Abstract
Objective
To investigate the dys-psychological stress effect on the growth of subcutaneous xenotransplanted tumor in nude mice bearing human epithelium ovarian carcinoma, and the influence on P53 and NFκBp65 expressions.
Methods
The subcutaneous tumor xenografts were established by implanting human epithelium ovarian carcinoma tissues into nude mice and the dys-psychological stress model was established with restraint. The mice were randomized into the following four treatment groups with each group six mice respectively: tumor group (group A), normal saline intraperitoneal injection; tumor with stress group (group B), normal saline intraperitoneal injection; tumor therapy group (group C), cisplatin intraperitoneal injection; and tumor therapy with stress group (group D), cisplatin intraperitoneal injection. The expressions of P53 and NFκBp65 in tumor tissues were determined by Western blotting.
Results
The expressions of P53 and NFκBp65 in each restraint group were enhanced compared with the control groups (P<0.05).
Conclusion
The dys-psychological stress may induce the high expressions of P53 and NFκBp65 proteins and further promote tumor growth.
Key words: Constraint stress, Oncoprotein, Ovarian cancer animal model
INTRODUCTION
Recently, following the transition of medical model from biomedicine to biological-psychological-social medicine, more and more researchers pay attention to the influence of psychosocial factors on tumor. Psychosocial factors impact on immune system mainly by functional disorder of hypothalamic-pituitary-adrenal axis and further make an influence on the progression of tumor[1], but the mechanism of its action is uncertain. With the further study on oncomolecularbiology, p53 and NFκBp65 gene are considered as biological factors correlation to the tumorigenesis and progression of many tumors. p53 is regarded as a tumor suppressor gene and its mutant is closely related to the tumorigenesis and progression of tumor[2], whereas, NFκBp65 is considered as an oncogene, which may suppress tumor cell apoptosis after activated. In this study, we determined the expressions of P53 and NFκBp65 proteins in tumor xenografts to investigate the dys-psychological stress effect on the growth of subcutaneous xenotransplanted tumor.
MATERIALS AND METHODS
Chemicals and Reagents
β-actin polyclonal antibody, NFκBp65 rat monoclonal antibody and P53 rabbit polyclonal antibody (Zhongshan Golden Bridge Biotechnology, China). Nucleoprotein Extraction Kit (Pulilai Gene Technology, China), RPMI 1640 medium (GIBCO, USA), methyl thiazolyl tetrazolium (MTT), dimethyl sulfoxide (DMSO) and pancreatic enzyme (Sigma, USA), collagenase III and hyaluronidase V (Like Biotechnology, China).
Animals
Female BALB/C nude mice of 6−8 weeks old, 18–25 g, were obtained from the Laboratory Animal Centre of National Institute for the Control of Pharmaceutical and Biological Products, China (certificate No. SCXK-2005-004) and maintained in the Individual Ventilation Cage System of Jiangxi Provincial Medical Laboratory Animal Center (certificate No. SYXK-2003-0003). Animals were supplied with sterilized food and water.
Tumor Tissues for Implantation
The metastatic tumor tissues for implantation (pathology No. 377879) were obtained from a patient with ovary serous cystadenocarcinoma stage IIIC, who received an operation in Jiangxi Provincial Maternal and Child Health Hospital.
Establishment of Dys-psychological Stress Model
The subcutaneous tumor xenografts were established by implanting tumor tissues into the nude mice[3]. After 12 d, when the subcutaneous tumors were found about 0.8 cm in diameter, the dys-psychological stress model was established by putting the mice bearing tumor xenografts respectively into a 50 ml centrifuge tube with a 3 mm hole for excellent ventilation[4]. The tails of the mice were not squeezed so that the mice could move to and fro in the tubes. Eight hours later, the animals were released from the tubes and then supplied with food and water. According to the improvement in the research of Amy[5], during the study, the mice of dys-psychological stress model were restrained 8 h/d (10 a.m. to 6 p.m.), 5 d/week for two weeks[6].
Treatment
When the subcutaneous tumors were found about 0.8 cm in diameter, the mice were randomly divided into the following four treatment groups (n=6/group): (A) tumor group, normal saline intraperitoneal injection; (B) tumor with dys-psychological stress group, normal saline intraperitoneal injection; (C) tumor group, cisplatin intraperitoneal injection; (D) tumor with dys-psychological stress group, normal saline and cisplatin intraperitoneal injection. The animals were treated with normal saline 0.2 ml/20 g and cisplatin 3 mg/kg. The reagents were administered 1/3 d for four times[7]. All experiments were approved by the Nanchang University Animal Care and Use Committee.
