Abstract
Objective
To explore the effects and mechanism of glycogen synthase kinase 3β (GSK-3β) inhibitor (2'Z,3'E)-6-bromo-indirubin-3'-oxime (BIO) on drug resistance in colon cancer cells.
Methods
The colon cancer SW480 and SW620 cells were treated with BIO, 5-fluorouracil (5-FU) and BIO/5-FU, separately. Cell cycle distribution, apoptosis level and efflux ability of rhodamine 123 (Rh123) were detected by flow cytometry. The protein expressions of P-glycoprotein (P-gp), multidrug resistance protein 2 (MRP2), thymidylate synthase (TS), β-catenin, E2F-1 and Bcl-2 were detected by Western blot. β-catenin and P-gp were stained with double immunofluorescence and observed under a confocal microscope.
Results
BIO up-regulated β-catenin, P-gp, MRP2 and TS, enhanced the efflux ability of Rh123, decreased Bcl-2 protein and gave the opposite effect to E2F-1 protein in SW480 and SW620 cells. Furthermore, BIO significantly inhibited cell apoptosis, increased S and G2/M phase cells, and reduced the cell apoptosis induced by 5-FU in SW480 cells, whereas the effects were slight or not obvious in SW620 cells.
Conclusion
GSK-3β was involved in drug resistance regulation, and activation of β-catenin and inhibition of E2F-1 may be the most responsible for the enhancement of 5-FU chemotherapy resistance induced by GSK-3β inhibitor BIO in colon cancer.
Key words: Colorectal neoplasms, Drug resistance, Glycogen synthase kinase 3β, Fluorouracil, β-catenin, E2F-1
INTRODUCTION
Colorectal cancer (CRC) is a type of common malignant tumor, and resistance to chemotherapy is one of the main obstacles, which leads to the failure of chemotherapy. Research showed there were multiple mechanisms of drug resistance to chemotherapy. The expression of ATP-binding cassette (ABC) trans- porters, DNA-repair capacity and reluctance to enter apoptosis are involved in resistance to chemo- therapy[1,2]. Some genes may be the potential multifunction drug resistance targets, which join two or more metabolic and signal transduction pathways[3]. Glycogen synthase kinase 3β (GSK-3β), as a multifunctional kinase, participates in the regulation of cell proliferation, apoptosis and drug resistance in tumor cells by influencing the downstream factors, and has become an attractive target for tumor therapy[4-7]. However, the role of GSK-3β in the drug resistance and biological properties of tumor cells remains controversial. Research has shown the paradoxical role of GSK-3β in various human cancers[8]. In certain types of tumors, inhibition of GSK-3β activity with a variety of techniques or pharmacological inhibitors of GSK-3β could attenuate the growth and development of tumors, and GSK-3β inhibitors seem to have a potential therapeutic function. However, inhibition of GSK-3β leads to cell-cycle progression and resistance to apoptosis in other certain types of tumors. What is more, emerging evidence indicated that some GSK-3β inhibitors influenced chemotherapy sensitivity[9,10]. Therefore, the function of GSK-3β and clinical application of GSK-3β inhibitors need to be carefully investigated for the potential harmful effects.
In this study, a small-molecule ATP competitive GSK-3β inhibitor (2'Z,3'E)-6-bromo-indirubin-3'-oxime (BIO) was used in primary colon cancer SW480 cells and lymph node metastatic lesion SW620 cells, and the effects and mechanism of BIO on drug resistance to 5-fluorouracil (5-FU, a basic chemotherapy drug for CRC[11,12]) were studied.
MATERIALS AND METHODS
Chemicals
BIO was purchased from Sigma (USA), and 5-FU was from Shanghai Xudong Haipu Pharmaceutical Co., Ltd (China).
Cell Culture
Human colon adenocarcinoma SW480 and SW620 cells were obtained from American Type Culture Collection. The cells were cultured in RPMI 1640 medium (Gibco, USA) with 10% fetal bovine serum (TBD, China) at 37°C under an atmosphere of 5% CO2.
Apoptosis Analysis
The cells were treated for 24 h with different concentrations of BIO (0, 1, 2, 4 μmol/L), 5-FU (50 μmol/L) and BIO (4 μmol/L) + 5-FU (50 μmol/L), separately. The cells were measured using FACS Aria flow cytometer (BD Biosciences, USA), and Annexin V (+) cells were counted for apoptotic cells after Annexin V- fluorescein isothiocyanate/propidium iodide (FITC/PI) (BD Pharmingen, USA) double staining. The experiment was performed in triplicate.
Cell Cycle Analysis
The cells were treated for 24 h with different concentrations of BIO (0, 1, 2, 4 μmol/L). The cells were harvested and fixed in 70% ice-cold ethanol at 4°C for 30 min. After washed with PBS, the cells were incubated with propidium iodide staining buffer (BD Pharmingen, USA) for 15 min and analyzed by flow cytometer. The experiment was performed in triplicate.
