Abstract
Objective
This study aimed to express a fusion protein of diphtheria toxin and human B cell-activating factor (DT388sBAFF) in Escherichia coli (E. coli) and investigate its activity in human B-lineage acute lymphoblastic leukemia 1 cells (BALL-1).
Methods
A fragment of DT388sBAFF fusion gene was separated from plasmid pUC57-DT388sBAFF digested with Nde I and Xho I, and inserted into the expression vector pcold II digested with the same enzymes. Recombinants were screened by the colony polymerase chain reaction (PCR) and restriction map. The recombinant expression vector was transformed into BL21 and its expression was induced by isopropyl β-D-1-thiogalactopyranoside (IPTG). The recombinant protein was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot, and then purified by Ni2+-NTA affinity chromatography. The expression level of B cell-activating factor receptor (BAFF-R) on BALL-1 cells was assessed by real-time PCR. The receptor binding capacity of recombinant protein was determined by cell fluorescent assay. The specific cytotoxicity of recombinant protein on BALL-1 cells was detected by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay.
Results
The expression level of recombinant protein was 50% of total bacterial proteins in E. coli, and the recombinant protein could bind to BAFF-R-positive BALL-1 cells and thereby produce a cytotoxic effect on the cells.
Conclusion
The fusion protein expression vector DT388sBAFF was successfully constructed and the recombinant protein with selective cytotoxicity against BALL-1 cells was obtained, providing foundation for further study of the therapy of human B-lineage acute lymphoblastic leukemia.
Key words: B cell-activating factor, B-lineage acute lymphoblastic leukemia, Diphtheria toxin, Fusion protein
INTRODUCTION
Immunotoxin is an artificial hybrid molecule with a targeted killing ability[1], consisting of a targeting moiety and a toxic payload. The targeting moiety falls into two categories: one is monoclonal antibody; the other is certain receptor or ligand with important physiological functions. The toxic payload is usually a protein toxin, either plant or bacterial. Immunotoxin commonly contains toxins such as diphtheria toxin and pseudomonas exotoxin[2]. Immunotoxins targeting hematological malignancies and solid tumors have been additionally demonstrated with excellent clinical activity[3], representing a second revolution in antibody-mediated cancer therapy[4].
B cell-activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS), TNF- and APOL-related leukocyte expressed ligand 1 (TALL-1), or TNF homolog that activates apoptosis, NKFB, and JNK (THANK), is a member of tumor necrosis factor (TNF) superfamily[5,6] and is expressed on the surface of monocytes, dendritic cells (DC), neutrophils, stromal cells, activated T cells, malignant B cells and epithelial cells[7,8]. BAFF can bind to three different receptors, BAFF receptor (BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B cell maturation protein (BCMA), which are expressed seperately at various times during B cell ontogeny[9]. BAFF-R specifically expresses on more mature B cells, starting at the T1 transitional B cell stage[7]. Nevertheless, a recent research[9] presented evidence that the BAFF-R is universally expressed on B-lineage acute lymphoblastic leukemia (ALL) cells which develop by transformation of normal B-cell progenitors. Other studies have demonstrated that the fusion protein of BAFF and gelonin, a plant toxin, had the killing effects against BAFF-R (+) B lymphoma cells[10,11].
To investigate the human B cell leukemia BALL-1 cell-targeting effect of BAFF/diphtheria toxin fusion protein DT388sBAFF and the feasibility of its clinical applications, in this study, we expressed DT388sBAFF in Escherichia coli (E. coli), purified the recombinant protein and investigated its biological activity in target BAFF-R (+) BALL-1 cells.
