Abstract
Objective
To examine the expressions of osteopontin (OPN), α ν β3 and Pim-1 in non-small cell lung cancer (NSCLC), and investigate their potential pathogenic roles in the development of NSCLC.
Methods
Immunohistochemistry was used to examine the expressions of OPN, α ν β3 and Pim-1 in cohort (136 cases) of NSCLC samples and their adjacent normal lung tissue specimens. Statistical analysis was performed to evaluate the relationships among expressions of OPN, α ν β3 and Pim-1 and their associations with patients clinico- pathological parameters.
Results
The expressions of OPN and Pim-1 were predominantly observed in cytoplasm. The expression of α ν β3 was mostly detected in cytoplasm and/or membrane. In NSCLC samples, the positive rates of OPN, α ν β3 and Pim-1 expressions were 68.4% (93/136), 77.2% (105/136) and 57.4% (78/136), respectively. In normal lung tissues, in contrast, the positive rates of OPN, α ν β3 and Pim-1 were 24.0% (12/50), 26.0% (13/50) and 16.0% (8/50), respectively. There were significant differences of the positive expression rates of OPN, α ν β3 and Pim-1 between NSCLCs samples and normal lung tissues (P<0.01). In addition, the positive expression of OPN, α ν β3 and Pim-1 in NSCLCs samples was significantly associated with increased pathological grade, lymph node metastasis and advanced clinical stage (P<0.01), and they were independent of other clinicopathological parameters (P>0.05). Furthermore, a significantly positive correlation between the expression of OPN and α ν β3 (r=0.38, P<0.01), OPN and Pim-1 (r=0.37, P<0.01), or α ν β3 and Pim-1 (r=0.20, P<0.05) was evaluated in our NSCLC cohort.
Conclusion
OPN, α ν β3 and Pim-1 proteins are frequently overexpressed in NSCLC, and they may play important roles in the development and/or progression of NSCLC.
Key words: NSCLC, OPN, ανβ3, Pim-1, Immunohistochemistry
INTRODUCTION
Primary bronchogenic carcinomas have the highest mortality rate of malignant tumors in the world. Among them, non-small cell lung cancer (NSCLC) accounts for 85%, most patients who have been already clinically diagnosed are in middle-advanced stage, and the 5-year survival rate is very low[1]. Thus, early diagnosis and early treatment are particularly important to improve the survival rate. It is now considered that various pathogenic factors were involved in the evolution of NSCLC.
Osteopontin (OPN) is a multifunctional secrete phosphorylated glycoprotein, and it can promote cell chemotaxis, adhesion and migration, which mediate invasion and metastasis of tumor cells, and it is closely related to the occurrence, development, metastasis and prognosis of a variety of cancers[2,3]. Recent studies have indicated that OPN is involved in NSCLC progression and metastasis through interaction with its receptor, alphaν beta3 (α ν β3) integrin, and overexpression of OPN is associated with progression and poor prognosis of NSCLC[2,3]. α νβ3 is a peptide of heparin binding factor and is an important molecule of integrin family. α νβ3 can mediate adhesion of cell to cell and cell to matrix, regulate intracellular signaling pathways, and induce the activation of protein dissolving enzymes, thereby contributing to extracellular matrix and basement membrane degradation, and promoting invasion and migration migration of tumor cells[4]. Previous study found that α νβ3 is detectable in a variety of tumors, and is closely associated with the tumorigenesis and the degree of malignancy of tumor[5]. Other factors which are closely related with genes of cell cycle regulation and proliferation, including Pim-1 (coded by pim-1 oncogene),are involved in tumorigenesis[6]. It has been reported that OPN acts through α νβ3 integrin, which in turn activates the FAK, PI3K, Akt, ERK, NF-κB and Pim-1 pathways, contributing to the migration of lung cancer cells[6]. Taken together, these results suggest OPN mediates migration in human lung cancer cells via the α νβ3 integrin, FAK, PI3K, Akt, ERK, NF-κB and Pim-1 signaling pathways. Therefore, we chose three factors of signaling pathways, OPN, α νβ3 integrin and Pim-1 in this study. To date, however, the expression dynamics of OPN, α νβ3 and Pim-1 in NSCLCs, and their potential biological roles in tumorigenesis of NSCLC have not been elucidated.
