Abstract
Objective
To investigate the effects of 5-Aza-2'-deoxycytidine (5-Aza-Cdr) and trichostatin A (TSA) combined with p53-expressing adenovirus (Ad-p53) on Hep-2 cell line in vivo and in vitro, in order to explore its possibility in biological treatment of laryngocarcinoma.
Methods
Effects of 5-Aza-Cdr and TSA in combination with Ad-p53 on Hep-2 cell line in vivo were determined by Cell Counting Kit-8 (CCK-8) assay. The effect of drug combination was calculated by Jin's formula. Effects on the cell line in vitro were investigated by establishing the nude mice model.
Results
5-Aza-Cdr and TSA showed inhibitory effects on the proliferation of Hep-2 cells in dose- and time-dependent manner. Ad-p53 can inhibit the growth of Hep-2 cells in vivo and in vitro. However, the combination of epigenetic reagents (5-Aza-Cdr/TSA) and Ad-p53 was less effective than individual use of Ad-p53. 5-Aza-Cdr and Ad-p53 inhibited the growth of transplanted tumors and reduced the volume of tumors, and the tumor volume of Ad-p53 group was significantly smaller than that of the control group (P<0.05).
Conclusion
Both epigenetic reagents (5-Aza-Cdr/TSA) and Ad-p53 can suppress cell proliferation on Hep-2 in vivo and in vitro and there may be some antagonistic mechanism between Ad-p53 and epigenetic reagents (5-Aza-Cdr/ TSA).
Key words: 5-Aza-2'-deoxycytidine, trichostatin A, p53-expressing adenovirus, Hep-2 cell line
INTRODUCTION
Genetic and epigenetic changes are the main mechanisms which can cause malignant tumor. Epigenetic modifications mainly involve DNA methy-lation and histone acetylation[1]. DNA methylation and histone acetylation have some synergies on gene transcription inhibition[2, 3]. 5-Aza-2'-deoxycytidine (5-Aza-Cdr) is a DNA methyltransferase (DNMT) inhibitor, which could specifically inhibit DNA methyltransferase, reverse gene methylation, restore the function of tumor suppressor genes and inhibit the growth of tumor cells. Trichostatin A (TSA) is a histone deacetylase (HDAC) inhibitor, which could inhibit the reverse activity of histone deacetylase, induce tumor cell cycle block, differentiate apoptosis, therefore, achieve an anti-tumor effect. p53 gene, which has the highest relativity with human tumor, has been discovered (p53 is an important tumor suppressor). p53 gene mutation is associated with more than 50% human carcinomas[4-6]. Previous studies have discovered that wild-type p53 is closely related to regulation of cell cycle and transformation and induction of apoptosis. This study adopted the method of combining 5-Aza-Cdr and TSA with p53-expressing adenovirus (Ad-p53) to act on Hep-2 cell line in vivo and in vitro, in order to explore its possibility in biological treatment of laryngocarcinoma.
Laryngocarcinoma is one of the most common malignant tumors in the head and neck regions, and the mechanism involves genetics and epigenetics. Many reports indicated DNA methylation patterns in the CpG island and aberrant histone acetylation modification lead to inactivation of some tumor suppressor genes in human laryngeal cancer. At the aspect of p53 gene, large numbers of research is made, but there is a few works about its combination with epigenetic reagents. Therefore, we make a study on the combination of Ad-p53 with DNMT inhibitor 5-Aza-Cdr and HDAC inhibitor TSA on the growth of human laryngeal cancer Hep-2 Cells in order to investigate the value of biological therapy of laryngeal cancer.
MATERIALS AND METHODS
Materials
5-Aza-Cdr and TSA were obtained from Sigma Chemicals, Co. 5-Aza-Cdr was dissolved in phosphate buffered saline (PBS, Thermo Fisher Scientific) as 20 mg/ml stock solution and stored at –20°C. TSA was dissolved in 1 ml dimethyl sulfoxide (DMSO) as 5 mg/ml stock solution and stored at –20°C.
Ad-p53 (Gendicine®) was obtained from Shenzhen Sibiono Bentech, China. It was stored at –20°C at a concentration of 1×1012 viral particles (VP)/ampoule. Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Laboratories, USA. RPMI 1640 culture medium and fetal bovine serum (FBS) were obtained from Thermo Scientific HyClone, USA. BALB/c mice (male, 11−13 g and 4 weeks old) raised under SPF conditions were obtained from Animal Laboratory, Capital Medical University, Beijing, China. The animal experiments were approved by the Animal Care and Use Committee of Beijing Institute of Otorhino-laryngology.
Cell lines and Culture Conditions
Human laryngeal carcinoma cell line Hep-2 was obtained from Cytobiology Laboratory of Beijing Institute of Otorhinolaryngology and cultured in RPMI 1640 medium supplemented with 10% FBS in a humidified atmosphere containing 5% CO2 at 37°C.