Western Blot Analysis
Following the treatment with different concen- trations of BIO (0, 1, 2, 4 μmol/L) for 24 h, the cells were collected and lysed. Protein content was measured by the BCA protein assay kit (Beyotime Institute of Biotechnology, China) and 20 μg protein per lane was separated by 8%–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. Specific protein bands were achieved with an ECL detection reagent (Pierce, USA). Anti-P-glycoprotein (P-gp) (Chemicon International, USA) dilution was 1:100. Anti-β-catenin and anti-Bcl-2 (Santa Cruz, USA) dilutions were 1:100 and 1:200. Anti-E2F-1 and anti-α- tubulin (Bioworld Technology, USA) dilutions were 1:400 and 1:1,000. Anti-multidrug resistance protein 2 (MRP2) and anti-thymidylate synthase (TS) (Abcam Ltd, UK) dilutions were 1:50 and 1:100. Horseradish peroxidase (HRP)-conjugated goat-anti-rabbit and goat-anti-mouse IgG antibodies (ProteinTech Group, USA) dilutions were 1:3,000. The experiment was performed in triplicate.
Double-lable Immunofluorescence and Confocal Microscopy
Cells were seeded onto glass cover-slips in 6-well plates at a density of 2×105/well and incubated for 48 h. The cells were treated with BIO (0, 4 μmol/L) for 24 h, and fixed in acetone at −20°C for 30 min. The cells were exposed to goat serum for 1 h and incubated with mouse anti-P-gp monoclonal antibody (1:100, Santa Cruz, USA) for 12 h at 4°C and Cy3-labelled goat-anti-mouse IgG (1:300, Beyotime Institute of Biotechnology, China) for 1 h at 37°C. After full washed, the cells were exposed to goat serum for 1 h and incubated with the secondary mouse monoclonal anti-β-catenin (1:100) for 12 h at 4°C and FITC-labelled rabbit-anti-mouse IgG (1:300, Dingguo Biotechnology, China) for 1 h at 37°C. The cells were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) (Dingguo Biotechnology, China) for 10 min and analyzed using a laser scanning confocal microscope.
Rh123 Efflux Assay for P-gp Function
After exposed to BIO (0, 4 μmol/L) for 24 h, the cells were incubated with 4 μg/ml rhodamine 123 (Rh123) (KeyGEN Biotech. Co., Ltd, China) for 30 or 60 min at 37°C. The fluorescence intensity of Rh123 in cells was detected by flow cytometry. The efflux rate of Rh123 (%) = the mean fluorescence intensity of Rh123 (30–60 min) / (30 min) %.
Statistical Analysis
Statistical analysis was performed with the SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA). Data are presented as‾x±s. One way analysis of variance (ANOVA) was used for apoptosis, cell cycle and Western blot data analyses, and paired t-test was used for Rh123 efflux. P<0.05 was considered statistically significant.
RESULTS
Effect of BIO on Apoptosis Induced by 5-FU in SW480 and SW620 Cells
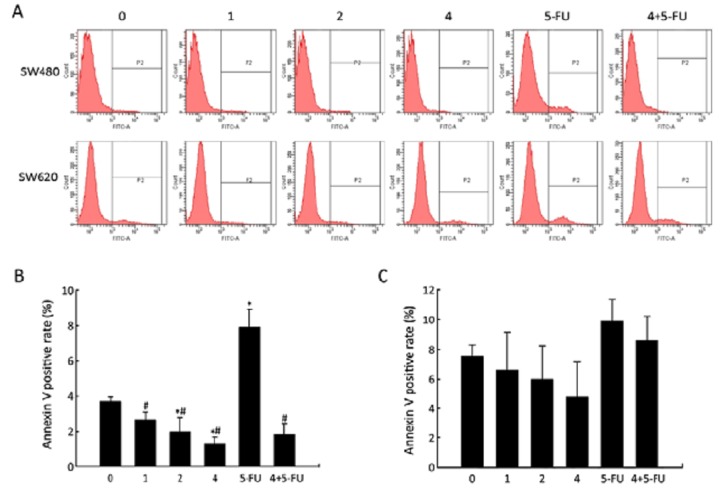
After incubating SW480 and SW620 cells with BIO in various concentrations (0, 1, 2, or 4 μmol/L), separately, the effect of BIO on the apoptosis of SW480 and SW620 cells was examined using flow cytometry after Annexin V-FITC/PI staining. As shown in Figure 1, in SW480 cells, there was a BIO dose-dependent decrease in the population of Annexin V (+) cells. In addition, we found that BIO could significantly reduce the apoptosis induced by 5-FU, whereas the effects were slight in SW620 cells.
Figure 1.
The effects of BIO on apoptosis of SW480 and SW620 cells. A: Flow cytometry analysis of Annexin V positive cells in SW480 and SW620 cells following BIO and (or) 5-FU treatment; B, C: The rate of Annexin V positive cells in SW480 cells (B) and SW620 cells (C) after BIO and (or) 5-FU treatment. All Annexin V positive cells were considered apoptotic cells, and their percentage was calculated among the total number of cells. Each bar represents the‾x±s of three experiments. *P<0.05 compared with the control (0 μmol/L); #P<0.05 compared with 5-FU-treated cells (50 μmol/L).
