Abstract
Objective
Experimental evidence suggests that the overexpression of breast cancer-specific tumor suppressor protein 1 (BRCA1) gene enhances sensitivity to docetaxel and resistance to cisplatin and ribonucleotide reductase M1 (RRM1) gene overexpression enhances resistance to gemcitabine. To further examine the effect of BRCA1 and RRM1 mRNA levels on outcome in advanced non-small cell lung cancer (NSCLC), we performed this non-randomized phase II clinical trial which tested the hypothesis that customized therapy would confer improved outcome over non-customized therapy.
Methods
RNA was isolated from fresh tumor tissue. Patients received chemotherapy regimen based on their BRCA1 and RRM1 mRNA levels: both low–cisplatin plus gemcitabine (GP); both high–vinorelbine plus cisplatin (NP); BRCA1 low and RRM1 high–cisplatin plus docetaxel (TP); BRCA1 high and RRM1 low–vinorelbine plus gemcitabine (GN).
Results
From Dec 2005 to Nov 2008, 94 metastatic and locally advanced NSCLC patients from our institute were enrolled in this study. The median age was 58 years old. Among them, 21 patients received GP, 30 patients received TP and 43 patients received NP chemotherapy. GP group had a higher response rate, and longer median time to progression (TTP) and median overall survival (OS) time than the other 2 groups. The response rates in the GP, TP and NP groups were 42.9%, 36.7% and 27.9%, respectively (P=0.568). The median TTP was 5.6, 5.0, 4.8 months (P=0.975), respectively, and the median OS time was 12.5, 11.0, 9.7 months (P=0.808), respectively.
Conclusion
Chemotherapy customized according to BRCA1 and RRM1 expression levels is associated with higher response rate and longer TTP and OS time in the GP group. This suggests that BRCA1 and RRM1 mRNA levels could be used as biomarkers in individual therapy in NSCLC.
Key words: Individualized chemotherapy, Non-small cell lung cancer, BRCA1, RRM1
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in China and throughout the world. More than 70% of non-small cell lung cancer (NSCLC) patients have advanced disease at the time of diagnosis[1, 2]. In the past decades, platinum-based chemotherapy has established itself as the standard treatment for the 1st line treatment of locally advanced or metastatic NSCLC. Compared with best supportive care, it provides slight but significant improvement of the overall survival (OS): the OS is only improved by 1−2 months and one year survival rate by 10%−20%. Many phase III trials and meta-analyses[3-5] showed that all the studied platinum-based doublets had similar efficacy in the 1st line therapy of advanced NSCLC. Response rate was about 15% to 30%, progression free survival about 46 months and OS about 8−10 months. Newer combination regimens did not further improve the efficacy when compared to platinum-based doublets. So, the 1st line chemotherapy treatment has reached its plateau in efficacy and the outcome of patients with advanced NSCLC is still poor[2].
With the recent advances in pharmacogenomics research, it is possible to tailor chemotherapy in advanced NSCLC patients to improve the efficacy and reduce the toxicity of chemotherapy according to expression levels or polymorphisms of one or several genes[6-10]. Aimed at improving efficacy of platinum-based chemotherapy, a phase III trial[11] based on chemotherapy tailored by excision repair cross-complementation group 1 (ERCC1) mRNA expression level in advanced NSCLC was carried out. Patients in the control arm received docetaxel plus cisplatin while patients with low ERCC1 level received docetaxel/ cisplatin and those with high levels of ERCC1 were given gemcitabine/docetaxel. The results showed that objective response rate (ORR), primary endpoint, was statistically improved in the genotypic arm compared with the control arm. Simon, et al.[12] also found that therapeutic decision making based on ribonucleotide reductase M1 (RRM1) and ERCC1 gene expression level for patients with advanced NSCLC is feasible and promising for improvement in patient outcome. Likewise, together with other authors, we[8-10,13-15] have previously described a strong association of both RRM1 and breast cancer-specific tumor suppressor protein 1 (BRCA1) genes to the therapeutic benefit derived from gemcitabine, platinum and taxane. Both genes are critical components in the DNA synthesis and DNA damage repair pathways[14-16]. This article provides results from a prospective, single-institution phase II clinical trial that utilized tumoral expression of the RRM1 and BRCA1 genes for selecting double-agent chemotherapy.
