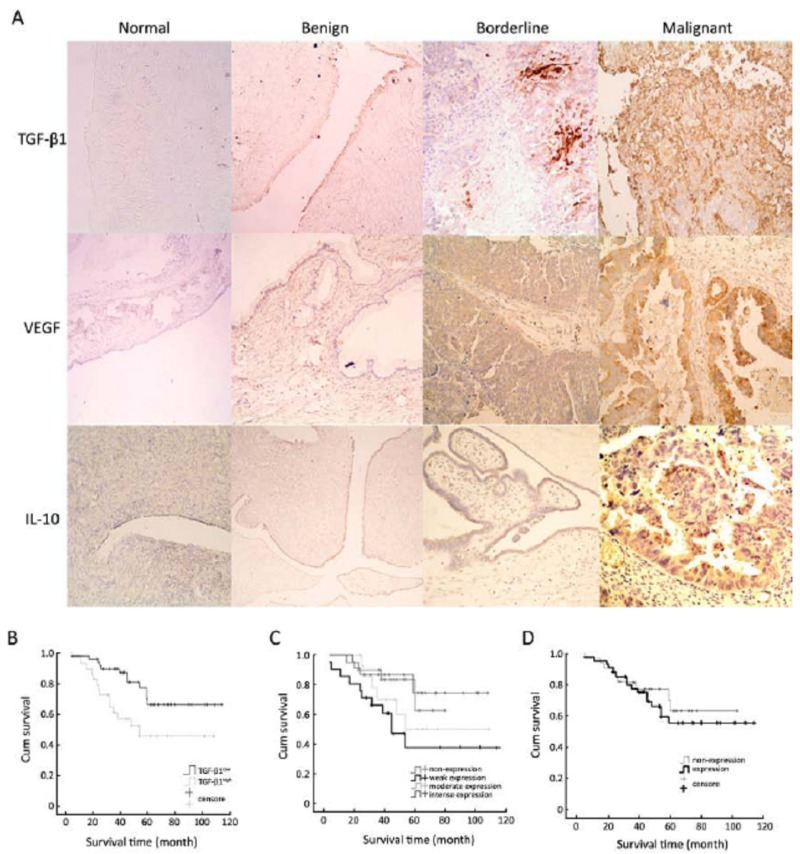
Positive staining for TGF-β1, VEGF, and IL-10 of epithelial cells are shown in Figure 1A. In the EOC group, the positive staining rates of TGF-β1, VEGF, and IL-10 were 100% (79/79), 74.69% (59/79), and 56.96% (45/79), respectively. Using the Kruskal-Wallis test, the expression and mean rank of the cytokines in different kinds of ovarian tissue were determined and are shown in Table 1. TGF-β1 expression in normal ovarian tissue was significantly lower than that in epithelial carcinoma, borderline, and benign ovarian tumors (P=0.009); while, the levels of VEGF and IL-10 in EOC tissue were significantly higher than those in the borderline, benign tumor, and normal tissues (P<0.001). Pearson correlation analysis showed IL-10 over- expression was correlated with VEGF expression (P<0.001), and the correlation coefficient was 0.327. TGF-β1 expression was not correlated with VEGF or IL-10, and the correlation coefficients were 0.104 and 0.102, respectively (P=0.101 and 0.119, respectively).
Figure 1.
TGF-β1, VEGF and IL-10 expression and survival analysis. A: TGF-β1, VEGF, and IL-10 expression in 4 types of ovarian tissue specimens (SP, ×200). TGF-β1 was not expressed in normal ovarian tissue, weakly expressed in ovarian mucinous cyst- adenoma and ovarian borderline serous cystadenoma, and intensely expressed in ovarian serous cystadenocarcinoma. VEGF was not expressed in normal tissue and mucinous cystadenoma, moderately expressed in borderline serous cystadenoma, and strongly expressed in ovarian serous cystadeno- carcinoma. IL-10 was not expressed in normal ovarian tissue, weakly expressed in ovarian mucinous cyst- adenoma, moderately expressed in borderline serous cystadenoma, and strongly expressed in ovarian serous cystadenocarcinoma. B: Survival curve of 79 EOC with low or high level of TGF-β1 expression; C: Survival curve of 79 EOC with different level of VEGF expression; D: Survival curve of 79 EOC with IL-10 non-expression of expression.