Drugs Treatment
Exponentially growing cells were randomly assigned into 7 groups (5-well in each group): blank control group (added in RPMI-1640 medium containing 100 g/L serum), negative control group (drug-free medium), 5-Aza-Cdr group (treated with 5-Aza-Cdr at different final concentrations of 0.5, 1.0 and 2.0 mg/L for 72 h, and fresh 5-Aza-Cdr was replaced every 24 h), TSA group (treated with TSA at different final concentrations of 50, 100 and 200 μg/L for 72 h, and fresh TSA was replaced every 24 h), 5-Aza-Cdr+TSA group (treated with 5-Aza-Cdr at different final concentrations of 0.5, 1.0 and 2.0 mg/L for first 24 h, then with TSA at different final concentrations of 50, 100 and 200 μg/L for later 48 h, fresh TSA was replaced every 24 h), Ad-p53 group (treated with Ad-p53 at final concentration of 1010 VP for 72 h), 5-Aza-Cdr+TSA+Ad-p53 group (treated with 5-Aza-Cdr at different final concentrations of 0.5, 1.0 and 2.0 mg/L for first 24 h, then with TSA at different final concentrations of 50, 100 and 200 μg/L for the second 24 h and with Ad-p53 at final concentration of 1010 VP for the last 24 h, drugs and fresh medium were replaced every 24 h).
In Vitro Cytotoxicity Assays
Hep-2 cells in logarithmic growth phase were inoculated in 96-well plate, with the amount of 100 μl per well and the cell suspension density of 5×104/ml. After 24 h of incubation, the cells were randomly divided into the control group and the test groups medially with five duplicates per group, and drugs and fresh medium were replaced every 24 h. All samples were taken every 24 h for 3 d. Accompanying with every sampling, 10 μl of CCK-8 was added to each well. After 2 h of incubation, the absorption value A of each well was detected at the wavelength of 450 nm in μQuant spectrophotometer (Bio-Tek Instruments, USA). Cell inhibition rate (I%) was calculated using the following equation:
I%=(Acontrol−Atreated)/(Acontrol−Ablank) × 100%
The results from the assays were analyzed for the combination effect between 5-Aza-Cdr and TSA according to Jin’s[7] method. In this method, Q<0.85 indicates antagonism, 0.85≤Q<1.15 indicates additivity, and Q≥1.15 indicates synergism. The formula is Q=Ea+b/(Ea+Eb−Ea×Eb), where Ea+b, Ea and Eb are the average effects of the combination treatment, 5-Aza-Cdr only, TSA only, respectively. All treatments were performed in quadruplication and the experiments were repeated three times.
Effect of 5-Aza-Cdr and Ad-p53 on Hep-2 Transplanted Tumor Growth in Nude Mice
Forty male BALB/c mice received subcutaneous injection with 0.1 ml of 2×107/ml Hep-2 cells. Three weeks later, 10 unqualified mice were weeded out, and the remained 30 mice were divided into 5 groups randomly, with 6 mice in each group. Group 1 (p53 group) was treated with Ad-p53 (0.1 ml to each one, every 3 d). Group 2 (combination group) was treated with Ad-p53 (0.1 ml each one, every 3 d) and 5-Aza-Cdr (0.1 mg/ml, 0.1 ml, three times a week). Group 3 (epigenetic group) was treated with 5-Aza-Cdr (0.1 mg/ml, 0.1 ml, three times a week). Group 4 (control group) was treated with normal saline (three times a week). Group 5 (blank group) was treated with nothing. Tumor size was monitored weekly by measuring the largest and smallest diameters of tumor and estimated according to the formula[8]: volume = 1/2 × (largest diameter) × (smallest diameter)2. On the 20th day, we sacrificed the mice, took out the tumors to weigh and calculated the inhibition rate of tumor.
Statistical Analysis
All statistical analyses were carried out using SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). All data were expressed as x±s and cell proliferation was compared with one-way analysis of variance (ANOVA) in different groups, and the tumor volume in groups was also analyzed with one-way ANOVA, and the pairwise comparison was performed with least significant difference (LSD) t test. P<0.05 was considered statistically significant.
RESULTS
Effect of Drugs Inhibition on Cell Proliferation
Hep-2 cells in vitro were treated with different concentrations of drugs, and the cell growth inhibition rate of Hep-2 was assessed by CCK-8 assay. The results showed that the inhibitory effect on the proliferation of Hep-2 cells increased with the increase of concentration and time. Different experimental groups (Ad-p53, 5-Aza-Cdr, TSA and 5-Aza-Cdr+TSA) all inhibited Hep-2 cell growth significantly, in which the inhibition action strengthened with the raised drug concentration and prolongation of the treatment. In each group, the inhibition rate was significantly different between different concentrations acting for the same period (P<0.05), and it was also significantly different from each other (P<0.05) at the same concentration for different periods. The relationship between the concentrations and time in cell proliferation is listed in Tables 1,2,3,4,5.