Effect of BIO on Cell Cycle Progression in SW480 and SW620 Cells
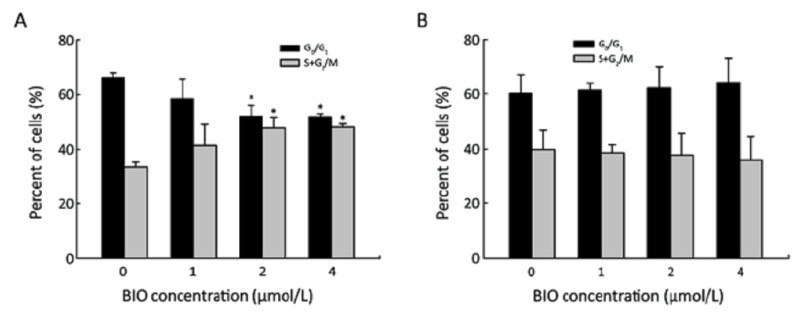
After incubating SW480 and SW620 cells with BIO in various concentrations (0, 1, 2, or 4 μmol/L), separately, cells were stained and cell cycle distribution was determined by flow cytometry. In SW480 cells, the percentage of G0/G1 phase cells was decreased, while the percentage of cells at S+G2/M phase of the cell cycle was increased (Figure 2A). However, these results were not shown in SW620 cells (Figure 2B).
Figure 2.
Flow cytometry analysis of cell cycle distribution of SW480 (A) and SW620 (B) cells treated with different doses of BIO. Results are presented as‾x±s of three experiments. *P<0.05 compared with the control (0 μmol/L).
Effect of BIO on Proteins Involved in Apoptosis and Multidrug Resistance in SW480 and SW620 Cells
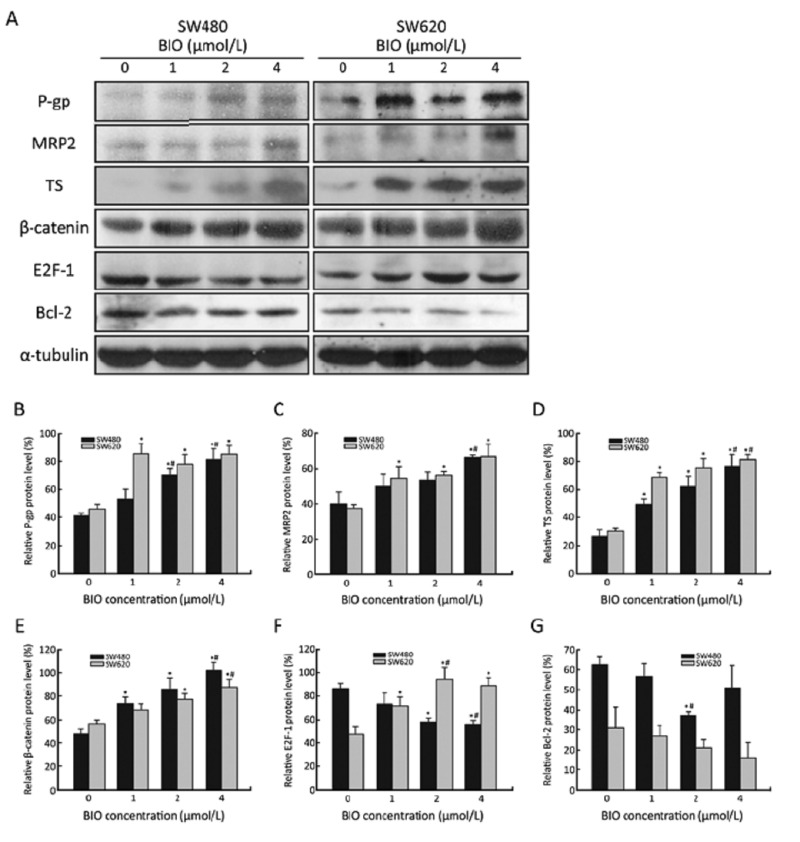
To further study the effect of BIO on proteins expression, total protein lysates were prepared and analyzed by Western blot. As shown in Figure 3, following 24 h treatment of BIO with different concentrations, the levels of β-catenin, P-gp, MRP2 and TS were dose-dependently elevated, while the levels of Bcl-2 were dose-dependently decreased in SW480 and SW620 cells. Interestingly, E2F-1 was dramatically reduced in SW480 cells, but increased in SW620 cells.
Figure 3.