In the present study, immunohistochemistry was used to examine the expression dynamics of OPN, α νβ3 and Pim-1 in cohort (136 cases) of NSCLC samples and their adjacent normal lung tissue specimens. Next, the potential correlations among the 3 protein expressions and their associations with NSCLC patients’ clinico- pathological features were evaluated.
MATERIALS AND Methods
Clinical Data
In this study, specimens were obtained from archived paraffin-embedded tissue sections of 136 patients with NSCLC at the Third Affiliated Hospital, Sun Yat-sen University, Guangzhou from January 1st, 2009 to December 31st, 2010. A group of 50 normal lung tissue cases was conducted as control. In this cohort of NSCLC patients, 97 were men and 39 were women, with a median age of 60 years (range 30−82 years). According to the World Health Organization criteria of lung cancer published in 2004[7], NSCLC patients were classified as follows: adenocarcinoma 72 cases, squamous cell carcinoma 40 cases, and other types 24 cases; well and moderately differentiated carcinoma 88 cases, and poorly differentiated carcinoma 48 cases. According to the staging standard of the TNM system of International Association for the Study of Lung Cancer[8], the NSCLC patients were classified as stage I/II 96 cases, and stage III/IV 40 cases. In 136 NSCLC cases of the present study, 35 had lymph node metastasis.
Protein Expression of OPN, α νβ3 and Pim-1 in NSCLC
Immunohistochemical technique using streptavidin- peroxidase (SP) was employed for OPN, α νβ3 and Pim-1 detection. Mouse-anti-human monoclonal antibodies of OPN, ανβ3, Pim-1 and SP kit were purchased from Maxim biological and technical company, Fuzhou, China; ready-to-use. All the sections were routinely deparaffinized and rehydrated, then the sections were rinsed in phosphate-buffered saline (PBS, pH=7.4), subsequently were treated for antigen retrieval. Sections were treated in EDTA buffer (pH=8.0) in an autoclave sterilizer. After cooling at room temperature for 20 min, the sections were rinsed in PBS, then immersed in 3% H2O2 for 15 min to block the endogenous enzymes. After being rinsed in PBS, the sections were incubated with normal goat serum at 37°C for 15 min to block nonspecific antibodies. After interaction with OPN antibody, ανβ3 and Pim-1 antibodies (monoclonal antibodies), the sections were rinsed in PBS, then incubated with biotinylated secondary antibodies and rinsed in PBS again. After interaction with streptavidin- horseradish peroxidase (HRP) and being rinsed in PBS, the sections were visualized by reaction with 3,3’-diami- nobenzidine and counterstained with hematoxylin. They were then dehydrated, transparentized and covered with coverslips and sealed with neutral gum. PBS substituting the primary antibody was used as negative control.
Judgment of Positive Result of OPN, α νβ3 and Pim-1
The judgment that whether the tumor and the normal tissues were positive or not was performed by two pathology doctors. The positive expression of OPN was mostly in cytoplasm with brown-yellow staining, α νβ3 was mostly in cytoplasm and membrane with brown- yellow staining, and Pim-1 was mostly in cytoplasm with brown-yellow staining. A tumor or normal tissue in which more than 10% of cells were stained with those antibodies was recognized as being positive; while less than 10% was recognized as being negative.
Statistical Analysis
Data were analyzed with computer aided SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). Chi- square test was used in comparing the expressions of OPN, α νβ3 and Pim-1 in NSCLC samples and in normal lung tissue specimens. The associations between the expressions of OPN, α νβ3 and Pim-1 and NSCLC patients’ clinicopathological parameters were also evaluated by the Chi-square test. The correlation between two variables was evaluated by the Spearman rank correlation test. A value of P<0.05 was considered statistically significant.