Table 1. Mean rank of TGF-β1, VEGF and IL-10 in 4 kinds of ovarian tissue.
| Pathologic Types | Mean rank TGF-β1 VEGF IL-10 |
||
|---|---|---|---|
| EOC | 75.59 | 87.47 | 87.63 |
| Borderline ovarian tumor | 92.16 | 57.84 | 66.22 |
| Benign ovarian tumor | 73.72 | 60.93 | 55.77 |
| Normal ovarian tissue | 46.38 | 46.08 | 46.50 |
| χ2 | 11.68 | 24.66 | 31.24 |
| P | 0.009 | <0.001 | <0.001 |
Relationship between TGF-β1, VEGF, and IL-10 Expression and Survival of Ovarian Cancer Patients
TGF-β1 expression showed neither a clear negative value nor a biphasic distribution; thus, the optimal cutoff point had to be determined[20]. The average optical density of TGF-β1 is 0.160–0.400; therefore, an interval of 0.001 from 0.160 to 0.400 was calculated and analyzed by Kaplan-Meier and log rank test to determine the cutoff point. The greatest difference occurred at A=0.358 (P=0.013). The survival time of the TGF-β1low group (A<0.358, 50/79) was 89.90±5.84 months; while, the survival of the TGF-β1high group (A≥0.358, 29/79) was 65.32±8.08 months (Figure 1B).
For VEGF, the survival time of the negative group (20/79), weak group (21/79), moderate group (15/79), and intense group (23/79) were 67.02±5.59, 62.20±10.36, 73.68±10.52, and 90.57±6.89 months, respectively. The difference among these groups is almost indistinctive (P=0.048) (Figure 1C).
IL-10 expression was detected in 45 of 79 cases (56.96%). The survival time of the negative group (34/79) was 78.858±6.722 months; while, that of the positive group (45/79) was 79.374±6.503 months (P=0.548) (Figure 1D).
Relationships between TGF-β1, VEGF, and IL-10 Expression and Clinicopathological Profiles and Patient Prognosis
Clinicopathological factors included age, histological grade, FIGO stage, residual tumor after surgery, lymphatic metastasis, and standard chemotherapy. The results showed that TGF-β1, residual tumor after surgery, and standard chemotherapy are prognostic factors for EOC (P=0.014, 0.001, and 0.042, respectively). TGF-β1 expression and its correlation with other clinicopathological factors are shown in Table 2. There were no significant differences in clinicopathological factors between the TGF-β1low group and TGF-β1high groups, which implies TGF-β1 is an independent factor of prognosis for EOC.
Table 2. Differences between high and low expression of TGF-β1 among several clinical data.
| Clinicopathological characteristic | TGF-β1low (n=50) | TGF-β1high (n=29) | P | |
|---|---|---|---|---|
| Age (year) | 53.96±11.03 | 54.86±11.62 | 0.732 | |
| Histological grade | I | 4 (8.0) | 5 (17.2) | 0.352 |
| [n (%) ] | II | 18 (36.0) | 12 (41.4) | |
| III | 28 (56.0) | 12 (41.4) | ||
| FIGO stage | FIGO I | 7 (14.0) | 4 (13.8) | 0.916 |
| [n (%) ] | FIGO II | 10 (20.0) | 4 (13.8) | |
| FIGO III | 30 (60.0) | 19 (65.5) | ||
| FIGO IV | 3 (6.0) | 2 (6.9) | ||
| Residual tumor | 0 | 21 (42.0) | 8 (27.6) | 0.256 |
| [n (%) ] | <2 cm | 11 (22.0) | 11 (37.9) | |
| >2 cm | 18 (36.0) | 10 (34.5) | ||
| Lymphatic metastasis | Negative | 21 (42.0) | 12 (41.4) | 0.840 |
| [n (%) ] | Positive | 18 (36.0) | 12 (41.4) | |
| Unclear | 11 (22.0) | 5 (17.2) | ||
| Chemotherapy | Standard | 39 (68.0) | 24 (82.8) | 0.450 |
| [n (%) ] | Nonstandard | 11 (22.0) | 5 (17.2) | |
The standard chemotherapy was defined as: at least 6 cycles of fist-line chemotherapy (cisplatin+taxol; carboplatin+taxol; or cisplatin+adriamycin+cyclophosphamide) after cytoreductive surgery.
