Abstract
Objective
Human carbonic anhydrases II (CAII) gene plays an important role in different cancer. However, its relevance to gastric cancer (GC) remains unclear. In the present study, we aimed to investigate the expression of CAII in GC and explore its correlation with some clinicopathologic characteristics of GC.
Methods
The expression of CAII in 20 specimens of normal gastric mucosa, 38 specimens of intraepithelial neoplasia and 112 specimens of gastric carcinoma were detected by immunohistochemical techniques. Survival in GC with CAII expression was studied.
Results
The positive rate of CAII protein in normal gastric mucosa was significantly higher than that in intraepithelial neoplasia and gastric carcinoma (100% vs. 63.16% and 28.57%, P<0.001). The positive rate of CAII protein was significantly higher in gastric carcinoma at early stages than that at advanced stages (70.0% vs. 19.57%, P<0.001). The positive rate of CAII protein was significantly lower in gastric carcinoma with lymph node metastases than that without lymph node metastases (10.81% vs. 37.33%, P<0.05). Furthermore, the positive rate of CAII protein was significantly lower in poorly-differentiated gastric carcinoma than in moderately- or well-differentiated gastric carcinoma (15.94% vs. 31.03% or 60.00%, P<0.05). Moreover, CAII expression was not related with sex, age and tumor size. The patients with CAII-positive tumors showed a better survival rate than those with CAII-negative tumors (P=0.024, log-rank test).
Conclusion
CAII expression was related with stages and lymph node metastases in gastric carcinoma. The reduction of CAII expression in GC might promote tumor cell motility and contribute to tumor growth and metastasis.
Key words: CAII, Gastric Carcinoma, Immunohistochemistry, Invasion, Metastasis
INTRODUCTION
Gastric cancer (GC) is the most common cancer in Eastern Asia and South America. Although the incidence in western countries is much lower than that in Asia, it is still a significant worldwide health burden, only second to lung tumors as a leading cause of cancer death[1]. The occurrence and development of gastric carcinoma is a complex multi-step process controlled by multiple factors. The activation of oncogenes (such as myc, K-ras, c-erbB-2 and K-sam) and deactivation of anti-oncogenes [such as p53, adenomatous polyposis coli (APC) and p16] play important roles. Therefore, it is important to identify new oncogenes involved in occurrence and development of gastric carcinoma for prognosis and clinical treatment of GC[2].
Carbonic anhydrase (CA) is a type of zinc-containing metalloenzyme. At least 16 types of CA isozymes have been reported in mammals with different tissue distribution and catalytic properties[3]. CAI, CAII, CAIX and CAXII are highly tumor-related[4,5], and among them, CAIX and CAXII are related to soft tissue sarcoma, breast cancer and non-small cell lung cancer[6-8]. Human CAII gene, which locates at chromosome 8q22, codes a cytoplasmic protein that has the highest turnover rate and widest tissue distribution among the known human CA isozymes[3]. CAII is correlated with several types of tumors[9-12]. In our previous study, the expression of CAII was down-regulated in GC[2]. However, the relationship between CAII expression and tumor invasion as well as metastasis in gastric carcinoma has not been examined. In the present study, we aimed to investigate the expression of CAII in GC and explore its correlation with some clinicopathologic characteristics of GC.
MATERIALS AND METHODS
Clinical Specimens
Retrospective analysis was performed on gastric carcinoma patients who had undergone gastrectomy between August 2004 and March 2007 in the pathological department of the 1st Hospital of Chenzhou. The mean age of the patients was 49 years, ranging from 23 to 79 years. A total of 78 males and 34 females (2.29:1) were included in our study, of which 65 patients were under 55 years old and 47 patients were over 55 years old. In terms of tumor size, 78 specimens were larger than 5 cm, and 36 specimens were smaller than 5 cm. According to tumor differentiation, we investigated 20 highly-differentiated tumors (17.86%), 29 moderately-differentiated tumors (25.89%) and 63 poorly-differentiated tumors (56.25%). In terms of the depth of tumor invasion, 20 (17.86%) and 92 (82.14%) patients were classified as early type and advanced type, respectively. Seventy-five (66.96%) patients were lymph node metastasis negative, whereas 37 (33.04%) were lymph node metastasis positive. No adjuvant radiotherapy or chemotherapy was administered on those patients before surgery. Multiple sections were microscopically examined to confirm the degree of differentiation, the depth of invasion and lymph node metastasis. One representative block was then selected for immunohistochemical study. Twenty normal mucosa and 38 intraepithelial neoplasia paraffin blocks derived from subtotal gastrectomy specimens because of duodenal ulcer. The study was approved by the Ethical Committee for Clinical Research of the Hospital.