RESULTS
Relationship between Protein Expression of OPN and Clinicopathological Parameters in NSCLC
The expression of OPN in NSCLC tissues was predominantly a cytoplasmic pattern (Figure 1 A, B). In our study, 93 (68.4%) of 136 NSCLC cases showed positive expression of OPN, while the positive rate of OPN in normal lung tissues was 24.0% (12/50). There was a significant difference of the expression of OPN between NSCLC and normal lung tissues (P<0.05) (Table 1). Further correlation analysis demonstrated that the expression of OPN was significantly associated with tumor differentiation degree, lymph node metastasis and clinical staging in NSCLC patients (P<0.01), but was not correlated with other clinicopathological parameters studied (P>0.05) (Table 2).
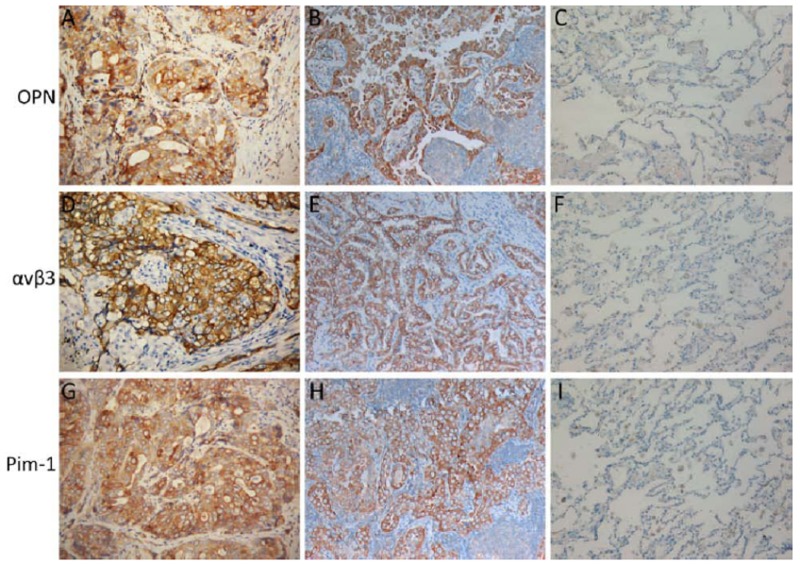
Figure 1.
Protein expressions of OPN, α ν β3 and Pim-1 in NSCLC. Protein expression of OPN is mostly in cytoplasm with yellow or brown- yellow staining. Positive expression of OPN was examined in a squamous carcinoma (A) and an adeno- carcinoma (B) of the lung. In normal lung tissue, the expression of OPN was negative (C). α ν β3 is mostly expressed in membrane or cytoplasm with yellow or brown- yellow staining. ανβ3 is positive in another squamous carcinoma (D) and adenocarcinoma (E) of the lung. In normal lung tissue, the expression of α ν β3 was negative (F). The expression of Pim-1 is pre- dominantly a cytoplasmic pattern. Pim-1 is positive in a squamous carcinoma (G) and an adeno- carcinoma (H) of the lung. In normal lung tissue, the expression of Pim-1 was negative (I) (SP ×200).
Table 1. The expression of OPN, α ν β3 and Pim-1 between NSCLC and normal lung tissue.
| Groups | n | OPN (%) | ανβ3 (%) | Pim-1 (%) |
|---|---|---|---|---|
| NSCLC | 136 | 93 (68.4) | 105 (77.2) | 78 (57.4) |
| Normal lung tissue | 50 | 12 (24.0) | 13 (26.0) | 8 (16.0) |
There was significant difference in the expression of OPN between NSCLC and normal lung tissue (χ2=29.29, P<0.01). There was significant difference in the expression of α ν β3 between NSCLC and normal lung tissue (χ2=41.33, P<0.01). There was significant difference in the expression of Pim-1 between NSCLC and normal lung tissue (χ2=25.15, P<0.01).