Expression levels of P-gp, MRP2, TS, β-catenin, E2F-1 and Bcl-2 in SW480 and SW620 cells before and after BIO treatment for 24 h. A: Protein expression by Western blot in SW480 and SW620 cells. α-tubulin was used as an internal loading control. B–G: The density of the protein band was quantitated using Quantity One software (Bio-Rad, USA). Values are expressed as protein/α-tubulin. The data are expressed as‾x±s of three experiments. *P<0.05 compared with the control (0 μmol/L); #P<0.05 compared with BIO-treated cells (1 μmol/L).
Sub-cellular Co-localization of β-catenin with P-gp in SW480 and SW620 Cells
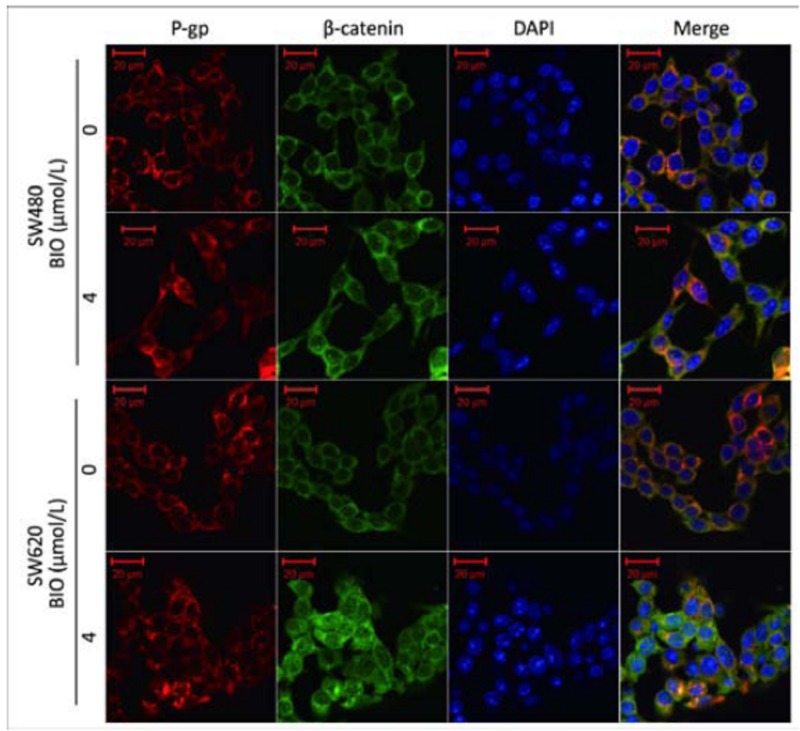
In order to investigate the localization of P-gp and β-catenin, a double-lable immunofluorescence was performed. As shown in Figure 4, before BIO treatment, the expressions of P-gp and β-catenin were primarily localized in the cell membrane and cytoplasm, and after BIO (4 μmol/L) treatment, we found the expressions of P-gp and β-catenin at the cell membrane and cytoplasm were increased and some cells showed a nuclear staining of β-catenin. β-catenin and P-gp were shown co-localization at the cell membrane and cytoplasm of SW480 and SW620 cells before and after BIO treatment.
Figure 4.
Co-localization of β- catenin (green) with P-gp (red) in SW480 and SW620 cells before and after BIO (4 μmol/L) treatment using confocal immunefluorescence microscopy. DAPI was used to stain the nucleus (blue).
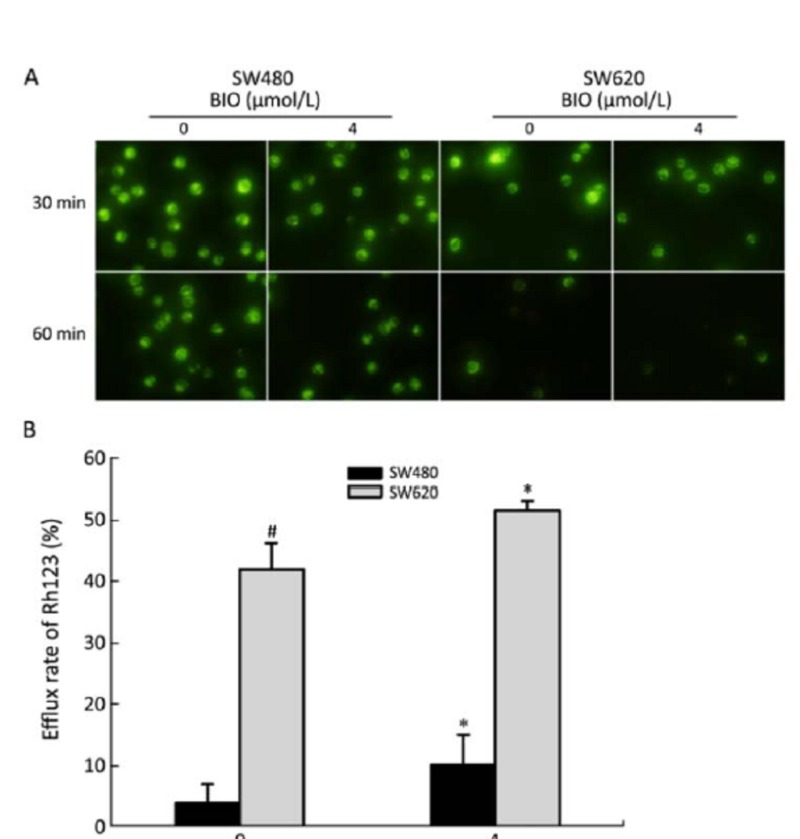
Effect of BIO on Rh123 Efflux in SW480 and SW620 Cells
To examine the effect of BIO on P-gp function, the fluorescence intensity of Rh123 was measured by flow cytometry and the efflux rate of Rh123 was calculated. As shown in Figure 5, the efflux rate of Rh123 was higher after 24 h BIO (4 μmol/L) treatment than that before BIO treatment in SW480 and SW620 cells. In addition, the efflux rate of Rh123 in SW620 cells was higher compared with SW480 cells before BIO treatment. These results were consistent with the expression of P-gp protein as determined by Western blot (Figure 3).