MATERIALS AND METHODS
Materials
The whole gene synthesis of pUC57-DT388sBAFF was carried out by Nanjing Genscript Biotechnology (Nanjing, China). The pcold II vector was constructed by the central laboratory of the Provincial Hospital Affiliated to Shandong University. The polymerase chain reaction (PCR) reagents, RNA isolation kit, and real-time PCR kits were purchased from Takara Biotechnology (Dalian, China). The DNA marker was purchased from Tiangen Biotech (Beijing, China). T4 DNA ligase, restriction enzymes Nde I, Xho I, protein marker and gel extraction kit were products of MBI Fermentas (Shenzhen, China). E. coli DH5α and BL21 were purchased from TransGen Biotech (Beijing, China). The plasmid extraction kit was prepared by the central laboratory of the Provincial Hospital Affiliated to Shandong University. Yeast extract, peptone and agar were purchased from Oxoid (Basingstoke, UK). The protein quantification kit was purchased from Shanghai Shenergy Biocolor BioScience & Technology (Shanghai, China). The anti-BAFF antibody, anti-His-Tag polyclonal antibody and goat anti-rabbit antibody were ABcam products (Cambridge, UK). The inducer isopropyl β-D-1-thiogalactopy-ranoside (IPTG) was a product of BBI Biotech (Berlin, Germany). Western chemiluminescent substrate was purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Monocytic cell line U937 and Hmy2.CIR B cells were kept by the authors’ group in the laboratory, and human acute lymphoblastic leukemia cell line BALL-1 was obtained from ATCC (Washington, USA). Modified RPMI 1640 medium (containing 2.05 mmol/L glutamine), IMDM medium and fetal bovine serum were purchased from HyClone Laboratories (Logan, USA). The 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl-tetrazolium bromide (MTT) kit was purchased from Keygen Biotech (Nanjing, China). Other reagents were imported products of analytical grade.
Primer Design and Synthesis
DT388sBAFF gene colony PCR segment primers (upstream primer 5'-CATATGAATCGTAAACTG-3'; downstream primer 5'-CTCGAGCAGCAGTTTCAGG-GCACCGAA-3') were synthesized by Sangon Biotech (Shanghai, China) according to the optimized diphtheria toxin-human BAFF (DT388sBAFF) gene sequence.
Recombinant DNA Technology
The gene fragment was isolated from the cloning vector pUC57-DT388sBAFF digested with Nde I and Xho I, inserted into an appropriate site of the expression vector pcold II, and then transformed into E. coli DH5α. After being identified by colony PCR, the positive clones were saved and the plasmid was extracted.
Expression and Identification of the Recombinant Protein
The positive recombinant expression vector pcold II/DT388sBAFF was transformed into competent strain E. coli BL21. Isolated colonies were selected and cultured to logarithmic phase. Inducer IPTG was then added to a final concentration of 1 mmol/L, followed by inducing at 15°C for 24 h. The expression level of the target protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The recombinant protein was identified by Western blot with anti-BAFF and polyclonal anti-His-Tag.
Protein Purification
In accordance with the instructions of QIAgenes E. coli Handbook, the fusion protein was purified with the non-denaturing method. The ultrasonic supernatant of recombinant strain was fully integrated with Ni2+-NTA column at 4°C for 1 h. After washing and elution, imidazole and other small molecules were removed by dialysis, and the recombinant protein was detected by SDS-PAGE.
Detection of BAFF Receptors Expression
The mRNA expression of human BAFF receptors (BAFF-R, TACI and BCMA) in BALL-1, Hmy2.CIR and U937 cell lines was assessed by real-time PCR[12]. The total mRNA of these cell lines was isolated by an RNAiso Plus kit (Takara, Dalian, China). The primers of BAFF-R, TACI and BCMA were as follows: BAFF-R: 5’-GGA GAA GGCAGG AAC CAC-3’ (sense) and 5’-AAG GCA AGC ACA CCA AA-3’ (anti-sense); TACI: 5’-CAC CCT AAG CAA TGTGC-3’ (sense) and 5’-TGG GAC TCA GAG TGC C-3’ (anti-sense); BCMA: 5’-TTA CTT GTC CTT CCA GGC TGT TCT-3’ (sense) and 5’-CATAGA AAC CAA GGA AGT TTC TAC C-3’ (anti-sense)[13]. The first strand cDNA was synthesized using these total mRNA. Real-time PCR was performed using Premix Ex Taq™ kit (Takara, Dalian, China) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous normalization control. Each 20 μl reaction solution contained 10 μl 1× Premix Ex Taq™, 0.4 μl 0.2 μmol/L forward primer, 0.4 μl 0.2 μmol/L reverse primer, 0.8 μl fluorescent probe, 0.4 μl 1× ROX Reference Dye II, 2.0 μl 100 ng cDNA and 6.0 μl dH2O. The PCR was performed using a 7500 Real-Time PCR System (ABI PRISM) to amplify human BAFF-R (300 bp), TACI (931 bp) and BCMA (806 bp). The cycling PCR conditions were: 94°C for 1 min; 40 cycles at 61°C (BAFF-R), 60°C (TACI) and 58°C (BCMA) respectively for 1 min; and 72°C for 10 min. The data were collected and analyzed using the ABI Sequence Detection Software version 1.3.1 (Applied Biosystems, USA).