Immunohistochemistry
Immunoperoxidase staining of formalin-fixed and paraffin-embedded tissue sections was performed by an ordinary biotin-streptavidin method. Briefly, sections were deparaffinized in xylene, heated with 10 mmol/L citrate buffer (pH 6.0) in a pressure cooker for 5 min and washed with phosphate-buffered saline (PBS, pH 7.2). In order to block endogenous peroxidase activity, sections were immersed in methanol containing 0.3% hydrogen peroxide (H2O2) at room temperature for 20 min. Then the sections were blocked with 10% normal calf serum in PBS for 10 min. Subsequently, the sections were incubated with anti-CAII mouse monoclonal antibody (1:200) (Santa Cruz Biotechnology Inc., USA) for 1 h in a humidified chamber. After incubating with secondary antibody and avidin-biotin complex reagent, color reaction was developed in 0.02% H2O2 in Tris buffer (pH 8.0). Hematoxylin was used for counterstaining. The positive control was positive slides bought from Santa Cruz Biotechnology Inc., USA. In addition, a parallel negative control without primary antibody was established.
Evaluation of Immunostaining
Yellow particles in the cytoplasm or cytomembrane meant positive staining. The scores were evaluated in terms of staining intensity as follows: 0, no reaction; +, weak reaction; ++, moderate reaction; and +++, strong reaction. In the statistical analyses, the specimens were grouped into two categories based on the staining intensity and positive cells: CAII(+) tumors, including more than 10% of neoplastic cells exhibiting moderate or strong reaction; and CAII(−) tumors, including less than 10% of neoplastic cells with moderate or strong reaction, or weak or negative immunostaining results.
Analysis of Survival Rate
We collected 112 patients’ postal address and telephone number from the medical record department of the 1st Hospital of Chenzhou. Then, we contacted patients or their family members, and collected 50 cases until July 2010. Finally, we drew the five-year survival curves.
Statistical Analysis
The correlation between CAII expression of neoplastic cells and clinicopathological factors was evaluated by chi-squared test or Fisher’s exact test. P<0.05 was considered statistically significant. The log-rank test was used in the survival analysis. All statistical analyses were carried out by using SPSS 13.0 (SPSS Inc., Chicago, IL, USA)
RESULTS
Immunostaining Analysis of CAII Expression in Normal Mucosa, Intraepithelial Neoplasia and GC
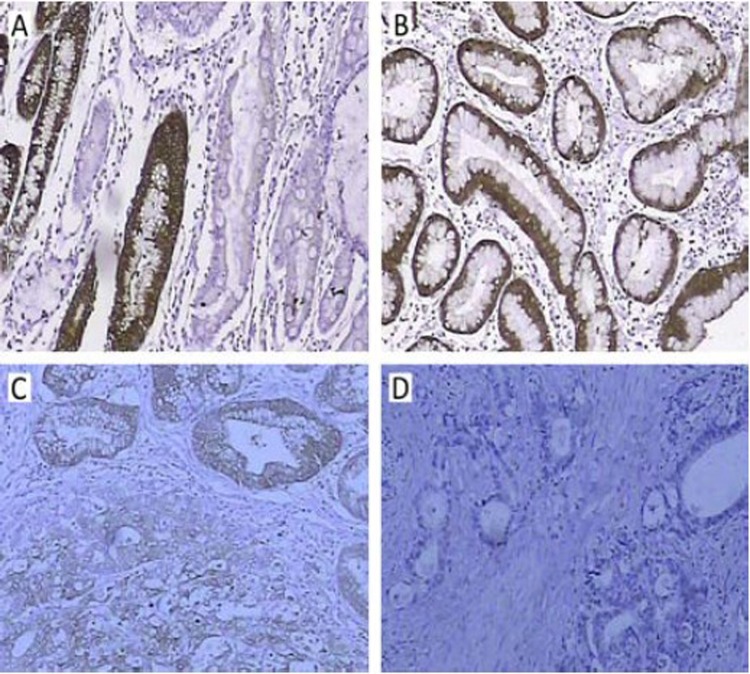
Table 1 shows that the positive rate of CAII protein was 28.57% (32/112) in 112 tumor specimens, 63.15% (24/38) in intraepithelial neoplasia, and 100% (20/20) in normal mucosa. Figure 1 shows that CAII protein was expressed in cytoplasm, and CAII expression at the protein level in normal mucosa was stronger than that in intraepithelial neoplasia and GC.