MATERIALS AND METHODS
Subjects
Patients were eligible if they were chemonaive staged IV or IIIB (with malignant pleural effusion) NSCLC patients with confirmed histopathological diagnosis. Other eligibility criteria included: Eastern Cooperative Oncology Group performance status (ECOG PS) of 0−2, aged over 18 years, adequate hematological function (hemoglobin >9 g/dl, neutrophil count >1,500/mm3, and platelet count >100,000/mm3), renal function (creatine clearance rate >50 ml/s) and liver function (bilirubin <1.5 times the normal upper limit, aspartate aminotransferase and alanine aminotransferase <2 times the normal upper limit), and a measurable disease. Patients with symptomatic brain metastases, spinal cord compression, uncontrolled massive pleural effusion, and previous chemotherapy were excluded. The histology diagnosis of tumors was based on criteria of the World Health Organization, and the TNM stage was determined according to criteria revised in 1997. The study was approved by the Ethics Committee of Tongji University Affiliated Shanghai Pulmonary Hospital and written informed consent was obtained from each participant before the initiation of any study related procedure.
Treatment
Trial participation required a dedicated biopsy of the tumor specifically for gene expression analysis performed by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR). Predetermined values for RRM1 and BRCA1 were used for decisions regarding use of the drugs gemcitabine, cisplatin and taxane. The cutoff values for RRM1 and BRCA1 mRNA expression, relative to the expression of the internal control housekeeping gene β-actin, were 2.65×10-2 and 1.1×10-3 adapted from the median value in a large sample examination in our institute.
This strategy resulted in four possible gene expression strata with the following doublet therapies: the low RRM1 and low BRCA1 group [gemcitabine and cisplatin (GP) group] was treated with gemcitabine (1,000 mg/m2 on d 1 and 8) and cisplatin (75 mg/m2 on d 1 or separated on d 1−2) every 21 d. The high RRM1 and high BRCA1 group [vinorelbine and cisplatin (NP) group] was treated with vinorelbine (25 mg/m2 on d 1 and 8) and cisplatin (75 mg/m2 on d 1 or separated on d 1−2) every 21 d. The high RRM1 and low BRCA1 group [docetaxel and cisplatin (TP) group] was treated with docetaxel (75 mg/m2 on d 1) and cisplatin (75 mg/m2 on d 1 or separated on d 1−2) every 21 d. The low RRM1 and high BRCA1 group [gemcitabine and vinorelbine (GN) group] was treated with gemcitabine (1,000 mg/m2 on d 1 and 8) and vinorelbine (25 mg/m2 on d 1 and 8) every 21 d.
Chemotherapy was repeated every 3 weeks for a maximum of six cycles. If patients developed more than grade 3 non-hematology toxicity (except alopecia and nausea/vomiting) and grade 4 hematology toxicity, febrile neutropenia or infection and/or thrombo-cytopenia associated with bleeding, doses of the cytotoxic agents in the following cycles were reduced by 25%. After the treatment, patients were followed up every 6 weeks up to disease progression, and then every 3 months up to death.
Clinical Assessments
All patients underwent staging procedures at baseline, including physical examination and a computed tomography (CT) scan of the thorax and upper abdomen. Bone scans, CT scans or brain magnetic resonance image (MRI) were performed if bone or brain metastases were suspected. Before each chemotherapy cycle, patients underwent physical examination, and biochemical and hematological testing.
Objective tumor responses were evaluated in accordance with the Response Evaluation Criteria in Solid Tumors guidelines[17] every two cycles. Response was confirmed after 4 weeks of treatment. Progression-free survival was calculated from the date of chemo-therapy to disease progression or death, whichever came first. OS was calculated from the date chemotherapy was started to the date of death or last clinical follow-up.
RNA Isolation and cDNA Synthesis
Total RNA isolation from fresh tumor tissue was performed according to a protocol by Shanghai Huashun Biotech Company, China. Extracted total RNA was dissolved in 50 μl of 5 mmol/L Tris-HCl. For cDNA synthesis, 2 μl of above solution was preserved in 70°C for 10 min, followed by adding 4 μl of 25 mmol/L MgCl2, 2 μl of 10× reverse transcriptase buffer, 2 μl of 10 mmol/L dNTP, 0.5 μl of RNAse inhibitor, 0.5 μg of Oligo(dT)15, and 15 U of avian myeloblastosis virus (AMV) reverse transcriptase to a total volume of 20 μl. Reverse transcription reaction was carried out at 42°C for 15 min.