Receptor-binding Assay
Binding ability of the recombinant protein to BAFF receptors on BALL-1 cell surface was detected by using fluorescin isothiocyanate (FITC). BAFF-R (+) Hmy2.CIR cells and BAFF-R (−) U937 cells were separately chosen as positive control and negative control. Free diphtheria toxin (not coupled to BAFF) was used as blank control. When grown to the logarithmic phase, these cells were counted, and 1×105/ml cell suspension was prepared with the culture medium. Every 100 μl recombinant protein was mixed with 2 μl fluorescent and incubated at room temperature for 30 min to prepare FITC recombinant protein. After preparing 100 μl cell suspensions from the three cell lines, 100 μl FITC recombinant proteins were added respectively, incubated at room temperature for 1 h, then washed three times (1 ml/time) by 5% fetal calf serum (FCS)/phosphate buffer solution (PBS), smeared and observed with fluorescent microscope. Free diphtheria toxin was detected through the same procedure as aforesaid.
Cytotoxicity Experiment
Cytotoxicity experiment was carried out to examine the killing effect of the recombinant protein DT388sBAFF on human BALL-1 cells and control cells. Firstly, in the logarithmic phase, BALL-1 cells, Hmy2.CIR cells and U937 cells (5×102/well) were seeded in flat-bottomed 96-well plates. Then the purified recombinant protein and diphtheria toxin of various concentrations (0.0 ng/ml as the non-drug group; 0.4 ng/ml, 0.8 ng/ml, 1.2 ng/ml, 1.6 ng/ml, 2.0 ng/ml and 2.4 ng/ml as the drug groups) were added to triplicate wells. Cells were cultured at 37°C in 5% CO2 for 96 h. After adding 20 μl MTT (5 mg/L) to each well, the cells were cultured for 4 h. After centrifugation and careful removal of the supernatant, 100 μl dimethyl sulfoxide was added to each well, then mixing and standing for 20 min at 37°C to fully dissolve crystals. Absorbance of each well was measured at 570 nm using a microplate reader, and the inhibition rate was computed according to the following formula: inhibition rate (%) = (1 − drug group/no-drug group) × 100%.
Statistical Analysis
All data were expressed as x±s. Differences of the mRNA expression levels of human BAFF receptors (BAFF-R, TACI and BCMA) in BALL-1, Hmy2.CIR and U937 cell lines were evaluated by analysis of variance (ANOVA). Comparison of the killing effects of the recombinant protein on BALL-1, Hmy2.CIR and U937 cells was also done by the ANOVA test. The data were processed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant.
RESULTS
Construction of Recombinant Expression Vector
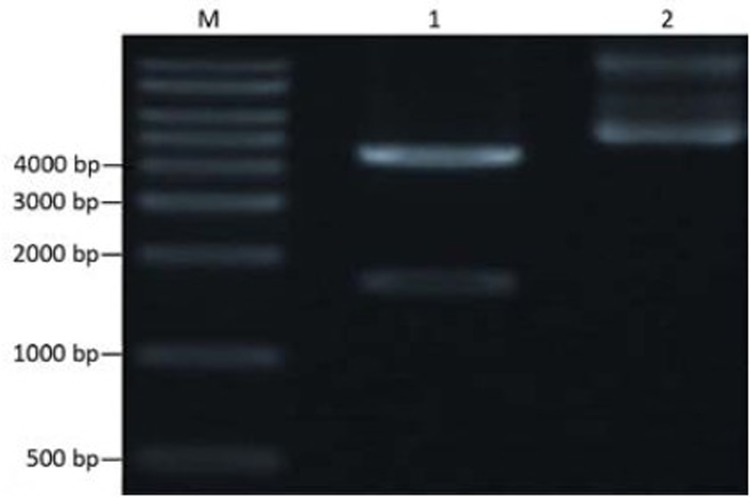
The cloning vector pUC57-DT388sBAFF was double digested with Nde I and Xho I, and the targeting fusion gene fragment, diphtheria toxin-human BAFF gene, was isolated. Agarose gel electrophoresis showed the fragment at 1,629 bp, which was consistent with the target gene (Figure 1). The isolated target gene fragment was inserted into the corresponding site of pcold II to construct the recombinant expression vector which was transformed into E. coli DH5α and then identified by colony PCR (Figure 2). The recombinant plasmid DNA was confirmed by the double restriction digestion analysis (Figure 3), and its sequencing result was identical with the optimal target gene sequence.