Figure 5.
The effects of BIO on efflux of Rh123 in SW480 and SW620 cells. A: The fluorescent signals of Rh123 before and after BIO treatment were observed by fluorescent microscope (×400) in SW480 and SW620 cells. B: The efflux rate of Rh123 before and after BIO treatment in SW480 and SW620 cells. All values are presented as‾x±s of three experi- ments. *P<0.05 compared with the control (0 μmol/L). #P<0.05 compared with SW480 (0 μmol/L).
DISCUSSION
GSK-3β has been known to be a key regulatory enzyme in Wnt/β-catenin, NF-κB, Rb/E2F-1 and other molecule signaling pathways, affecting cell proliferation, apoptosis and chemoresistance[13-16]. As a consequence, the role of GSK-3β and therapeutic potential of GSK-3β inhibitors have become one of the key areas of investigation. Through competing with ATP and influencing the phosphorylation of GSK-3β, the small molecule BIO could evidently inhibit the activity of GSK-3β[17,18]. Recent studies have shown that inhibition of GSK-3β activity by BIO or LiCl (another specific inhibitor of GSK-3β) could induce chemoresistance to interferon-α/5-FU, etoposide and camptothecin in hepatocellular carcinoma, hepatoblastoma, lung carcinoma and breast carcinoma cells[9,10], while renewing GSK-3β activity could increase the sensitivity to apoptosis induced by chemotherapy agents in liver cancer cells[19]. In this study, we found that BIO could reduce the apoptosis induced by 5-FU in SW480 and SW620 cells, while the effect was particularly evident in SW480 cells. These results indicated that GSK-3β was involved in drug resistance regulation in colon cancer.
The mechanism of drug resistance to chemotherapy is complex, and the cell apoptosis resistance plays an important role in drug resistance and sensitivity. Previous work has suggested that GSK-3β participated in cell apoptosis and the proliferation process in many human cancers. However, the role of GSK-3β remains controversial. In Tseng, et al.’s study[18], the GSK-3β inhibitor BIO could promote dedifferentiation and increase the proliferation capacity of cardiomyocytes in vitro, and Sinha, et al.[13] have observed that BIO could promote renal epithelial cell survival by inhibiting apoptosis. An analogous study has shown that the suppression of GSK-3β could inhibit the apoptosis and increase the S phase cells in human lung cancer cells[8]. Conversely, Shakoori et al.[20] have observed that suppression of GSK-3β by chemical inhibitors or RNA interference in colon cells could induce cell apoptosis and reduce the growth of cancer cells. In this study, it is obvious that the GSK-3β inhibitor BIO could inhibit apoptosis and promote cell cycle progression in SW480 cells, while there was less effect in SW620 cells. As mentioned above, the inhibition of GSK-3β activity could lead to different effects on different tumor cells, even in the original colon primary tumor and metastatic tumor cells[11]. This phenomenon might be related to the cell type[8] and the different function of downstream signaling pathways of GSK-3β in different cell lines.