Western Blotting Analysis of P53 and NFκBp65
Two days after the last treatment, the mice were sacrificed, then tumors were collected for Western blotting to determine the expressions of P53 and NFκBp65 proteins. Total protein extracted from tumor tissues was determined by spectrophotometer, then 50 g total protein sample was electrophoresed on polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes. The transferred nitrocellulose blot was blocked with 5% fat-free milk powder in TBS-T (pH 7.6; 20 mmol/L Tris-HCl, 100 mmol/L NaCl, and 0.01% Tween 20) at room temperature for 2 h. After being washed with TBS-T solution three times, the membrane was incubated with 1:200 β-actin polyclonal antibody for rat β-actin, 1:200 P53 rabbit polyclonal antibody for rat P53 and 1:100 NFκBp65 rat monoclonal antibody for rat NFκBp65 in TBS-T at 4˚C overnight. Then the membrane was washed three times and hybridized with horseradish peroxidase-conjugated secondary antibody (dilution factor, 1:2,000) at room temperature for 2 h. At last detection was performed using the reagents provided in the Enhanced Chemiluminescence Plus kit. The experiment was performed in triplicate.
Statistical Analysis
Western blotting fragments were scanned and quantified by Image Tool software (Shanghai Furi company). β-actin was used as an internal control. Measurement data were expressed as x±s, while enumeration data were expressed as relative number. Statistical comparisons were performed with analysis of variance (ANOVA) or nonparametric tests, and transactional analysis was performed with ANOVA of factorial design using SPSS 13.0 software (SPSS Inc., Chicago, USA). P<0.05 was considered as statistical significance.
RESULTS
P53 and NFκBp65 Protein Expressions of Tumor Tissues
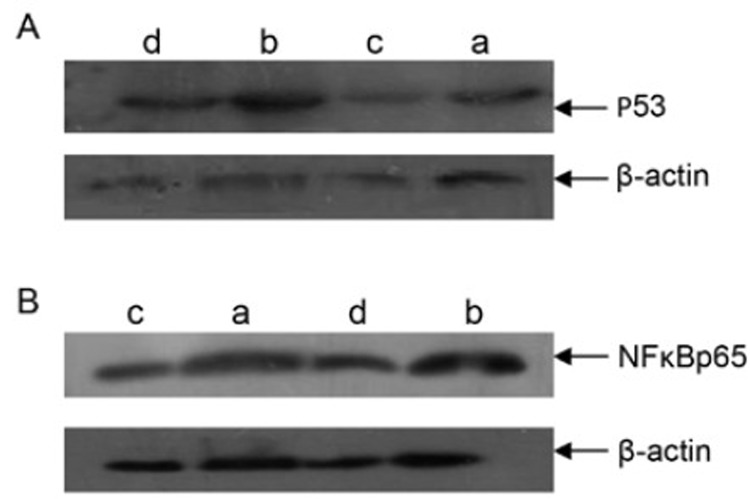
After the treatment, the expressions of P53 and NFκBp65 proteins in tumor tissues were determined by Western blotting. The results are shown in Table 1 and Figure 1. The expressions of P53 and NFκBp65 proteins in Group B were both dramatically increased and there was significant difference compared to the other groups. We could conclude that it was the dys-psychological stress that resulted in the increased expressions of P53 and NFκBp65 proteins in tumor xenografts while chemotherapy with cisplatin could induce the decreased expressions of P53 and NFκBp65 proteins. However, when cisplatin and dys-psychological stress were used simultaneously, the expressions of P53 and NFκBp65 proteins increased.
Table 1. The levels of P53 and NFκBp65 in tumor tissues.
| Group | n | P53/β-actin intensity | NFκBp65/β-actin intensity |
|---|---|---|---|
| A | 6 | 1.285±0.060 | 2.008±0.083 |
| B | 6 | 3.880±0.070*† | 3.033±0.073*† |
| C | 6 | 1.177±0.071* | 1.700±0.075* |
| D | 6 | 3.718±0.110*†‡ | 2.278±0.107*†‡ |
*P<0.05 compared with Group A; †P<0.05 compared with Group C; ‡P<0.05 compared with Group B.
Figure 1.
The expressions of P53 and NFκBp65 proteins in tumor xenografts. A: The expression of P53 protein in tumor tissues; B: The expression of NFκBp65 protein in tumor tissues. a: Group A; b: Group B; c: Group C; d: Group D.