TGF-β1, VEGF and IL-10 Expression Levels in Concen- trated SKOV3.ip Culture Supernatants
SKOV3.ip cells secreted high levels of TGF-β1 and VEGF in culture, which were not detected in the negative control medium. IL-10 was not detected in either the SKOV3.ip supernatants or the control medium. TGF-β1 and VEGF concentrations in SKOV3.ip supernatants were (1,276.990±331.487) pg/ml and (27,732.777±534.825) pg/ml, respectively.
Ovarian Cancer Immunosuppressive Responses Induced by TGF-β1, VEGF and IL-10 In Vitro
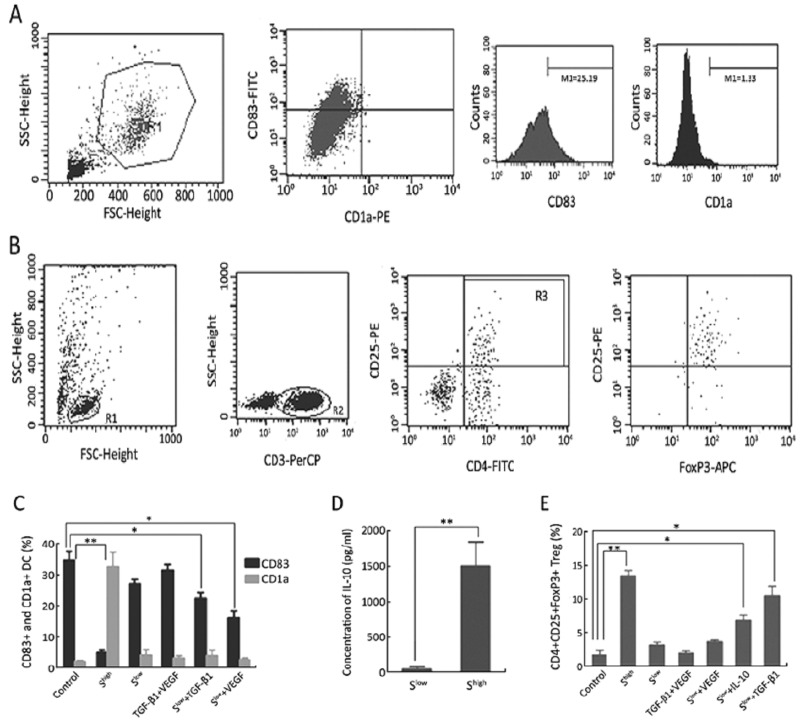
Different concentrations of SKOV3.ip supernatants were added to the DC and lymphocyte cultured system. DC maturation and regulatory T lymphocytes (Treg) were detected by flow cytometry (Figures 2). When the supernatant was sufficient (Shigh, final concentration: TGF-β1 >300 pg/ml, VEGF >6 ng/ml), CD83+ mature DCs decreased, while CD1a+ imDCs increased significantly (Figure 2C). Moreover, the percentage of CD4+CD25+FoxP3+ Tregs clearly increased (Figure 2E). These responses could not be induced by TGF-β1 (>300 pg/ml) and VEGF (>6 ng/ml) without the cancer cell supernatant (Figures 2C and 2E).
Figure 2.
Immunosuppressive response. A: DCs were gated in R1, CD83+ DCs and CD1a+ DCs were counted in R1. B: Lymphocytes were gated in R1, CD3+ T lymphocytes were gated in R2, and CD4+ CD25+ T lymphocytes were counted and gated in R3. FoxP3+ Tregs were counted in R3. C: CD83+ DCs and CD1a+ DCs in different groups (**P<0.01, *P<0.05). D: Concentration of IL-10 in DC cultured supernatants with Slow or with Shigh (**P<0.01). E: The percentage of CD4+CD25+FoxP3+ Tregs in different groups (**P<0.01, *P<0.05).