Figure 1.
Restriction map of cloning vector pUC57-DT388sBAFF. M: DNA marker; 1: Plasmid digested by Nde I and Xho I; 2: pUC57-DT388sBAFF.
Figure 2.
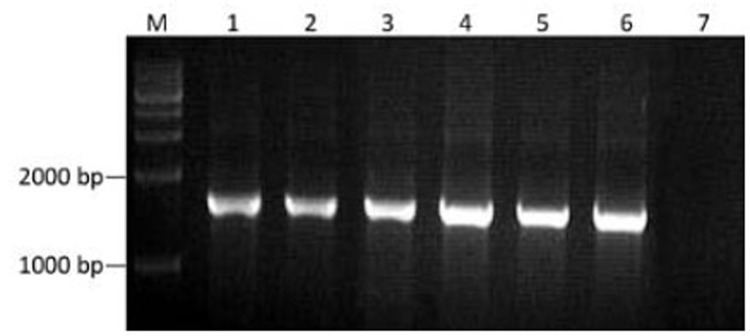
Bacterial colony PCR identification of the recom-binant expression vector. M: DNA marker; 1–6: Positive recombinant; 7: Negative recombinant.
Figure 3.
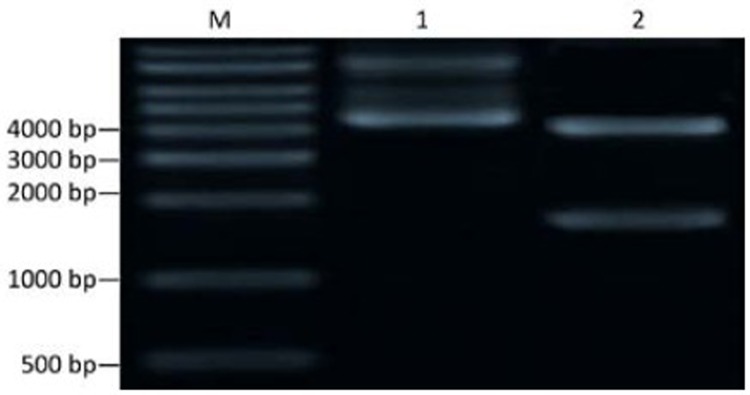
Restriction map of recombinant plasmid pcold II/ DT388sBAFF. M: DNA marker; 1: pcold II/DT388sBAFF; 2: Plasmid digested by Nde I and Xho I.
Expression of Recombinant Protein
SDS-PAGE showed that pcold II-DT388sBAFF was positively induced, and a distinct protein band with the relative molecular mass of 58.4 kD was visible, which was consistent with the expected protein size (Figure 4). Image analysis demonstrated that the target protein accounted for about 50% of the total bacterial proteins.
Figure 4.
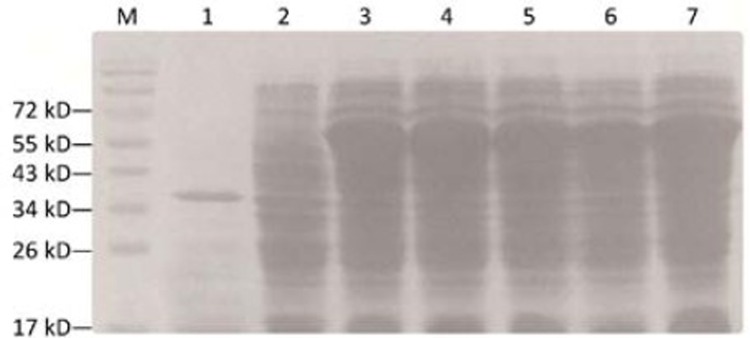
SDS-PAGE of recombinants. M: Protein marker; 1: pcold II induced at 18°C for 24 h; 2: pcold II/DT388sBAFF before induced; 3–7: pcold II/DT388sBAFF induced at 18°C for 24 h.
Identification of Recombinant Protein
The recombinant protein was identified by Western blot with anti-BAFF and polyclonal anti-His-Tag. The results showed that the two antibodies reacted specifically with the target protein, indicating that the recombinant protein was the specific DT388sBAFF fusion protein (Figure 5).
Figure 5.