Table 1. Expression of CAII in normal mucosa, intraepithelial neoplasia and GC.
| Item | Cases | CAII positive (n, %) | CAII negative (n, %) | χ2 | P |
|---|---|---|---|---|---|
| Normal mucosa | 20 | 20 (100) | 0 (0) | ||
| Intraepithelial neoplasia | 38 | 24 (63.16) | 14 (36.74) | 41.765 | 0.000 |
| GC | 112 | 32 (28.57) | 80 (71.43) |
Figure 1.
Immunohistochemical staining of CAII protein was located in cytoplasm. A, B: normal gastric mucosa; C: intraepithelial neoplasia; D: GC.
Correlation between CAII Expression and Age or Sex
The expression rates of CAII was 29.48% and 26.47% in male and female GC specimens, respectively, and there was no correlation between CAII expression and age.
Correlation between CAII Expression and Tumor Size
We showed that 28.95% of the cases whose tumor sizes were smaller than 5 cm expressed CAII and 27.78% of the cases whose tumor sizes were larger than 5 cm expressed CAII. There was no correlation between CAII expression and tumor size.
Correlation between CAII Expression and Differentiation, Stage as well as Metastasis
Table 2 shows that differentiation, stage and lymph node metastasis were positively correlated with CAII expression among the 112 specimens with gastric carcinoma. The positive rate of CAII protein was significantly lower in poorly-differentiated gastric carcinoma than that of moderately- or well- differentiated gastric carcinoma (15.94% vs. 31.03% or 60.00%, P<0.05). The positive rate of CAII protein was significantly higher in gastric carcinoma at early stages than that at advanced stages (70.00% vs. 19.57%, P<0.001). Furthermore, the positive rate of CAII protein was significantly lower in gastric carcinoma with lymph node metastasis than that without lymph node metastasis (10.81% vs. 37.33%, P<0.05).
Table 2. Correlations between CAII expression and clinicopathologic characteristics of GC.