When enough TGF-β1 or VEGF was present in inadequate supernatants (Slow+TGF-β1 or Slow+VEGF), TGF-β1 or VEGF interfered with DC maturation (Figure 2C); TGF-β1 induced Treg generation (Figure 2E). Furthermore, the IL-10 concentration in the supernatant of cultured DCs was detected by ELISA. The results showed that DCs in Shigh secreted higher levels of IL-10 than those in Slow (Figure 2D). Moreover, when 1,500 pg/ml IL-10 was added to Slow (Slow+IL-10), CD4+ CD25+FoxP3+ Tregs also increased (Figure 2E).
DISCUSSION
EOC is the most leading cause of gynecologic malignancies. Most patients present with advanced disease with little prospect for cure. Compelling evidence indicates an effective immune response against ovarian cancer may play an important role in disease control[21]; but, cancer cells always create a suppressive microenvironment to escape the immune attack. A series of cytokines secreted by cancer cells may play important roles in the tumor micro- environment. For example, TGF-β1, VEGF, and IL-10 were overexpressed in ovarian cancer specimens in comparison with normal ovarian tissue in several reports[18,22,23]. However, these studies focused on a single cytokine and were reported by different authors, making it hard to compare or evaluate the roles and relationships of these three cytokines in ovarian cancer.
In this study, TGF-β1, VEGF, and IL-10 expression levels were detected in 79 cases of EOC, 16 cases of ovarian borderline tumors, 30 cases of ovarian benign tumors, and 20 cases of normal ovarian tissue using immunohistochemistry. The computer assessment method has been widely used in the assessment of immunohistochemical staining and has the advantages of being user-friendly and easy to operate. Especially for nuclei stained counting, the computer assessment method is consistent with traditional semi-quantitative counting but more reliable than the latter. In our study, the immunohistochemical reactivity of TGF-β1 in the tumor cells was granular and located in the cytoplasm and nucleus; while, VEGF and IL-10 staining was almost confined to the cytoplasm of cells. Therefore, the computer assisted method was used to evaluate TGF-β1, and traditional semi-quantitative counting was used to quantify VEGF and IL-10.
The expression of TGF-β1 in EOC, borderline, and benign ovarian tumor tissues was significantly higher than that in normal ovarian tissue; while, the expressions of VEGF and IL-10 in EOC were significantly higher than those in borderline and benign tumors and normal tissues. These results confirmed that these three cytokines are frequently overexpressed in EOC cases. Recently, some studies indicated the expression of these cytokines was correlated in other malignancies; for example, TGF-β1 induced IL-10 expression in melanoma[24] and TGF-β1 was concomitantly overexpressed with IL-10 and VEGF in esophageal squamous cell carcinoma[25]. In contrast, in our study, TGF-β1 expression did not correlate with VEGF and IL-10; while, IL-10 was concomitantly overexpressed with VEGF in different ovarian tissues. In this study, survival analysis showed the total survival rate was related to the expression of TGF-β1, but not to IL-10 or VEGF expression. Logistic regression revealed the expression of TGF-β1, residual tumor, and normative chemotherapy were important prognostic factors for EOC. In further evaluation, no correlations were observed between the expression of TGF-β1 and age, FIGO stage, histological grade, residual tumor, lymphatic metastasis, or normative chemo- therapy. Therefore, TGF-β1 may be an independent prognostic factor for EOC.
TGF-β1, VEGF, and IL-10 are important factors that have been found to induce immunosuppression; therefore, their overexpression in EOC implies they may participate in the construction of an immune- suppressive microenvironment and play important roles in it. A series of in vitro experiments were performed to investigate the immunosuppressive functions and interactions of these three cytokines. In the immune system, DCs are considered the most powerful APCs. Both circulating and tumor-infiltrating DCs from cancer patients appear to be phenotypically and functionally defective, for the most part due to imDC accumulation and abnormal differentiation[26-30]. Several studies have indicated that VEGF is the most important factor for preventing DCs from progressing beyond the immature phenotype and for inhibiting T cell immune responses[7]. CD4+CD25+ Tregs, defined by Foxp3 expression[31,32], were reported to maintain self-tolerance by downregulating the immune response to self and nonself antigens in an antigen-nonspecific manner[33]. Tregs were increased in patients with carcinoma and were associated with a poor prognosis[20,34-40]. TGF-β1 in the tumor microenviron- ment may promote generation of Tregs from CD4+ CD25− cells[41].