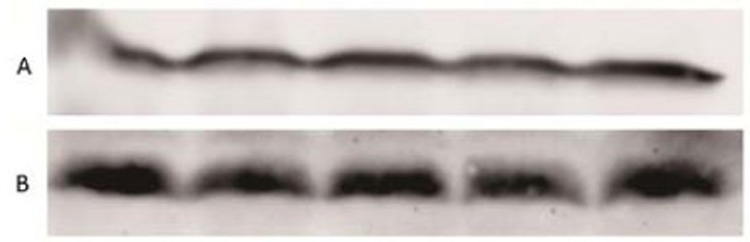
Western blot identification of recombinant protein. A: Anti-BAFF polyclonal antibody; B: Anti-His-Tag polyclonal antibody
Purification of the Recombinant Protein
Recombinant bacterial lysate, ultrasonic super-natant and ultrasonic inclusion bodies were analyzed by SDS-PAGE, showing that the recombinant protein was present in the supernatant and suggesting that the expression of DT388sBAFF was in a soluble form in strain cytoplasm. After purification by Ni2+-NTA affinity chromatography, the purity of the recombinant protein DT388sBAFF was more than 90% (Figure 6).
Figure 6.
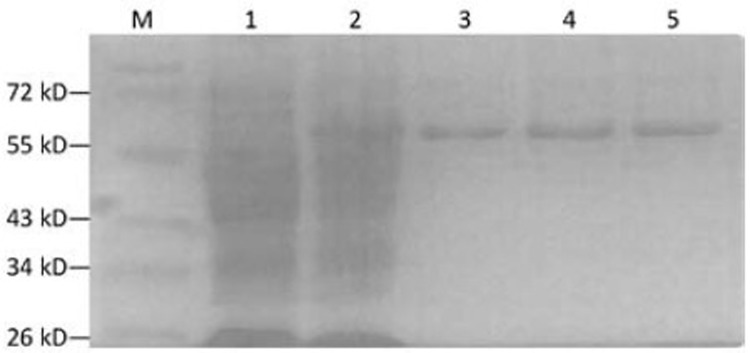
SDS-PAGE analysis of purified products. M: Protein marker; 1: pcold II/DT388sBAFF before induced; 2: pcold II/ DT388sBAFF induced at 18°C for 24 h; 3–5: Purified protein.
Expression of BAFF-R mRNA
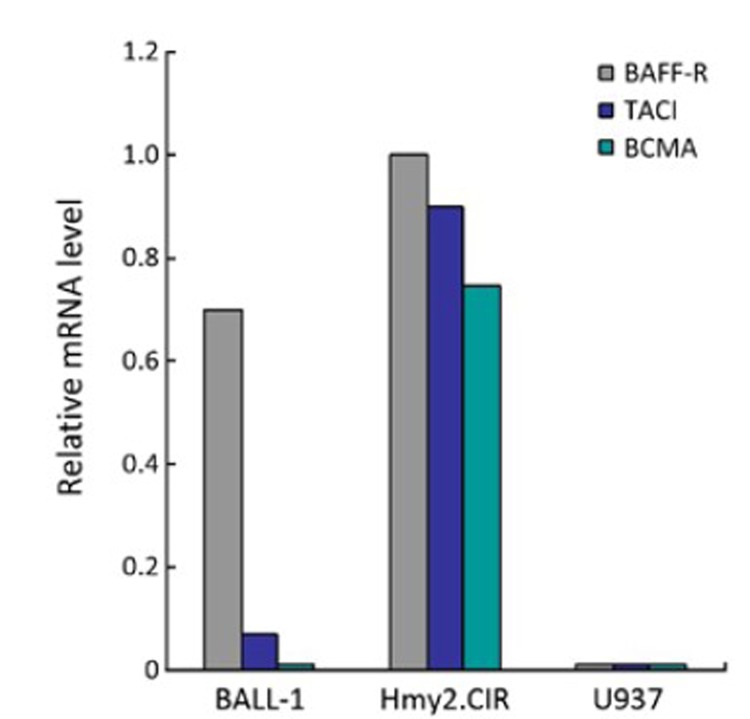
The mRNA expression of BAFF receptors (BAFF-R, TACI and BCMA) in the three cell lines was examined by real-time PCR. Hmy2.CIR and U937 cells were used as positive and negative controls. Surprisingly, BALL-1 cells showed high expression of BAFF-R (relative expression level=0.7, relative expression level of Hmy2.CIR cells=1), weakly positive expression for TACI (relative expression level=0.1), but negative for BCMA. U937 cells showed no or undetectable mRNA expression of BAFF receptors (Figure 7).