| Item | Cases | CAII positive (n, %) | CAII negative (n, %) | χ2 | P |
|---|---|---|---|---|---|
| Sex | 0.745 | 0.106 | |||
| Male | 78 | 23 (29.48) | 55 (70.56) | ||
| Female | 34 | 9 (26.47) | 25 (73.53) | ||
| Age (year) | |||||
| <55 | 65 | 18 (27.69) | 47 (72.31) | 0.809 | 0.059 |
| 55 | 47 | 14 (29.79) | 33 (70.21) | ||
| Tumor size (cm) | |||||
| <5 | 76 | 22 (28.95) | 54 (71.05) | 0.898 | 0.016 |
| 5 | 36 | 10 (27.78) | 26 (72.12) | ||
| Differentiation | |||||
| Well | 20 | 12 (60.00) | 8 (40.00) | 13.577 | 0.001 |
| Moderate | 29 | 9 (31.03) | 20 (68.97) | ||
| Poor | 63 | 11 (15.94) | 52 (84.06) | ||
| Stage | |||||
| Early | 20 | 14 (70.00) | 6 (30.00) | 20.477 | 0.000 |
| Advanced | 92 | 18 (19.57) | 74 (80.43) | ||
| Lymph node metastasis | |||||
| No | 75 | 28 (37.33) | 47 (63.67) | 8.540 | 0.003 |
| Yes | 37 | 4 (10.81) | 33 (89.19) |
Analysis of Survival Rate
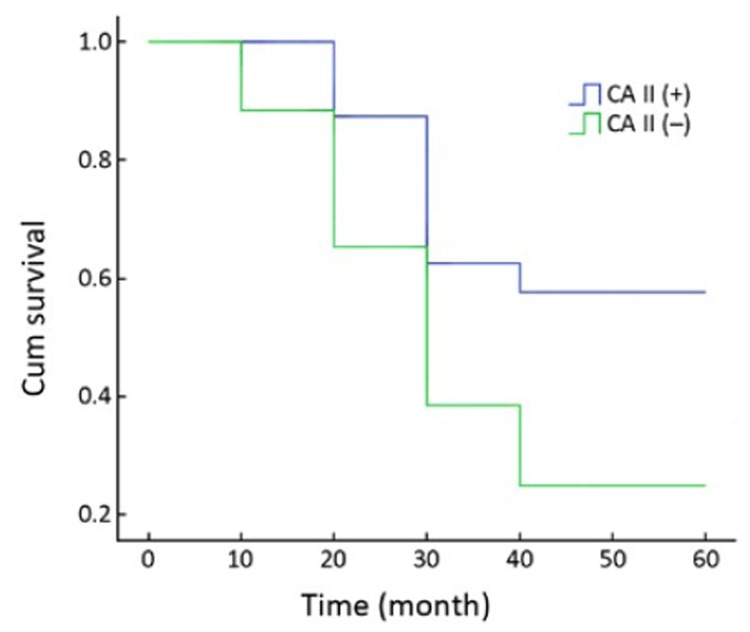
We collected 50 GC patients until July 2010. Fourteen patients were CAII(+), and 36 patients were CAII(−) tumors. Three CAII(+) patients are alive. The CAII(+) patients had longer survival time. Figure 2 shows that the patients with CAII(+) tumors had a slightly higher survival rate than those with CAII(−) tumors, and the difference was statistically significant (P=0.024, log-rank test).
Figure 2.
Survival of GC with CAII expression. The patients with CAII(+) tumors showed a better survival rate than those with CAII(−) tumors (P=0.024, log-rank test).
DISCUSSION
Tumorigenesis is a multi-step process accompanied with genetic alterations of precancerous cells and the simultaneous microenvironment building up, which promotes transformation[13]. CA is a type of zinc-containing metalloenzyme. CAII is a cytoplasmic protein that has the highest turnover rate and widest tissue distribution among the known human CA isozymes. In tumor, CAII is involved in acidifying the extracellular microenvironment, and tumors show much stronger invasiveness in acidified micro-environment[14-16].
A previous study showed that the extracellular pH of solid tumor was more acidic than that of adjacent normal tissue[17]. In contrast, the intracellular pH estimated by 31P-magnetic resonance spectroscopy is identical or slightly higher in tumor compared with normal tissue[18]. In order to establish the pH gradient between the extracellular and intracellular com-partments, tumor cells express ion transport proteins, including vacuolar H+-ATPase, Cl−/HCO3− exchanger and Na+/H+ exchanger[19,20]. Many tumors also express CAs, which catalyze the production of H+ and HCO3− ions in a reversible reaction: H2O+CO2 H++HCO3− [21-23]. Recently, Ivanov, et al. hypothesized that tumor-associated transmembrane isozymes CAII and XII may be involved in acidifying the extracellular microenvironment surrounding cancer cells. Conse-quently, a microenvironment, which is suitable for tumor growth and spread, is created[24]. CAIV, CAIX or CAXII may produce bicarbonate ions outside of the cells. Then the bicarbonate ions are transported inside the cells by bicarbonate/chloride exchangers and catalyzed by CAII to titrate protons. On the other hand, CAII binds to the carboxyl terminus of human bands 3 protein, which catalyzes the exchange of bicarbonate for chloride[25]. Buffering protons facilitate proton secretion and protect the cell from intracellular acidification[26-28]. CAII is interrelated to antigen of vascular endothelium, and its expression is related to amplification of epidermal growth factor receptor (EGFR). The anti-tumor mechanism of CAII is similar to that of EGFR. Yoshiura, et al.[29] suggested that endothelial CAII can be a potential target antigen for dendritic cell therapy.