Impact of Constraint Stress and Cisplatin on the Expressions of P53 and NFκBp65
The impact of constraint stress and cisplatin on the expressions of P53 and NFκBp65 were evaluated with transactional analysis by ANOVA of factorial design. As shown in Table 2, constraint stress could result in the increased expression of P53 in tumor xenografts while cisplatin therapy could induce the decreased expression of P53 and there was no significant difference between them (P=0.424); as to NFκBp65, constraint stress could result in the increased expression of NFκBp65 in tumor xenografts while cisplatin therapy could induce the decreased expression of NFκBp65, and furthermore there was significant difference between them (P<0.05).
Table 2. Impact of constraint stress and cisplatin on the expressions of P53 and NFκBp65.
| Variable | Degree of freedom | P53 expression |
NFκBp65 expression |
||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Cisplatin therapy | 1 | 17.064 | <0.050 | 231.855 | <0.05 |
| Constraint stress | 1 | 6,176.023 | <0.050 | 527.133 | <0.05 |
| Interaction between cisplatin therapy and constraint stress | 1 | 0.666 | 0.424 | 40.911 | <0.05 |
DISCUSSION
Stress is a known risk factor for human diseases, such as autoimmune diseases, infectious diseases and cancer[8,9]. Epidemiologic and experimental animal studies have shown that stress may alter tumor growth[10-13]. The stress-activated signaling pathways, P53 and NFκB, have a major role in the regulation of cellular senescence and organismal aging[14]. It has been reported that psychological stress can mediate the suppression of tumor immunity by activating NFκB[15,16]. Zhang[17] observed that restraint stress significantly enhanced the level of NFκB binding activity, suggesting that restraint stress stimulates activation of the NFκB signaling pathway. NFκB mainly exsits as the dipolymer consisting of a subunit (p65) and a subunit (p50). When NFκB combined with inhibitory protein IκB as an inactive trimer, it located in the cytoplasm at silent period and couldn’t promote transcription. However, when cells respond to a wide variety of diverse stimuli including cytokines such as tumor necrosis factor (TNF), interleukin (IL) and growth factor (GF), viral or bacterial infections and irradiation, phosphorylation and ubiquitination of serines of polymer IκB lead to the degradation of IκB resulting in the exposure of nuclear localization signals (NLS), leading to nuclear translocation and binding to a specific sequence in the DNA, which in turn results in gene transcription, so the expression of NFκB in the nucleus could be considered as a mark of the activation of NFκB[18]. The activation of NFκB can suppress the transcription of p53, promote apoptosis gene transcription and further induce cell proliferation involved in tumor promotion. It was indicated by most researches that the cause for diseases induced by NFκB is that the transcription activation of cytokines or their receptors induces non-regulative lymphocyte proliferation or blocks its apoptosis. In addition, NFκB may lead to tumorigenesis by directly affecting the cell cycle or DNA replication.
Another research indicated that missing or mutant of gene is a factor for tumorigenesis. As an important tumor suppressor gene, p53 gene plays an important role in the tumorigenesis and progression of tumor. p53 of 16−20 kb consisting of 11 extrons and 10 introns, locates in human chromosome 17p13, and encodes 393 amine acids. There are two phenotypes of p53. One is wild-type p53 (wtp53), which is involved in the regulation of cell cycle and plays a role in promoting cell growth and inhibiting tumor proliferation; the other is mutant-type p53 (mtp53), which is related to tumorigenesis and progression of tumor[2]. Wtp53 is relevant to apoptosis, and as it mutates into mtp53, mtp53 cannot suppress cell growth but can promote cell excessive proliferation and further induce tumorigenesis[19]. Owing to the low content and short half life period, wtP53 protein is not easy to determine. Contrarily, mtP53 protein may cause a serial changes to P53 such as the loss of inhibitory effect on tumor, molecular conformation change, the extension of half life period and the ability of tumor promotion, which lead to transforming it to an oncoprotein, and can be determined[20]. Accordingly, the proteins usually determined by us is the products of mtp53. The prevalence of mtP53 protein in epithelial ovarian cancers was 50%−70% and did not show any association with the disease stage at present[21].