Based on the roles of DCs and Tregs in immuno- suppressive responses, the immunosuppressive functions of TGF-β1, VEGF, and IL-10 were evaluated by DC maturation and Treg expression. In our study, the ovarian cancer cell line SKOV3.ip secreted TGF-β1 and VEGF, which were detected in cell supernatants by ELISA. Consistent with some reports[17], IL-10 was absent in SKOV3.ip cell supernatants. In vitro experiments revealed that VEGF and TGF-β1 influenced DC maturation, increased IL-10 levels secreted by imDCs; IL-10 and TGF-β1 had the ability to promote Treg generation. Although these cytokines are important factors in the immunosuppressive responses, they could not execute these responses alone. In our study, the supernatant of SKOV3.ip cells was necessary. This finding indicated the tumor microenvironment is a complicated network that involves many mechanisms which are not completely understood. Our study revealed that in this network, TGF-β1 induced CD4+CD25+FoxP3+ Tregs to increase in a non-antigen- specific manner (without antigen presenting); while, the remaining CD4+CD25− T helper cells required excitation by the MHC-II antigenic complex. However, mature DCs present antigens to the MHC-II molecule. When DC maturation was blocked by VEGF and TGF-β1, CD4+CD25− helper T cells activation was blocked as well. Furthermore, imDCs generate large amounts of IL-10 and induce Treg generation. All of these events lead to a local immune system around the tumor, leading to magnification of the immune- suppressive effects.
This study confirmed overexpressions of TGF-β1, VEGF, and IL-10 play an important role in EOC and consequently lead to frequent immune evasion events. Targeting these cytokines for tumor treatment, especially in the early stages, might prevent tumor progression to advanced stages.
ACKNOWLEDGMENTS
The authors thank Ni J, An LP, Shen DH, Zou SL, Gao L, Yu X and Chong X for offering specimens or technical support.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 1990;63:245-7 [DOI] [PubMed] [Google Scholar]
- 3.Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99-146 [DOI] [PubMed] [Google Scholar]
- 4.Kriegel MA, Li MO, Sanjabi S, et al. Transforming growth factor-beta: recent advances on its role in immune tolerance. Curr Rheumatol Rep 2006;8:138-44 [DOI] [PubMed] [Google Scholar]
- 5.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992;359:693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350-8 [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996;2:1096-103 [DOI] [PubMed] [Google Scholar]
- 8.Furlan D, Sahnane N, Carnevali I, et al. Up-regulation of the hypoxia- inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum Pathol 2008;39:1483-94 [DOI] [PubMed] [Google Scholar]
- 9.Huang CL, Liu D, Ishikawa S, et al. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer 2008;44:2680-8 [DOI] [PubMed] [Google Scholar]
- 10.Oh SY, Kwon HC, Kim SH, et al. Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer 2008;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon HC, Kim SH, Oh SY, et al. Clinicopathological significance of p53, hypoxia-inducible factor 1alpha, and vascular endothelial growth factor expression in colorectal cancer. Anticancer Res 2010;30:4163-8 [PubMed] [Google Scholar]
- 12.Abu-Jawdeh GM, Faix JD, Niloff J, et al. Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Lab Invest 1996;74:1105-15 [PubMed] [Google Scholar]
- 13.Shen GH, Ghazizadeh M, Kawanami O, et al. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer 2000;83:196-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan TJ, Al-Attar A, Rolland P, et al. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res 2008;14:3030-5 [DOI] [PubMed] [Google Scholar]
- 15.Volk H, Asadullah K, Gallagher G, et al. IL-10 and its homologs: important immune mediators and emerging immunotherapeutic targets. Trends Immunol 2001;22:414-7 [DOI] [PubMed] [Google Scholar]
- 16.Wakkach A, Cottrez F, Groux H.Can interleukin-10 be used as a true immunoregulatory cytokine? Eur Cytokine Netw 2000;11:153-60 [PubMed] [Google Scholar]