Recently, Supuran, et al.[30] showed that the pH value of tumor environment can be changed through modulating the CA activity. This effect may favorably influence the anti-tumor effect of sulfonamide CA inhibitor or other concomitantly used anti-tumor agents. Thus, it is very important to examine the potential of CA inhibitor/activator in treating tumor and inhibiting tumor progression[31,32]. However, its relevance to GC remains unclear. Based on these considerations, our study showed that there was a significant reduction of cytosolic CAII expression in GC. The positive rate of CAII protein was 28.57% (32/112), 63.60% (24/38) and 100% (20/20) in gastric carcinoma, intraepithelial neoplasia and normal mucosa, respectively. This is consistent with the previous study[33]. In 112 gastric carcinoma specimens, the differentiation, stage and lymph node metastasis were positively correlated with CAII expression. Because of less metastatic gastric carcinoma specimens, we did not compare difference between primary and metastatic gastric carcinoma. In order to know whether CAII is an independent prognosis index, we analyzed the survival rate of patients with GC. In the present study, the patients with CAII(+) tumors had a slightly higher survival rate than those with CAII(−) tumors, and the difference was statistically significant (P=0.024, log-rank test). Therefore, we think that CAII may be a good prognosis index.
Since CAII affects the tumor development by changing tumor’s microenvironment and energy metabolism, the reduction of CAII expression may promote GC cell motility and contribute to tumor growth and metastasis.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94:153-6 [DOI] [PubMed] [Google Scholar]
- 2.Xie HL, Li ZY, Gan RL, et al. Differential gene and protein expression in primary gastric carcinomas and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. J Dig Dis 2010; 11:167-75 [DOI] [PubMed] [Google Scholar]
- 3.Mau M, Kaiser TM, Südekum KH. Carbonic anhydrase II is secreted from bovine parotid glands. Histol Histopathol 2010; 25:321-9 [DOI] [PubMed] [Google Scholar]
- 4.Kuo WH, Yang SF, Hsieh YS, et al. Differential expression of carbonic anhydrase isoenzymes in various types of anemia. Clin Chim Acta 2005; 351:79–86 [DOI] [PubMed] [Google Scholar]
- 5.Nordfors K, Haapasalo J, Korja M, et al. The tumour-associated carbonic anhydrases CAII, CAIX and CAXII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CAIX with poor prognosis. BMC Cancer 2010; 10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Måseide K, Kandel RA, Bell RS, et al. Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin Cancer Res 2004; 10:4464-71 [DOI] [PubMed] [Google Scholar]
- 7.Brennan DJ, Jirstrom K, Kronblad A, et al. CAIX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 2006; 12:6421-31 [DOI] [PubMed] [Google Scholar]
- 8.Le QT, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 2006; 12:1507-14 [DOI] [PubMed] [Google Scholar]
- 9.Kuo WH, Chiang WL, Yang SF, et al. The differential expression of cytosolic carbonic anhydrase in human hepatocellular carcinoma. Life Sci 2003; 73:2211-23 [DOI] [PubMed] [Google Scholar]
- 10.Haapasalo J, Nordfors K, Järvelä S, et al. Carbonic anhydrase II in the endothelium of glial tumors: a potential target for therapy. Neuro Oncol 2007; 9:308-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inada A, Nienaber C, Fonseca S, et al. Timing and expression pattern of carbonic anhydrase II in pancreas. Dev Dyn 2006; 235:1571-7 [DOI] [PubMed] [Google Scholar]