Table 2. Relationship between expressions of OPN, α ν β3 and Pim-1 and clinicopathological parameters in NSCLC.
| Parameter | n | OPN (%) | P* | ανβ3 (%) | P* | Pim-1 (%) | P* |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 97 | 68 (70.1) | 0.496 | 75 (77.3) | 0.960 | 56 (57.7) | 0.888 |
| Female | 39 | 25 (64.1) | 30 (76.9) | 22 (56.4) | |||
| Age (year) | |||||||
| <60 | 61 | 40 (65.6) | 0.525 | 47 (77.0) | 0.969 | 36 (59.0) | 0.724 |
| ≥60 | 75 | 53 (70.7) | 58 (77.3) | 42 (56.0) | |||
| Tumor size (cm) | |||||||
| <3 | 51 | 34 (66.7) | 0.739 | 38 (74.5) | 0.562 | 28 (54.9) | 0.654 |
| ≥3 | 85 | 59 (69.4) | 67 (78.8) | 50 (58.8) | |||
| Lymph node metastasis | |||||||
| Have | 45 | 39 (86.7) | 0.001 | 43 (95.6) | 0.000 | 33 (73.3) | 0.008 |
| No | 91 | 54 (59.3) | 62 (68.1) | 45 (49.5) | |||
| Differentiation | |||||||
| Well/moderate | 88 | 51 (58.0) | 0.000 | 60 (68.2) | 0.001 | 37 (42.1) | 0.000 |
| Low | 48 | 42 (87.5) | 45 (93.8) | 41 (85.4) | |||
| Pathological typing | |||||||
| Squamous carcinoma | 40 | 26 (65.0) | 0.854 | 31 (77.5) | 0.960 | 22 (55.0) | 0.837 |
| Adenocarcinoma | 72 | 50 (69.4) | 55 (76.4) | 43 (59.7) | |||
| Other types | 24 | 17 (70.8) | 19 (79.2) | 13 (54.2) | |||
| Tumor location | |||||||
| Peripheral | 60 | 41 (68.3) | 0.991 | 46 (76.7) | 0.894 | 34 (56.7) | 0.886 |
| Central | 76 | 52 (68.4) | 59 (77.6) | 44 (57.9) | |||
| TNM stage | |||||||
| I+II | 96 | 57 (59.4) | 0.000 | 68 (70.8) | 0.006 | 47 (49.0) | 0.002 |
| III+IV | 40 | 36 (90.0) | 37 (92.5) | 31 (77.5) |
*Chi-square test
Relationship between Protein Expression of α νβ3 and Clinicopathological Parameters in NSCLC
In NSCLC, we observed that α νβ3 protein was mostly expressed in membrane or cytoplasm (Figure 1 D, E). The positive rate of α νβ3 expression in NSCLCs was 77.2% (105/136), which was significantly higher than that (26.0%, 13/50) in normal lung tissues (P<0.05) (Table 1). In addition, the expression of α νβ3 was also positively associated to NSCLC histopathological differentiation, lymph node metastatic status and clinical stage (P<0.01) (Table 2).
Relationship between Protein Expression of Pim-1 and Clinicopathological Parameters in NSCLC
The expression of Pim-1 was mostly observed in cytoplasm (Figure 1 G, H). In our NSCLC cohort, 78 (57.4%) of the 136 cases had positive expression of Pim-1. The positive rate of Pim-1 in normal lung tissues was 16.0% (8/50). There was a significant difference of Pim-1 expression between NSCLC and normal lung groups (P<0.05) (Table 1). The expression of Pim-1 was significantly correlated to differentiation degree, lymph node metastasis and clinical staging in NSCLC (P<0.01), while no correlations were observed between the expression of Pim-1 and other clinicopathological parameters studied (P>0.05) (Table 2).