Figure 7.
mRNA levels of BAFF receptors in BALL-1, Hmy2.CIR and U937 cells assessed by real-time PCR. The mRNA levels were normalized with GAPDH. BALL-R relative expression levels in BALL-1, Hmy2.CIR and U937 cells were respectively 0.7, 1.0, and 0/undetectable, P<0.05; TACI relative expression levels were respectively 0.07, 0.9 and 0/undetectable, P<0.05; BCMA relative expression levels were respectively 0, 0.75 and 0/undetectable, P<0.05.
Biological Activity of Recombinant Protein
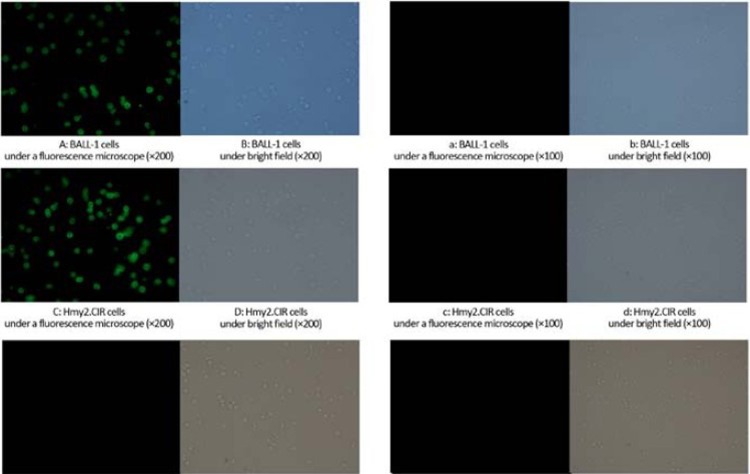
Detection of the fluorescent-labeled recombinant protein showed its ability of binding to cell surface receptors. Under the inverted fluorescence microscope, strong green fluorescence could be observed in BALL-1 and Hmy2.CIR cells, but the negative control group did not show fluorescence in U937 cells (Figure 8), indicating that there was a specific binding ability between the recombinant protein and surface receptors on BALL-1 and Hmy2.CIR cells. Whereas the BALL-1, Hmy2.CIR and U937 cells with fluorescent-labeled free diphtheria toxin did show fluorescence not all. Cytotoxicity assay demonstrated a killing effect of the recombinant protein on BALL-1 and Hmy2.CIR cells. The results showed a strong inhibitory effect in a dose-
Figure 8.
Cell binding activity of recom-binant protein. A–F: Cell binding activity of fluorescent-labeled recombinant protein; a–f: Cell binding activity of fluorescent-labeled free diphtheria toxin. Under the inverted fluorescence micros-cope, strong green fluorescence could be observed in BALL-1 (Figure 8A) and Hmy2.CIR (Figure 8C) cells with fluorescent-labeled recombinant protein (P<0.05), whereas the negative control group did not show fluorescence in U937 cells (Figure 8E, P<0.05) and fluorescent-labeled free diphtheria toxin BALL-1 (Figure 8a, P<0.05), Hmy2.CIR (Figure 8c, P<0.05) and U937 cells (Figure 8e, P<0.05), indicating that there was a specific binding ability between the recombinant protein and surface receptors on BALL-1 and Hmy2.CIR cells.
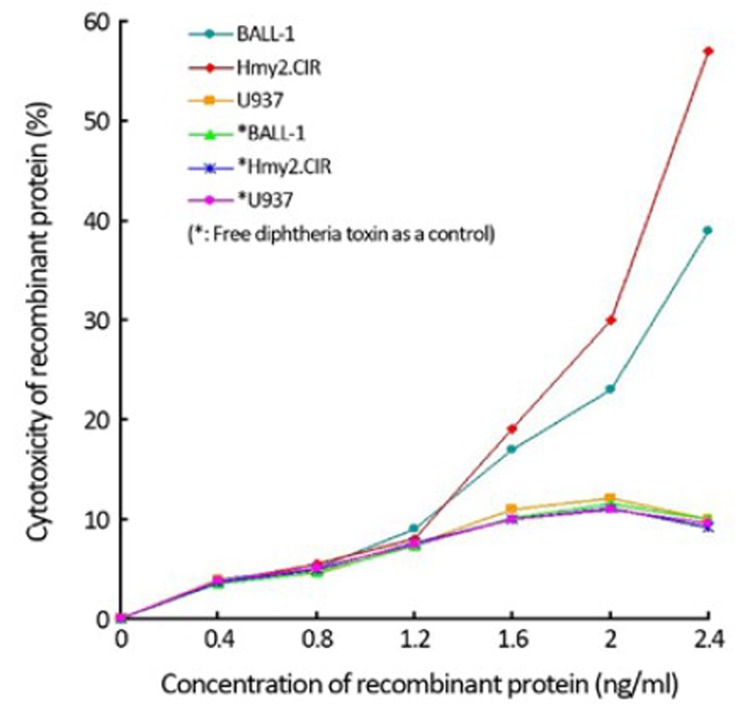
dependent manner, meaning that such inhibitory effect increased with higher protein concentration and was higher in Hmy2.CIR cells than in BALL-1 cells. The negative control groups BALL-1 and Hmy2.CIR with free diphtheria toxin and U937 cells did not exhibit such inhibitory effect (Figure 9).