β-catenin is a key factor of Wnt/β-catenin signaling pathways, which can be activated by the suppression of GSK-3β[21,22]. High expression of β-catenin could promote cell cycle process and inhibit apoptosis through the downstream nuclear transcription factors, especially in CRC[23]. It was exhibited in this study that the GSK-3β inhibitor BIO could up-regulate the levels of β-catenin protein and promote nuclear translocation of β-catenin in SW480 and SW620 cells. Similar results were also reported that pharmacologic inhibitors of GSK-3β could up-regulate β-catenin expression and promote cell proliferation in breast cancer[21] and cardiomyocytes[18]. Bcl-2, as the downstream factor of NF-κB, plays an important role in the resistance to apoptosis induction of cancer cells. GSK-3β might regulate NF-κB-mediated gene transcription[14]. It has been observed in the normal intestinal mucosa radioprotective effect experiment that GSK-3β inhibitors could up-regulate Bcl-2 expression and inhibit apoptosis[24]. Conversely, pharmacological inhibition of GSK-3β led to a decrease in expression of NF-κB target genes Bcl-2 and a subsequent increase in apoptosis in pancreatic cancer, renal cancer and chronic lymphocytic leukemia B cells[14,15,25]. In this study, we found that the level of Bcl-2 protein in SW480 and SW620 cells was down-regulated with increasing doses of BIO, and the change of Bcl-2 protein in colon cancer was different from that in normal intestinal cells[24], while consistent with the above tumor cells[14,15,25]. E2F-1 is the extremely important transcription factor in Rb/E2F-1 pathway, affecting cell proliferation, apoptosis and cell cycle process. Recently, several studies have indicated that GSK-3β could affect the regulation of E2F-1. However, the experiment results were inconsistent. GSK-3β inhibitor could suppress E2F transactivation by interrupting the interaction of E2F-1 factor with its target gene promoter[26], while the opposite results were reported that depletion of the levels of GSK-3β protein using siRNA could activate E2F-1 transcript activity[16]. In this study, the level of E2F-1 was down-regulated in SW480 cells and up-regulated in SW620 cells after the treatment of BIO. In other words, BIO might cause different effects on primary and metastatic colon cancer cells. Our results revealed when β-catenin was up-regulated and Bcl-2 was down-regulated by BIO treatment, the low expression of E2F-1 was conducive to cell cycle process and apoptosis inhibition, while high expression of E2F-1 gave the opposite effect. This evidence indicated that E2F-1 mainly acted as the tumor inhibition factor in SW480 and SW620 cells. Consistently, it was also found that activation of E2F-1 initiated apoptosis and induced cell cycle arrest, thus suppressed growth in colon cancer cells[27,28]. In this study, although Bcl-2 protein expression was down-regulated by BIO, the apoptosis of cancer cells still appeared to be inhibited. This phenomenon shed light on the role of these proteins that high expression of β-catenin might play the central role in apoptosis and cell cycle process, and the different expression of E2F-1 was likely to be one of the pathways leading to the different outcome in the SW480 and SW620 cells.
The high expression of multidrug resistance relevant proteins is another important chemo- resistance mechanism. P-gp and MRP2, as the important transmembrane protein components of ABC transporter system, can restrict the drug effect on intracellular target through reducing the intracellular drug concentration or changing the intracellular distribution, which results in a certain drug-resistance in cancer cells[29,30]. In this study, we observed that GSK-3β inhibitor BIO could increase P-gp and MRP2 expression and enhance the efflux function of P-gp, which was represented by efflux function of Rh123 (a substrate of P-gp)[31]. It has been suggested that the enhancement of efflux capacity of transmembrane proteins was responsible for the apoptosis resistance to 5-FU, and P-gp function level was accompanied by promotion of Rh123 efflux and resistance to 5-FU in the colon adenocarcinoma cells[32]. Until the present, the mechanism for the inhibition of GSK-3β activity up-regulating transmembrane proteins remains unclear. Several previous studies have found that GSK-3β inhibitors up-regulate P-gp and other transporters, and the mechanism might be related to regulation of some signaling pathways, including β-catenin, cSrc, CD95, NF-κB[10,33,34], especially the β-catenin signaling pathway. The activation of β-catenin signaling by GSK-3β inhibitor could increase P-gp expression in rat brain endothelial cells, while decreased expression of multidrug efflux transporters in the brains of GSK-3β transgenic mice[35,36]. The consistency between the nuclear accumulation of β-catenin protein and the multidrug resistance 1 (MDR1) gene product (P-gp) expression has been observed in the human familial adenomatous polyposis intestinal tumor and experimental mice intestinal tumor[37,38]. Yamada, et al.[37] have discovered that MDR1 gene contained multiple β-catenin-T-cell factor4-binding elements in its promoter and was one of the immediate targets of the complex by a large-scale comparison of the expression profiles. It has been reported that Wnt pathway activation led to the high expression of ABC transporter gene, while silencing β-catenin appeared to the ABC transporter gene downregulation[39]. In this study, we found the up-regulation of P-gp and MRP2 with the treatment of GSK-3β inhibitor BIO was synchronized with the increase of β-catenin, and the co-localization phenomenon was shown between P-gp and β-catenin protein expression. These results suggested that there was a close relationship between the up-regulation of P-gp and the increase of β-catenin. This is very important to evaluate the expression of multidrug efflux transporters in CRC with high expression of β-catenin.