Correlations among Protein Expression of OPN, α ν β3 and Pim-1 in NSCLCs
In our study, the potential correlations among protein expression of OPN, α νβ3 and Pim-1 in NSCLC were further evaluated. Spearman rank correlation analysis showed that the expression of OPN was positively correlated ανβ3 expression in our NSCLC cohort (r=0.38, P<0.01) (Table 3). The expression of OPN in NSCLC was also positively correlated with Pim-1 expression (r=0.37, P<0.01) (Table 4). Furthermore, a significant positive correlation between expressions of ανβ3 and Pim-1 was observed (r=0.20, P<0.05) (Table 5).
Table 3. Relativity of protein expressions of OPN and α ν β3 in NSCLC.
| OPN |
α
ν
β3 |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 82 | 11 | 93 |
| Negative | 23 | 20 | 43 |
| Total | 105 | 31 | 136 |
There are relationships to expressions of OPN and α ν β3 in NSCLC (P<0.01, χ2=20.10), correlation coefficient, r=0.38.
Table 4. Relativity of protein expressions of OPN and Pim-1 in NSCLC.
| OPN | Pim-1 |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 65 | 28 | 93 |
| Negative | 13 | 30 | 43 |
| Total | 78 | 58 | 136 |
There are relationships to expressions of OPN and Pim-1 in NSCLC (P<0.01, χ2=18.91), correlation coefficient, r=0.37.
Table 5. Relativity of α ν β3 and Pim-1 expressions in NSCLC.
| α ν β3 | Pim-1 |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 62 | 43 | 105 |
| Negative | 16 | 15 | 31 |
| Total | 78 | 58 | 136 |
There are relationships to expressions of α ν β3 and Pim-1 in NSCLC (P<0.05, χ2=5.41), correlation coefficient, r=0.20.
DISCUSSION
It is known that malignant transformation of cells needs changes of gene phenotype and angiogenesis. OPN is considered to be the most important factor. OPN is an extracellular matrix-secreted phosphorylated glycoprotein which is also called the transformation- related protein phosphatase, and it was found by Senger, et al.[9] for the first time in the epithelial cell strain of malignant transformation. In recent years, studies have confirmed that overexpression of OPN can promote tumor growth, invasion and/or metastasis[3].
Previously, it was reported that the expression of OPN was 68.8% in NSCLC[10]. Other groups reported that OPN expression was associated with tumor growth, tumor staging, and lymph node invasion of patients with NSCLC[11]. In the present study, the expression of OPN protein was detected in 68.4% of NSCLC, while only 24.0% of normal lung tissues expressed OPN protein, suggesting that the positive expression of OPN may provide a selective advantage for the development of NSCLC. In our study, further statistical analysis showed that the expression of OPN was correlated closely to NSCLC’s differentiation degree, lymph node metastasis and clinical staging, and it was independent of other clinicopathological parameters. The positive expression of OPN was more frequently observed in poorly differentiated cancer, tumors with lymph node metastasis and/or tumors in more advanced clinical stage III/IV. A current study suggested that OPN can produce a marked effect by binding to receptors on endothelial cell membrane. After OPN binds to the receptors such as ανβ3 integrins, it can directly stimulate the differentiation and proliferation of lung cancer cells, and might be capable of regulating genesis and migration of lung cancer cells, increase vascular permeability, change extracellular matrix, induce angiogenesis, activate intracellular signaling pathways and promote the growth of NSCLC[12]. These data suggested that the increased expression of OPN may facilitate the development and/or progression of NSCLC and OPN might be used as a therapeutic target and useful biomarker for NSCLC.