Figure 9.
Specific cytotoxicity of recombinant protein on B cell line. The figure showed a strong inhibitory effect in a dose-dependent manner, suggesting that such inhibitory effect increased with increased protein concentration and Hmy2.CIR cells were more remarkable than BALL-1 cells (P<0.05). The negative control group BAFF-R (−) U937 cells did not exhibit such inhibitory effect (P<0.05); and BALL-1, Hmy2.CIR and U937 cells with free diphtheria toxin did not also show such inhibitory effect (P<0.05).
DISCUSSION
Immunotoxin has both specific recognition and anti-toxin function. Therefore, they have been extensively researched for applications in cancer, autoimmune diseases, transplant rejection and many other disorders. Some drugs have been approved by the US Food and Drug Administration for production, whereas many others are in pre-clinical or clinical studies[14]. Carried into the target cells by the targeting protein of immunotoxin, the toxin inhibits protein synthesis in these cells and thereby causes the death of tumor cells, achieving a specific killing effect without damage to normal tissues[15, 16].
In 1990s, the recombinant immunotoxin technology emerged with the development of molecular biology techniques[17-19]. In this technology, the encoding gene of a toxin and the ligand gene of a specific tumor cell surface molecule are fused into a recombinant plasmid. After expression in prokaryotic or eukaryotic cells and purification, a new type of immunotoxin which is stabile, uniform, more permeable, less immunogenic, less molecularly weighted and easy for large-scale production is obtained. The immunotoxin technology brings a new hope to the treatment of autoimmune diseases, as immunotoxins play important roles in the immune process of killing target cells.
BAFF is a type II transmembrane protein with N terminal intracellular domain, absent signal peptide, and C terminal extracellular domain which is highly homologous with TNF- and APOL-related leukocyte expressed ligand 2 (APRIL) of the TNF superfamily[20]. BAFF exists in two forms: membrane-bound and soluble ligands. The former consists of 285 amino acids, among which amino acid 47 to 73 are transmembrane domain and 74 to 285 are the extracellular region which is formed by the extracellular domain falling off under the action of the protease and has the functions of binding and activating B cells. Studies have shown that BAFF has three receptors: BCMA, TACI and BAFF-R (BR3), all of which are expressed mainly on B cells. BAFF and its receptor BAFF-R are important for survival and growth of mature normal B cells.
B-lineage acute lymphoblastic leukemia (ALL) originates from transformation of progenitor (pre-B) cells, and it is generally believed that BAFF-R is not expressed on pre-B cells. But a recent study[9] had found that BAFF-R is a pre-B leukemia-specific cell surface antigen and thus opened the possibility of using the receptor-mediated pathway to deliver a certain fusion toxin to ALL cells as an adjuvant therapeutic strategy. Another research[21] had demonstrated a diphtheria toxin interleukin-3 fusion protein [DT(388)IL3] can improve the effectiveness of tyrosine kinase inhibitors (TKIs), such as imatinib and dasatinib, against Ph chromosome-positive [Ph(+)] acute leukemia cells. The target cell line in this study was human BALL-1 cells, B-lineage human acute lymphoblastic leukemia cell line; and the positive and negative control cells were human B lymphoblast cells Hmy2.CIR and human monoblastic leukemia cells U937, respectively.