- 12.Chiang WL, Chu SC, Yang SS, et al. The aberrant expression of cytosolic carbonic anhydrase and its clinical significance in human non-small cell lung cancer. Cancer Lett 2002; 188:199-205 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JP, Meltzer SJ. A review of the genomics of gastric cancer. Clin Gastroenterol Hepatol 2006; 4:416-25 [DOI] [PubMed] [Google Scholar]
- 14.Bhatt D, Tu C, Fisher SZ, et al. Proton transfer in a Thr200His mutant of human carbonic anhydrase II. Proteins 2005; 61:239-45 [DOI] [PubMed] [Google Scholar]
- 15.Kivela AJ, Parkkila S, Saarnio J, et al. Expression of von Hippel-Lindau tumor suppressor and tumor-associated carbonic anhydrases IX and XII in normal and neoplastic colorectal mucosa. World J Gastroenterol 2005; 11:2616-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74 [DOI] [PubMed] [Google Scholar]
- 17.Webb SD, Sherrantt JA, Fish RG. Mathematical modelling of tumour acidity: regulation of intracellular pH. J Theor Biol 1999; 196:237-50 [DOI] [PubMed] [Google Scholar]
- 18.Gerweck LE. Tumor pH: implications for treatment and novel drug design. Semin Radiat Oncol 1998; 8:176-82 [DOI] [PubMed] [Google Scholar]
- 19.Montcourrier P, Silver I, Farnoud R, et al. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp Metastasis 1997; 15:382-92 [DOI] [PubMed] [Google Scholar]
- 20.Lee AH, Tannock IF. Heterogeneity of intracellular pH and of mechanism that regulate intracellular pH in populations of cultured cells. Cancer Research 1998; 58:1901-8 [PubMed] [Google Scholar]
- 21.Peng CX, Gao YM. Studies of the physiological function of carbonic anhydrase. Beijing Da Xue Xue Bao (in Chinese)2007; 39:210-2 [PubMed] [Google Scholar]
- 22.Pastorekova S, Parkkila S, Zavada J.Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 2006; 42:167-216 [PubMed] [Google Scholar]
- 23.Halmi P, Parkkila S, Honkaniemi J.Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem Int 2006; 48:24-30 [DOI] [PubMed] [Google Scholar]
- 24.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 2001; 158:905-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl–/HCO3– exchanger. J Biol Chem 1998; 273:28430-7 [DOI] [PubMed] [Google Scholar]
- 26.Cammer W, Zhang H.Carbonic anhydrase II in microglia in forebrains of neonatal rats. J Neuroimmunol 1996; 67:131-6 [DOI] [PubMed] [Google Scholar]
- 27.Leppilampi M, Saarnio J, Karttunen TJ, et al. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol 2003; 9:1398-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mafra D, Cozzolino SM. Erythrocyte zinc and carbonic anhydrase levels in nondialyzed chronic kidney disease patients. Clin Biochem 2004; 37:67-71 [DOI] [PubMed] [Google Scholar]
- 29.Yoshiura K, Nakaoka T, Nishishita T, et al. Carbonic anhydrase II is a tumor vessel endothelium-associated antigen targeted by dendritic cell therapy. Clin Cancer Res 2005; 11:8201-7 [DOI] [PubMed] [Google Scholar]
- 30.Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003; 23:146-89 [DOI] [PubMed] [Google Scholar]
- 31.Parkkila S, Lasota J, Fletcher JA, et al. Carbonic anhydrase II: A novel biomarker for gastrointestinal stromal tumors. Mod Pathol 2010; 23:743-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 2003; 9:802-11 [PubMed] [Google Scholar]
- 33.Liu DP, Zheng YG, Jiang RL. The expression of carbonic anhydrase I and II in the tissue of early gastric carcinoma. Lin Chuang Xiao Hua Bing Za Zhi (in Chinese)2000; 12:109-10 [Google Scholar]