TS is a key enzyme in DNA synthesis and metabolism, and is also the target enzyme of 5-FU. As we know, the activated form of 5-FU can bind to the nucleotide-binding site of TS and affect the DNA replication and repair[40,41]. Several studies have indicated that intratumoral TS protein expression was inversely correlated to 5-FU sensitivity in CRC[42-44], although some studies have shown that 5-FU adjuvant chemotherapy might be appropriate for colorectal carcinoma with high expression of TS[45,46]. We observed that the GSK-3β inhibitor BIO up-regulated the TS expression and reduced the cell apoptosis induced by 5-FU in different degrees. These results support the hypothesis that high TS protein expression induced by BIO may be another resistance mechanism to 5-FU. However, there has been little explanation about the regulation mechanism on TS expression by inhibition of GSK-3β. Dong, et al.[47] discovered that the human TS promoter region includes at least two potential E2F binding sites within the inverted repeat region. The relationship between TS and E2F-1 has not been clearly defined[48,49]. In our study, the increase of TS protein was accompanied by the increase of E2F-1 protein in SW620 cells and the decrease of E2F-1 protein in SW480 cells. The results of the above studies suggested that the increase of TS was likely related to E2F-1 or non-E2F-1 pathways, and the mechanism needs to be further studied.
In conclusion, the results revealed that the GSK-3β inhibitor BIO, at least through the enhancement of cell apoptosis resistance and the increase of P-gp, MRP2 transporter protein and TS protein expression, enhanced the drug resistance to 5-FU in colon cancer SW480 and SW620 cells. β-catenin, E2F-1 and Bcl-2 jointly participated in the regulation of cell drug resistance in this process. It has been indicated that the effects on cell drug resistance induced by BIO depended on a self-stabilizing interaction among a series of activation and inhibition pathways, and GSK-3β acted as a multifunctional drug resistance target. The activation of β-catenin and inhibition of E2F-1 may be the most responsible factor for 5-FU chemotherapy resistance induced by GSK-3β inhibitor BIO in colon cancer.
REFERENCES
- 1.Ahmed FE. Molecular markers that predict response to colon cancer therapy. Expert Rev Mol Diagn 2005; 5:353-75 [DOI] [PubMed] [Google Scholar]
- 2.Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006; 5:219-34 [DOI] [PubMed] [Google Scholar]
- 3.Chao SY, Chiang JH, Huang AM, et al. An integrative approach to identifying cancer chemoresistance-associated pathways. BMC Med Genomics 2011; 4:23-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phukan S, Babu VS, Kannoji A, et al. GSK3beta: role in therapeutic landscape and development of modulators. Br J Pharmacol 2010; 160:1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J.Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 2009; 273:194-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeste-Velasco M, Folch J, Trullas R, et al. Glycogen synthase kinase-3 is involved in the regulation of the cell cycle in cerebellar granule cells. Neuropharmacology 2007; 53:295-307 [DOI] [PubMed] [Google Scholar]
- 7.Seo SB, Hur JG, Kim MJ, et al. TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases. Mol Cancer 2010; 9:199-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra R.Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer 2010; 9:144-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noda T, Nagano H, Takemasa I, et al. Activation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer 2009; 100:1647-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beurel E, Kornprobst M, Blivet-Van EM, et al. GSK-3beta inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp Cell Res 2004; 300:354-64 [DOI] [PubMed] [Google Scholar]
- 11.Leibovitz A, Stinson JC, McCombs WR, et al. Classification of human colorectal adenocarcinoma cell lines. Cancer Res 1976; 36:4562-9 [PubMed] [Google Scholar]
- 12.Li Y, Li J, Lu M, et al. Capecitabine maintenance therapy after first-line chemotherapy in patients with metastatic colorectal cancer. Chin J Cancer Res 2010; 22:181-5 [Google Scholar]
- 13.Sinha D, Wang Z, Ruchalski KL, et al. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am J Physiol Renal Physiol 2005; 288:F703-13 [DOI] [PubMed] [Google Scholar]
- 14.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, et al. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res 2005; 65:2076-81 [DOI] [PubMed] [Google Scholar]
- 15.Ougolkov AV, Bone ND, Fernandez-Zapico ME, et al. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood 2007; 110:735-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Alvarez G, Ventura V, Ros O, et al. Glycogen synthase kinase-3beta binds to E2F1 and regulates its transcriptional activity. BiochimBiophysActa 2007; 1773:375-82. [DOI] [PubMed]
- 17.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci 2007; 64:1930-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol 2006;13:957-63 [DOI] [PubMed] [Google Scholar]
- 19.Beurel E, Kornprobst M, Blivet-Van EM, et al. GSK-3beta reactivation with LY294002 sensitizes hepatoma cells to chemotherapy-induced apoptosis. Int J Oncol 2005; 27:215-22 [PubMed] [Google Scholar]
- 20.Shakoori A, Ougolkov A, Yu ZW, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun 2005; 334:1365-73 [DOI] [PubMed] [Google Scholar]