The immunotoxin designed in this research comprised smBAFF, the mutant of soluble BAFF in which amino acid 217 to 224 were deleted, as the targeting moiety which was necessary to maintain the activation of BAFF[22]. The mutant retained the activity of binding to receptors, whereas the B-cell proliferation activity was significantly reduced, thus eliminating the potential side effects of the immunotoxin stimulated B-cell proliferation. In this study, the active fragment of diphtheria toxin DT388, a cytotoxic molecule and BAFF, a targeting molecule, between which a flexible linker (GGGGS) was inserted, could effectively maintain protein conformation at both ends to facilitate their respective functions. To improve the expression level of fusion protein in E. coli, the codon of target gene and the mRNA secondary structure had been optimized to enhance the expression of recombinant immunotoxins. The target protein obtained from the genetically engineered strain accounted for about 50% of the bacterial proteins.
Biological activity studies have shown that the DT388sBAFF fusion protein possessed the receptor-specific binding activity and targeted cytotoxicity to BALL-1 and Hmy2.CIR cells. Among BAFF receptors, only BAFF-R was detected to express highly on both BALL-1 and Hmy2.CIR cells. TACI and BCMA showed weakly or undetectable mRNA on BALL-1 cells. This finding indicates that BAFF-R plays a key role in the inhibitory effect, and established an experimental foundation for its application in the biological treatment of B-lineage acute lymphoblastic leukemia.
REFERENCES
- 1.Kreitman RJ. Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs 2009; 23:1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J 2006; 8:E532-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyu MA, Cao YJ, Mohamedali KA, et al. Cell-targeting fusion constructs containing recombinant gelonin. Methods Enzymol 2012; 502:167-214 [DOI] [PubMed] [Google Scholar]
- 4.FitzGerald DJ, Wayne AS, Kreitman RJ, et al. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res 2011; 71:6300-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166:6-10 [DOI] [PubMed] [Google Scholar]
- 6.Zhou T, Zhang J, Carter R, et al. BLyS and B cell autoimmunity. Curr Dir Autoimmun 2003; 6:21-37 [DOI] [PubMed] [Google Scholar]
- 7.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol 2007; 19:327-36 [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Davidson A.BAFF and selection of autoreactive B cells. Trends Immunol 2011; 32:388-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossen C, Schneider P.BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol 2006; 18:263-75 [DOI] [PubMed] [Google Scholar]
- 10.Ng LG, Sutherland AP, Newton R, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 2004; 173: 807-17 [DOI] [PubMed] [Google Scholar]
- 11.Parameswaran R, Müschen M, Kim YM, et al. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res 2010; 70:4346-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmanapalli R, Lyu MA, Du M, et al. The growth factor fusion construct containing B-lymphocyte stimulator (BLyS) and the toxin rGel induces apoptosis specifically in BAFF-R-positive CLL cells. Blood 2007; 109:2557-64 [DOI] [PubMed] [Google Scholar]
- 13.Lyu MA, Cheung LH, Hittelman WN, et al. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol Cancer Ther 2007; 6:460-70 [DOI] [PubMed] [Google Scholar]
- 14.Langat DL, Wheaton DA, Platt JS, et al. Signaling pathways for B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) in human placenta. Am J Pathol 2008; 172:1303-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern C, Cornuel JF, Billard C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 2004; 103:679-88 [DOI] [PubMed] [Google Scholar]
- 16.Cohen KA, Liu TF, Cline JM, et al. Safety evaluation of DT388IL3, a diphtheria toxin/interleukin 3 fusion protein, in the cynomolgus monkey. Cancer Immunol Immunother 2005; 54:799-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descotes J.Methods of evaluating immunotoxicity. Expert Opin Drug Metab Toxicol 2006; 2:249-59 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Song S, Kou G, et al. Treatment of hepatocellular carcinoma in a mouse xenograft model with an immunotoxin which is engineered to eliminate vascular leak syndrome. Cancer Immunol Immunother 2007; 56:1775-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansfield E, Amlot P, Pastan I, et al. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood 1997; 90:2020–6 [PubMed] [Google Scholar]
- 20.Liu Y, Xu L, Opalka N, et al. Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell 2002; 108:383-94 [DOI] [PubMed] [Google Scholar]
- 21.Kim HP, Frankel AE, Hogge DE. A diphtheria toxin interleukin-3 fusion protein synergizes with tyrosine kinase inhibitors in killing leukemic progenitors from BCR/ABL positive acute leukemia. Leuk Res 2010; 34:1035-42 [DOI] [PubMed] [Google Scholar]
- 22.Chiron MF. Recombinant immunotoxins and chimeric toxins for targeted therapy in oncology. Bull Cancer 1997; 84:1135-40 [PubMed] [Google Scholar]