- 17.Nash MA, Lenzi R, Edwards CL, et al. Differential expression of cytokine transcripts in human epithelial ovarian carcinoma by solid tumour specimens, peritoneal exudate cells containing tumour, tumour- infiltrating lymphocyte (TIL)-derived T cell lines and established tumour cell lines. Clin Exp Immunol 1998;112:172-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinovich A, Medina L, Piura B, et al. Expression of IL-10 in human normal and cancerous ovarian tissues and cells. Eur Cytokine Netw 2010;21:122-8 [DOI] [PubMed] [Google Scholar]
- 19.Law AK, Lam KY, Lam FK, et al. Image analysis system for assessment of immunohistochemically stained proliferative marker (MIB-1) in oesophageal squamous cell carcinoma. Comput Methods Programs Biomed 2003;70:37-45 [DOI] [PubMed] [Google Scholar]
- 20.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005;11:8326-31 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13 [DOI] [PubMed] [Google Scholar]
- 22.Gordinier ME, Zhang HZ, Patenia R, et al. Quantitative analysis of transforming growth factor beta 1 and 2 in ovarian carcinoma. Clin Cancer Res 1999;5:2498-505 [PubMed] [Google Scholar]
- 23.Shi HR, Song WJ, Chen ZM, et al. Expression and clinical significance of endostatin and vascular endothelial growth factor in ovarian carcinoma. Ai Zheng 2005;24:1127-31 [PubMed] [Google Scholar]
- 24.Diaz-Valdés N, Basagoiti M, Dotor J, et al. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFbeta1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Res 2011;71:812-21 [DOI] [PubMed] [Google Scholar]
- 25.Gholamin M, Moaven O, Memar B, et al. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg 2009;33:1439-45 [DOI] [PubMed] [Google Scholar]
- 26.Troy A, Davidson P, Atkinson C, et al. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol 1998;160:214-9 [PubMed] [Google Scholar]
- 27.Troy AJ, Summers KL, Davidson PJ, et al. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res 1998;4:585-93 [PubMed] [Google Scholar]
- 28.Pinzon-Charry A, Ho CS, Laherty R, et al. A population of HLA-DR+ immature cells accumulates in the blood dendritic cell compartment of patients with different types of cancer. Neoplasia 2005;7:1112-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergeron A, El-Hage F, Kambouchner M, et al. Characterisation of dendritic cell subsets in lung cancer micro-environments. Eur Respir J 2006;28:1170-7 [DOI] [PubMed] [Google Scholar]
- 30.Baleeiro RB, Anselmo LB, Soares FA, et al. High frequency of immature dendritic cells and altered in situ production of interleukin-4 and tumor necrosis factor-alpha in lung cancer. Cancer Immunol Immunother 2008;57:1335-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryczek I, Liu R, Wang G, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 2009;69:3995-4000 [DOI] [PubMed] [Google Scholar]
- 32.Zou W.Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nat Rev Cancer 2005;5:263-74 [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151-64 [PubMed] [Google Scholar]
- 34.Zhu X, Ma LL, Ye T. Expression of CD4(+)CD25(high)CD127(low/-) regulatory T cells in transitional cell carcinoma patients and its significance. J Clin Lab Anal 2009;23:197-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Ding T, Pan W, et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 2009;125:1640-8 [DOI] [PubMed] [Google Scholar]
- 36.Li L, Chao QG, Ping LZ, et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 2009;24:357-67 [DOI] [PubMed] [Google Scholar]
- 37.Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favorable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkrolf P, Landmeier S, Altvater B, et al. A high proportion of bone marrow T cells with regulatory phenotype (CD4+CD25hiFoxP3+) in Ewing sarcoma patients is associated with metastatic disease. Int J Cancer 2009;125:879-86 [DOI] [PubMed] [Google Scholar]
- 39.Hinz S, Pagerols-Raluy L, Ober HH G, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 2007;67:8344-50 [DOI] [PubMed] [Google Scholar]
- 40.Li X, Ye DF, Xie X, et al. Proportion of CD4+CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Invest 2005;23:399-403 [PubMed] [Google Scholar]
- 41.Li X, Ye F, Chen H, et al. Human ovarian carcinoma cells generate CD4(+)CD25(+) regulatory T cells from peripheral CD4(+)CD25(-) T cells through secreting TGF-beta. Cancer Lett 2007;253:144-53 [DOI] [PubMed] [Google Scholar]