Real-time PCR for ERCC1 mRNA Expression
Relative cDNA quantitation for RRM1 and BRCA1 was done using a fluorescent, real-time detection method (Light 2 Cycler 2.0 from Roche Company Hoffmann-LaRoche Ltd., Swiss) with an internal reference gene (β-actin) as control. The primers and probe sequences used are shown in Table 1. Amplification was carried out in a total volume of 25 μl containing 0.25 μmol/L of each primer, 0.02 mmol/L dNTPs and 1 mmol/L MgCl2, 1.25U Taq polymerase and 5× PCR buffer. The PCR program initiated with 1 min denaturation at 95ºC. The DNA was amplified by one cycle of 95ºC for 5 s and 50 cycles of 92ºC for 40 s, followed by elongation at 60ºC for 40 s. The gene expression analysis was performed in a blinded fashion where the laboratory investigators were unaware of the clinical data.
Table 1. Primes and probes for gene analysis.
| RRM1 | BACR1 | |
|---|---|---|
| Probe | 5′-AAGGTGGGAACAAGCGTCCTGGG-3′ | 5′-GACTGGGTCACCCAGAAATA-3′ |
| Forward primer | 5′-TGGCCTTGTACCGATGCTG-3′ | 5′-CCCATTTTCCTCCCGCA-3′ |
| Reverse primer | 5′-GCTGCTCTTCCTTTCCTGTGTT-3′ | 5′-GGACCTTGGTGGTTTCTTCCA-3′ |
Statistical Analyses
The association between RRM1 or BRCA1 mRNA level and efficacy variables was evaluated using the χ2-test or Fisher’s exact test. The logistic regression model was used for multivariate analysis of response. Time to progression (TTP) was measured from time at which chemotherapy was started to the time of disease progression or last visit. OS was measured from the start of chemotherapy to the date of death from any cause or the date of last visit. Survival curves were plotted using the Kaplan-Meier curve and compared with the log-rank test. The association between the different mRNA expression and survival was estimated by computing the hazard ratios and its 95% confidence interval (CI) from both univariate and multivariate Cox regression models. Statistical significance was set at 5%. All tests were two-sided and analyses were carried out with SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient Characteristics
A total of 97 patients with stage IIIB or IV NSCLC were registered in the trial and underwent the required biopsy: 45 had CT-guided lung biopsies, 36 had bronchoscopy-guided lung biopsies, and 16 had biopsies from organs other than lung. However, samples from 3 patients were not available for gene expression analysis because the samples contained necrosis cells or inflammatory cells. Finally, 94 patients were included in this study. There was one procedure associated complication due to the CT-guided lung biopsy which resulted in a small pneumothorax that spontaneously resolved.
Patient baseline characteristics are shown in Table 2. Of the patients, 18.1% had stage IIIB disease with pleural effusion and 81.9% had stage IV disease. Median age was 58 years (range: 40−78 years). Among them, 75.5% were male, 45.5% had adenocarcinoma and 64.9% had a history of smoking. No patient received thoracic radiotherapy before enrollment into this study.
Table 2. Patient characteristics at the baseline.
| Characteristics | N (%) |
|---|---|
| Median age (year) | 58 |
| Gender | |
| Male | 71 (75.5) |
| Female | 23 (24.5) |
| Smoking history | |
| Never-smoker | 33 (35.1) |
| Smoker | 61 (64.9) |
| Histopathology | |
| Squamous carcinoma | 38 (40.4) |
| Non-squamous | 56 (59.6) |
| ECOG Performance status | |
| 0 | 38 (40.4) |
| 1 | 56 (59.6) |
| Stage | |
| IIIB | 17 (18.1) |
| IV | 77 (81.9) |
Efficacy
RRM1 and BRCA1 mRNA were detectable in all 94 samples analyzed. The cutoff values of RRM1 and BRCA1 mRNA expression, relative to the expression of the internal control housekeeping gene β-actin, were 2.65×10-2 and 1.1×10-3 adapted from the median value in a large sample examination in our institute. The expression of RRM1 mRNA was significantly correlated with the expression of BRCA1 mRNA (P<0.01). Twenty- one patients were treated with GP regimen while 30 and 43 patients were treated with TP and NP respectively; no patients were treated with GN regimen.