Now there have been many studies on the impact of dys-psychological stress effect on tumor growth, however, the mechanism is still unknown. In this report, we investigated the dys-psychological stress effect on the growth of subcutaneous xenotrans planted tumor in nude mice and the influence on P53 and NFκBp65 expressions. And the effect was confirmed by our results. The expressions of P53 and NFκBp65 in the constraint stress group were significantly higher than those in the control groups. Chemotherapy with cisplatin induced the decreased protein expressions of P53 and NFκBp65, however, under treatment with the dys-psychological stress, the expressions of the two proteins were both higher than those before treatment, thus further affecting the efficacy. Therefore, the dys-psychological stress may induce the high expressions of P53 and NFκBp65 proteins, which lead to promoting tumor growth. And it was also indicated that constraint stress induces tumor growth through P53 and NFκB pathways. But there were not big samples. During the chemotherapy, we should pay more attention to psychotherapy for good efficacy.
REFERENCES
- 1.Antoni MH. Psychoneuroendocrinology and psychoneuroimmunology of cancer: plausible mechanisms worth pursuing. Brain Behav Immun 2003; 17(Supple 1):S84-91 [DOI] [PubMed] [Google Scholar]
- 2.Ambros RA, Vigna PA, Figge J, et al. Observation on tumor and metastatic suppressor gene status in endometrial carcinoma with particular emphasis on p53. Cancer 1994; 73:1686-92 [DOI] [PubMed] [Google Scholar]
- 3.Gao GL, Zou CF, Zou XS, et al. Establishment of Subcutaneous Xenotransplanted Tumor Model of Human Epithelium Ovarian Cancer in Nude Mice and its Biological Characteristics. Shi Yong Ai Zheng Za Zhi (in Chinese)2004; 19:454-7 [Google Scholar]
- 4.Manfredi B, Sacerdote P, Gaspani L, et al. IL-6 knockout mice show modified basal immune functions, but normal immune responses to stress. Brain Behav Immun 1998; 12:201-11 [DOI] [PubMed] [Google Scholar]
- 5.Sieve AN, Steelman AJ, Young CR, et al. Chronic restraint stress during early Theiler's virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol 2004; 155:103-18 [DOI] [PubMed] [Google Scholar]
- 6.Yu LQ, Liu FJ, Gao GL, et al. Establishment of Dys-psychological stress model of human epithelial ovarian carcinoma in nude mice. Zhong Guo Zhong Liu Lin Chuang (in Chinese)2009; 36:1187-9 [Google Scholar]
- 7.Shi HR, Hou LJ, Qiao YH, et al. Anticancer effects of cisplatin and topotecan on ovarian cancer in nude mice. Zheng Zhou Da Xue Xue Bao (Yi Xue Ban) (in Chinese)2004; 39:563-5 [Google Scholar]
- 8.Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav Immun 2004; 18:114-9 [DOI] [PubMed] [Google Scholar]
- 9.Frick LR, Arcos ML, Rapanelli M, et al. Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 2009; 12:134-43 [DOI] [PubMed] [Google Scholar]
- 10.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004; 5:617-25 [DOI] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939-44 [DOI] [PubMed] [Google Scholar]
- 12.Li J, Hu S, Zhang CQ, et al. Impact of chronic restraint stress on splenocyte immunity and growth of mouse forestomach carcinoma xenografts in Kunming mice. Ai Zheng (in Chinese)2008; 27:471-5 [PubMed] [Google Scholar]
- 13.Gao J, Gao G, Zhang Y, et al. Proteomic analysis of human epithelial ovarian cancer xenografts in immunodeficient mice exposed to chronic psychological stress. Sci China Life Sci 2011; 54:112-20 [DOI] [PubMed] [Google Scholar]
- 14.Salminen A, Kaarniranta K.Control of p53 and NF-κB signaling by WIP1 and MIF: role in cellular senescence and organismal aging. Cell Signal 2011; 23:747-52 [DOI] [PubMed] [Google Scholar]
- 15.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004; 130:601-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagabhushan M, Mathews HL, Witek-Janusek L. Aberrant nuclear expression of AP-1 and NFkappaB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav Immun 2001; 15:78-84 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Foster R, Sun X, et al. Restraint Stress Induces Lymphocyte Reduction through p53 and PI3K/NF-κB Pathways. J Neuroimmunol 2008; 200:71-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 2002; 16: 1053-68 [DOI] [PubMed] [Google Scholar]
- 19.Zhang LH, Hou ZJ. Progress in study of p53 in pulmonary cancer. Lin Chuang Fei Ke Za Zhi (in Chinese)2006; 11:59-60 [Google Scholar]
- 20.Bressac B, Galvin KM, Liang TJ, et al. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA 1990; 87:1973-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosari S, Viale G, Radaelli U, et al. p53 accumulation in ovarian carcinomas and its prognostic implications. Hum Pathol 1993; 24:1175-9 [DOI] [PubMed] [Google Scholar]