The reason why OPN is highly expressed in NSCLC is that it is regulated by some transcription factors. Among them, ανβ3 and Pim-1 are more important. Previous studies have demonstrated that ανβ3 regulates the expressions of Pim-1 and VEGF, and mediates tumor angiogenesis through the signaling pathways of PI13/AKT. In turn, they also promote the expression of OPN[13,14]. Recently, it has been reported that the overexpression of ανβ3 integrin on tumor is associated with an aggressive phenotype of several solid tumor types[15,16]. In this study, we further found that the expression rate of ανβ3 was 77.2% in NSCLC, and significantly higher than that (26.0%) in normal lung tissue, suggesting that ανβ3 might be involved in the development of NSCLC. In addition, the statistical analysis showed that the protein expression of ανβ3 was positively associated with NSCLC’s differentiation degree, lymph node metastasis and clinical staging. These results further suggested that the expression of ανβ3 might be involved in the development of NSCLC, and closely related to the degree of malignancy, invasion and metastasis of NSCLC. Possible molecular mechanism why ανβ3 promotes the development of NSCLC is that ανβ3 integrin plays an important role in promoting angiogenesis, and adhesion of cell to matrix so that it enhances the development of NSCLC[17]. Thus, ανβ3 can be used as an important indicator to evaluate the malignant degree and invasion of NSCLC. These results also suggest that ανβ3 could be a surrogate marker in the diagnosis of NSCLC and the prediction of therapeutic response for treatment with anti-ανβ3 antibody in NSCLC.
The gene, pim-1, is a potential oncogene, which is located on chromosome 6p21.2. The encoded protein of Pim-1 is a serine/threonine kinase[18]. Recent studies have confirmed that it is highly expressed in some malignant tumors[19,20]. It is involved in the regulation of proliferation, differentiation and apoptosis of lung cancer cells[21]. In our present study, the positive expression of Pim-1 was detected in the majority of NSCLC, while in few normal lung tissues. This result was in consistent with the literature reported by Zhang, et al. and He, et al.[22, 23]. It has been documented that Pim-1 can promote G2/M process of cell cycle, activate growth, differentiation and proliferation of cells[6]. In addition, we also found that the expression of Pim-1 in NSCLC with poor differentiation, clinical stage of III and IV and lymph node metastasis was significantly higher than that in clinical stage of I and II and without lymph node metastasis. Based on the results mentioned above, collectively, we proposed that Pim-1 might also be involved in the tumorigenesis/progression of NSCLC.
In this study, Spearman rank correlation analysis showed that there was an obvious positive correlation of protein expression between OPN and ανβ3, in which the higher the expression level of OPN, the higher the expression of ανβ3. It was suggested that OPN acts through ανβ3 integrin, which in turn activates the FAK pathways, contributing to the overexpression of ανβ3[24]. Other groups also revealed that OPN is involved in tumor growth and angiogenesis of lung cancer by upregulating vascular endothelial cell migration and proliferation via interacting with ανβ3 integrin[25]. Upregulation of OPN specifically activates the activity of integrin receptor ανβ3, and may accelerate tumor angiogenesis, and induce activation of a variety of kinases such as Pim-1, PI3K, and AKT[13,14]. Therefore, when the expression level of OPN is higher, it can elevate the expressions of ανβ3 and Pim-1 through regulating signaling pathways. In NSCLC, the expression of OPN, Pim-1 and the expression of ανβ3, Pim-1 are also positively correlated. Therefore, our results, together with others’ findings, suggested that OPN plays a crucial role for tumor growth and/or progression of human lung cancer cells by interacting with ανβ3 integrin, and thus, regulating signaling pathways and activating Pim-1[13,14,18,24], resulting in the overexpression of OPN, ανβ3 and Pim-1.