The median number of treatment cycles was 4. Ninety-four patients were available for tumor response assessment and there was no complete response. Thirty-two (34%) patients achieved partial response (PR) and 38 (40.4%) patients had stable disease. A total of 78 patients were dead on the last date of follow up (Oct 10, 2010). All the 16 remaining live patients had progressive disease. The overall response rate was 34% and disease control rate (DCR, the rate of response plus stable disease,) was 74.4%. The median OS was 11 months (95% CI, 9.4 to 12.6 months) and the median TTP was 5 months (95% CI, 4.4 to 5.7 months) (Table 3).
Table 3. Efficacy of patients according to the treatment groups.
| Regimen | N | Response |
TTP | OS | ||
|---|---|---|---|---|---|---|
| PR | SD | PD | ||||
| TP | 30 | 11 | 10 | 9 | 5.0 | 11.0 |
| GP | 21 | 9 | 9 | 3 | 5.6 | 12.5 |
| NP | 43 | 12 | 19 | 12 | 4.8 | 9.7 |
| Total | 94 | 32 | 38 | 24 | 5.0 | 11.0 |
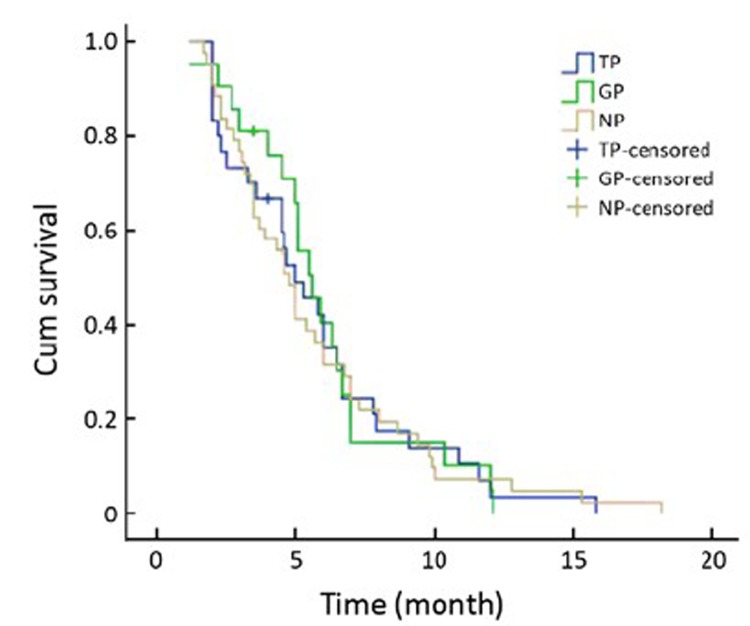
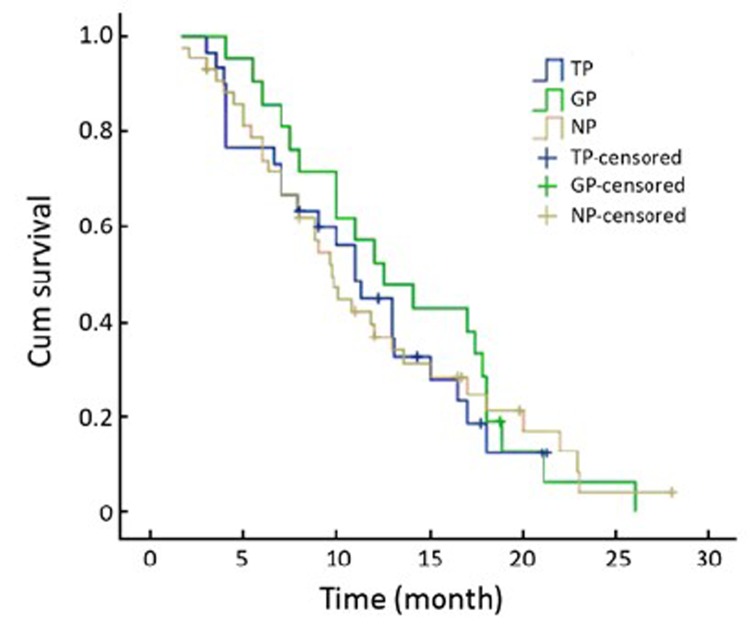
There was no significant difference in the response to therapy and the TTP and OS according to different expression level of RRM1 and BRCA1 mRNA. However, the gemcitabine-cisplatin treatment group experienced a higher response rate, and longer median TTP and median OS time when compared to the other 2 groups. The response rates in the GP, TP and NP groups were 42.9%, 36.7% and 27.9%, respectively (P=0.568). The median TTP was 5.6, 5.0, and 4.8 months (P=0.975), respectively, and the median OS was 12.5, 11.0, and 9.7 months (P=0.808), respectively (Table 3, Figure 1, 2).