- 21.Farago M, Dominguez I, Landesman-Bollag E, et al. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res 2005; 65:5792-801 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Si L, Li Y, et al. Expressions of GSK-3beta, Beta-Catenin and PPAR-Gamma in Medulloblastoma. Chin J Cancer Res 2009; 21:235-9 [Google Scholar]
- 23.Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis? Am J Pathol 2002; 160:389-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thotala DK, Geng L, Dickey AK, et al. A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int J Radiat Oncol Biol Phys 2010; 76:557-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilim V, Ougolkov A, Yuuki K, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer 2009; 101:2005-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun A, Shanmugam I, Song J, et al. Lithium suppresses cell proliferation by interrupting E2F-DNA interaction and subsequently reducing S-phase gene expression in prostate cancer. Prostate 2007; 67:976-88 [DOI] [PubMed] [Google Scholar]
- 27.Elliott MJ, Dong YB, Yang H, et al. E2F-1 up-regulates c-Myc and p14(ARF) and induces apoptosis in colon cancer cells. Clin Cancer Res 2001; 7:3590-7 [PubMed] [Google Scholar]
- 28.Vorburger SA, Pataer A, Yoshida K, et al. The mitochondrial apoptosis-inducing factor plays a role in E2F-1-induced apoptosis in human colon cancer cells. Ann Surg Oncol 2003; 10:314-22 [DOI] [PubMed] [Google Scholar]
- 29.Cheng SC, Zhou J, Xie Y. P-glycoprotein expression induced by glucose depletion enhanced the chemosensitivity in human hepatocellular carcinoma cell-lines. Cell Biol Int 2005; 29:269-75 [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki M, Makino T, Masuzawa T, et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer 2011; 104:707-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitchakarn P, Ohnuma S, Pintha K, et al. Kuguacin J isolated from Momordicacharantia leaves inhibits P-glycoprotein (ABCB1)-mediated multidrug resistance. J NutrBiochem 2012; 23:76-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao H, Duan ZH, Wang MH, et al. Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet Cytogenet 2009; 190:81-7 [DOI] [PubMed] [Google Scholar]
- 33.Liu YY, Gupta V, Patwardhan GA, et al. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer 2010; 9:145-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourguignon LY, Spevak CC, Wong G, et al. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem 2009; 284:26533-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim JC, Kania KD, Wijesuriya H, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem 2008; 106:1855-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JC, Mickute Z, Zaman M, et al. Decreased expression of multidrug efflux transporters in the brains of GSK-3beta transgenic mice. Brain Res 2009; 1276:1-10 [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Takaoka AS, Naishiro Y, et al. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res 2000; 60:4761-6 [PubMed] [Google Scholar]
- 38.Yamada T, Mori Y, Hayashi R, et al. Suppression of intestinal polyposis in Mdr1-deficient ApcMin/+ mice. Cancer Res 2003; 63:895-901 [PubMed] [Google Scholar]
- 39.Chikazawa N, Tanaka H, Tasaka T, et al. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res 2010; 30:2041-8 [PubMed] [Google Scholar]
- 40.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3:330-8 [DOI] [PubMed] [Google Scholar]
- 41.Patel PA. Evolution of 5-fluorouracil-based chemoradiation in the management of rectal cancer. Anticancer Drugs 2011; 22:311-6 [DOI] [PubMed] [Google Scholar]
- 42.Cascinu S, Aschele C, Barni S, et al. Thymidylate synthase protein expression in advanced colon cancer: correlation with the site of metastasis and the clinical response to leucovorin-modulated bolus 5-fluorouracil. Clin Cancer Res 1999; 5:1996-9 [PubMed] [Google Scholar]
- 43.Okumura K, Mekata E, Shiomi H, et al. Expression level of thymidylate synthase mRNA reflects 5-fluorouracil sensitivity with low dose and long duration in primary colorectal cancer. Cancer Chemother Pharmacol 2008; 61:587-94 [DOI] [PubMed] [Google Scholar]
- 44.Yeh KH, Cheng AL, Wan JP, et al. Down-regulation of thymidylate synthase expression and its staedy-state mRNA by oxaliplatin in colon cancer cells. Anticancer Drugs 2004; 15:371-6 [DOI] [PubMed] [Google Scholar]
- 45.Takenoue T, Nagawa H, Matsuda K, et al. Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemicalmethod. Ann SurgOncol 2000; 7:193-8 [DOI] [PubMed] [Google Scholar]
- 46.Edler D, Glimelius B, Hallstrom M, et al. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol 2002; 20:1721-8 [DOI] [PubMed] [Google Scholar]
- 47.Dong S, Lester L, Johnson LF. Transcriptional control elements and complex initiation pattern of the TATA-less bidirectional human thymidylate synthase promoter. J Cell Biochem 2000; 77:50-64 [DOI] [PubMed] [Google Scholar]
- 48.Kasahara M, Takahashi Y, Nagata T, et al. Thymidylate synthase expression correlates closely with E2F1 expression in colon cancer. Clin Cancer Res 2000; 6:2707-11 [PubMed] [Google Scholar]
- 49.Belvedere O, Puglisi F, Di Loreto C, et al. Lack of correlation between immunohistochemical expression of E2F-1, thymidylate synthase expression and clinical response to 5-fluorouracil in advanced colorectal cancer. Ann Oncol 2004;15:55-8 [DOI] [PubMed] [Google Scholar]