With regards to the molecular targets studied in this report, it was concluded that OPN can promote extracellular matrix degradation, inhibit apoptosis, and accelerate tumor angiogenesis in the process of invasive growth and metastasis. The other one, Pim-1 may promote cell cycle G2/M process[6], and promote cell proliferation through enhancing the cell cycle promoter activity, such as Cdc25A and Cdc25C, so that the decline of homotypic adhesion, degradation of extracellular matrix and cell proliferation can be a vicious cycle and promote each other. This may indicate that a synergistic effect among the three working together might be capable of regulating migration of lung cancer cells and promote the occurrence, invasion and metastasis of NSCLC. The expressions of OPN, ανβ3 and Pim-1 may be early diadynamic criteria of NSCLC. Taken together, our results suggested that targeting the interaction among OPN, ανβ3 and/or Pim-1 could be effective for future development of anti-angiogenic therapeutic agents for patients with NSCLC.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96 [DOI] [PubMed] [Google Scholar]
- 2.Macrì A, Versaci A, Lupo G, et al. Role of osteopontin in breast cancer patients. Tumori 2009;95:48-52 [DOI] [PubMed] [Google Scholar]
- 3.Bao LH, Sakaguchi H, Fujimoto J, et al. Osteopontin in metastatic lesions as a prognostic marker in ovarian cancers. J Biomed Sci 2007;14:373-81 [DOI] [PubMed] [Google Scholar]
- 4.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2002;2:91-100 [DOI] [PubMed] [Google Scholar]
- 5.Verbisck NV, Costa ET, Costa FF, et al. ADAM 23 negatively modulates alpha(v)beta(3) integrin activation during metastasis. Cancer Res 2009;69:5546-52 [DOI] [PubMed] [Google Scholar]
- 6.Bachmann M, Kosan C, Xing PX, et al. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol 2006;38:430-43 [DOI] [PubMed] [Google Scholar]
- 7.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9 [PubMed] [Google Scholar]
- 8.Di Cristofano A, Pesce B, Cordon-Cardo C, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet 1998;19:348-55 [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, Wirth DF, Hynes RO.Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979;16:885-93 [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Takahashi K, Takahashi F, et al. Differential osteopontin expression in lung cancer. Cancer Lett 2001;171:215-22 [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res 2005;11:4646-52 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues LR, Teixeira JA, Schmitt FL, et al. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16:1087-97 [DOI] [PubMed] [Google Scholar]
- 13.Chetty C, Lakka SS, Bhoopathi P, et al. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer 2010;127:1081-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan N, Pan H, Buckley DJ, et al. Prolactin-regulated pim-1 transcription: identification of critical promoter elements and Akt signaling. Endocrine 2003;20:123-30 [DOI] [PubMed] [Google Scholar]
- 15.Beer AJ, Niemeyer M, Carlsen J, et al. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med 2008;49:255-9 [DOI] [PubMed] [Google Scholar]
- 16.Lössner D, Abou-Ajram C, Benge A, et al. Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription. J Cell Physiol 2009;220:367-75 [DOI] [PubMed] [Google Scholar]
- 17.Hsu AR, Hou LC, Veeravagu A, et al. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol Imaging Biol 2006;8:315-23 [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Bhattacharya N, Weaver M, et al. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci 2001;2:167-79 [PubMed] [Google Scholar]
- 19.Guo S, Mao X, Chen J, et al. Overexpression of Pim-1 in bladder cancer. J Exp Clin Cancer Res 2010;29:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnecke-Eberz U, Bollschweiler E, Drebber U, et al. Prognostic impact of protein overexpression of the proto-oncogene PIM-1 in gastric cancer. Anticancer Res 2009;29:4451-5 [PubMed] [Google Scholar]
- 21.Kim DS, Sung JS, Shin ES, et al. Association of single nucleotide polymorphisms in PIM-1 gene with the risk of Korean lung cancer. Cancer Res Treat 2008;40:190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Z, Magnuson NS.Pim-1 kinase-dependent phosphorylation of p21Cip1/WAF1 regulates its stability and cellular localization in H1299 cells. Mol Cancer Res 2007;5:909-22 [DOI] [PubMed] [Google Scholar]
- 23.He HC, Bi XC, Zheng ZW, et al. Real-time quantitative RT-PCR assessment of PIM-1 and hK2 mRNA expression in benign prostate hyperplasia and prostate cancer. Med Oncol 2009;26:303-8 [DOI] [PubMed] [Google Scholar]
- 24.Fong YC, Liu SC, Huang CY, et al. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung Cancer 2009;64:263-70 [DOI] [PubMed] [Google Scholar]
- 25.Cui R, Takahashi F, Ohashi R, et al. Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer 2007;57:302-10 [DOI] [PubMed] [Google Scholar]