Figure 1.
Median TTP according to treatment groups in the 94 patients.
Figure 2.
Median OS according to treatment groups in the 94 patients.
Toxicity
No symptomatic toxicities or complications requiring intervention were observed after tumor biopsy. No unexpected chemotherapy related side effect occurred when the patient received the treatment according to the different gene mRNA expression level of RRM1 and BRCA1. Grade 3/4 myelosuppression included neutropenia (28 patients), thrombocytopenia (11 patients) and anemia (6 patients). No symptomatic grade 4 toxicities were noted. Severe grade 3 symptomatic toxicities included fatigue (6 patients), pain (3 patients), nausea and vomiting (3 patients), hypersensitivity reaction (1 patient), haemoptysis (1 patient) and dyspnea (1 patient).
DISCUSSION
It is well known that the current standard first-line chemotherapies where paclitaxel, gemcitabine, docetaxel, or vinorelbine is used in combination with a platinum compound, provide only modest improvement regarding the objective response rate and survival when compared with best supportive care. First line therapy has reached an “efficacy plateau” in NSCLC[3-5] and individualized chemotherapy based on the molecular biomarkers could help increase the response rate and prolonged survival[11,12,14]. A retrospective study indicated that expression of ERCC1 and class III beta-tubulin might be useful for predicting survival in NSCLC patients receiving carboplatin and paclitaxel for recurrent disease after radical tumor resection. This observation could be applied in personalized chemotherapy and it inspired us to explore the predictive value of other genes[17].
To test whether the selection of chemotherapy based on gene expression level is feasible and if it will improve patients’ survival, we conducted a phase II single-institution treatment trial in patients with advanced and incurable NSCLC. In this study, the decision for choosing the double-agent chemotherapy regimen was based on the level of expression of the RRM1 and BRCA1 genes. A fresh lung cancer tissue obtained from core needle biopsy, fibroscopy or biopsy from the metastatic lesion was required for study participation. Specimens were immediately frozen, sectioned, and subjected for tumor cell collection and mRNA expression analysis of RRM1 and BRCA1.
RRM1, located on chromosome segment 11p15.5, a region with a frequent loss of heterozygosity in NSCLC, was found to be associated with gemcitabine resistance. Published data already suggested that patients with low RRM1 level benefited significantly from cisplatin/gemcitabine in resected lung cancer. Rosell, et al.[14] also found RRM1 mRNA expression is a crucial predictive marker for the survival of the advanced NSCLC patients receiving gemcitabine/cisplatin. BRCA1 mRNA overexpression increases the tumor sensitivity to paclitaxel or docetaxel and causes resistance to platinum. On the contrary, decreased BRCA1 expression could enhance cisplatin sensitivity and resistance to antimicrotubule agents[16]. Several studies found that when combining the detection of DNA repair enzyme expression in tumor cells, BRCA1 could possibly affect the sensitivity to anti-cancer agents[18-20] and a simple molecular assay to determine its genotype could be useful for customizing chemotherapy[21]. BRCA1 expression leads to resistance to cisplatin and etoposide and sensitivity to paclitaxel, docetaxel and vinorelbine[22].
Our data suggest that treatment of patients with advanced NSCLC based on the intratumoral expression of RRM1 and BRCA1 results in promising outcome with a response rate of 42.9%, a median TTP of 5.6 months, and a median OS time of 13.3 months in the GP subgroup. Due to the controversial functional knowledge about the BRCA1 mRNA levels when this trial is initiated, there were drawbacks regarding the choice of chemotherapy regimen for the TP and NP group. Our data also tell us that NP group, which was an opposite regimen choice based on the current understanding of RRM1 and BRCA1 mRNA functions, had the worst response rate, shortest TTP and OS time. These results revealed that individual therapy strategy was favorable when comparing with our own prior experience in clinical data[23] for similar patient populations.
However, the following mentioned limitations of our study must be acknowledged. First, in this study, there were some restraints in chemotherapy regimen selection, especially the NP group. However, this contributed to the importance of gene guided chemotherapy through the opposite side. Second, this study consisted of 94 candidates in only one institute, and the relatively small number of patients may have limitation to be generalized in NSCLC patients. Third, this study is just a single arm observation phase II trial, lacking the high evidence of large number randomized data.
In conclusion, chemotherapy customized according to BRCA1 and RRM1 mRNA expression levels is associated with numerically higher response rate and longer TTP and OS time in the GP group, suggesting that BRCA1 and RRM1 mRNA levels could be used as biomarkers in individual therapy in NSCLC. A randomized clinical trial is undergoing in our institute to further confirm the finding in this article.
REFERENCES
- 1.Chen WQ, Zhang SW, Zou XN, et al. Cancer incidence and mortality in China, 2006. Chin J Cancer Res 2011; 23:3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camps C, Sirera R, Iranzo V, et al. Gene expression and polymorphisms of DNA repair enzymes: cancer susceptibility and response to chemotherapy. Clin Lung Cancer 2007; 8:369-75 [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92-8 [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3543-51 [DOI] [PubMed] [Google Scholar]
- 5.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007; 99:847-57 [DOI] [PubMed] [Google Scholar]
- 6.Ota S, Ishii G, Goto K, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer 2009; 64:98-104 [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Ishii G, Goto K, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer 2009; 65:105-11 [DOI] [PubMed] [Google Scholar]
- 8.Su C, Zhou S, Zhang L, et al. ERCC1, RRM1 and BRCA1 mRNA expression levels and clinical outcome of advanced non-small cell lung cancer. Med Oncol 201128:1411-7 [DOI] [PubMed] [Google Scholar]
- 9.Ren S, Zhou S, Zhang L, et al. High-level mRNA of excision repair cross-complementation group 1 gene is associated with poor outcome of platinum-based doublet chemotherapy of advanced nonsmall cell lung cancer patients. Cancer Invest 2010; 28:1078-83 [DOI] [PubMed] [Google Scholar]
- 10.Guo QZ, Wang J, Bai H, et al. High expression of ERCC1 is a poor prognostic factor in Chinese patients with non-small cell lung cancer receiving cisplatin-based therapy. Chin J Cancer Res 2010; 22:296-302 [Google Scholar]
- 11.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol 2007; 25:2747-54 [DOI] [PubMed] [Google Scholar]
- 12.Simon G, Sharma A, Li X, et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J Clin Oncol 2007; 25:2741-6 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007; 356:800-8 [DOI] [PubMed] [Google Scholar]
- 14.Rosell R, Danenberg KD, Alberola V, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 2004; 10:1318-25 [DOI] [PubMed] [Google Scholar]
- 15.Boukovinas I, Papadaki C, Mendez P, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One 2008; 3:e3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One 2007; 2:e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer lnst 2000; 92:205-16. [DOI] [PubMed]
- 18.Azuma K, Sasada T, Kawahara A, et al. Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer 2009; 64:326-33 [DOI] [PubMed] [Google Scholar]
- 19.Takenaka T, Yoshino I, Kouso H, et al. Combined evaluation of Rad51 and ERCC1 expressions for sensitivity to platinum agents in non-small cell lung cancer. Int J Cancer 2007; 121:895-900 [DOI] [PubMed] [Google Scholar]
- 20.de las Peñas R, Sanchez-Ronco M, Alberola V, et al. Polymorphisms in DNA repair genes modulate survival in cisplatin/gemcitabine-treated non-small-cell lung cancer patients. Ann Oncol 2006; 17:668-75 [DOI] [PubMed] [Google Scholar]
- 21.Vilmar A, Sørensen JB. Excision repair cross-complementation group 1 (ERCC1) in platinum-based treatment of non-small cell lung cancer with special emphasis on carboplatin: a review of current literature. Lung Cancer 2009; 64:131-9 [DOI] [PubMed] [Google Scholar]
- 22.Reguart N, Cardona AF, Carrasco E, et al. BRCA1: a new genomic marker for non-small-cell lung cancer. Clin Lung Cancer 2008; 9:331-9 [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Leaw SJ, Xu Y, et al. Comparison of cisplatin- and carboplatin-based third-generation chemotherapy in 1,014 Chinese patients with advanced non-small-cell lung cancer. Med Oncol 2011; 28:1418-24 [DOI] [PubMed] [Google Scholar]