Table 1. Effects of 5-Aza-Cdr on Hep-2 cell proliferation.
| 5-Aza-Cdr (mg/L) | A450 (x̄±s) |
Inhibition rate (%) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0 | 0.749±0.034 | 1.200±0.104 | 1.902±0.058 | 0 | 0 | 0 |
| 0.5 | 0.673±0.049 | 1.067±0.077 | 1.663±0.055 | 10.1 | 11.1 | 12.6 |
| 1 | 0.580±0.042 | 0.943±0.052 | 1.571±0.071 | 22.6 | 23.6 | 55.8 |
| 2 | 0.549±0.077 | 0.712±0.105 | 0.830±0.047 | 26.7 | 40.7 | 56.4 |
Table 2. Effects of TSA on Hep-2 cell proliferation.
| TSA (μg/L) | A450 (x̄±s) |
Inhibition rate (%) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0 | 0.749±0.034 | 1.200±0.104 | 1.902±0.058 | 0 | 0 | 0 |
| 50 | 0.449±0.047 | 0.514±0.012 | 0.760±0.147 | 40.1 | 57.2 | 60.0 |
| 100 | 0.395±0.426 | 0.481±0.081 | 0.738±0.100 | 47.3 | 59.9 | 61.0 |
| 200 | 0.373±0.472 | 0.340±0.031 | 0.354±0.007 | 50.2 | 71.7 | 81.4 |
Table 3. Effects of Ad-p53 on Hep-2 cell proliferation.
| Ad-p53 (VP) | A450 (x̄±s) |
Inhibition rate (%) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0 | 0.749±0.034 | 1.200±0.104 | 1.902±0.058 | 0 | 0 | 0 |
| 1010 | 0.447±0.064 | 0.257±0.025 | 0.329±0.063 | 40.3 | 78.6 | 82.7 |
Table 4. Effects of 5-Aza-Cdr and TSA on Hep-2 cell proliferation.
| 5-Aza-Cdr+TSA (mg/L+μg/L) | A450 (x̄±s) |
Inhibition rate (%) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0+0 | 0.749±0.034 | 1.200±0.104 | 1.902±0.058 | 0 | 0 | 0 |
| 0.5+50 | 0.673±0.049 | 0.953±0.125 | 1.489±0.092 | 10.1 | 20.6 | 21.8 |
| 1+100 | 0.580±0.042 | 0.769±0.026 | 0.598±0.020 | 22.6 | 35.9 | 68.6 |
| 2+200 | 0.549±0.077 | 0.684±0.011 | 0.412±0.058 | 26.7 | 43.0 | 78.3 |
Table 5. Effects of 5-Aza-Cdr and TSA combined with Ad-p53 on Hep-2 cell proliferation.
| 5-Aza-Cdr+TSA+Ad-p53 (mg/L+μg/L+VP) | A450 (x̄±s) |
Inhibition rate (%) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0+0+0 | 0.749±0.034 | 1.200±0.104 | 1.902±0.058 | 0 | 0 | 0 |
| 0.5+50+1010 | 0.673±0.049 | 0.953±0.125 | 1.172±0.045 | 10.1 | 20.6 | 38.4 |
| 1+100+1010 | 0.580±0.042 | 0.769±0.026 | 1.071±0.014 | 22.6 | 35.9 | 43.7 |
| 2+200+1010 | 0.549±0.077 | 0.684±0.011 | 0.982±0.036 | 26.7 | 43.0 | 48.4 |
Analysis of Combination Effect
We analyzed the combination effect according to the Jin’s method after calculating the inhibition rate of cell proliferation. Table 6 presents analysis on combination effect between 5-Aza-Cdr and TSA, We found all Q values were under 0.85, so it seemed to be an antagonistic effect. However, with the increment of the drug concentration and prolongation of the exposed time, Q value became higher and higher even to 0.85 and then the two drugs tended toward an additive effect. Table 7 presents analysis on combination effect between epigenetic reagents (5-Aza-Cdr and TSA) and Ad-p53, all of the Q values were less than 0.85, and the maximum one is only 0.5. It showed an antagonistic effect as well.
Table 6. Relationship between combination effect and different dose and time.
| 5-Aza-Cdr+TSA (mg/L+μg/L) | Q |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 0.5+50 | 0.22 | 0.33 | 0.34 |
| 1+100 | 0.38 | 0.52 | 0.83 |
| 2+200 | 0.42 | 0.52 | 0.85 |
Table 7. Analysis on combination effect between epigenetic reagents and Ad-p53.
| Group | Concentration | 72 h inhibition rate (%) | Q |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| Ad-p53 (VP) | 1010 | 82.0 | |
| 5-Aza-Cdr+TSA (mg/L+ug/L) | 0.5+50 | 21.8 | |
| 1+100 | 68.6 | ||
| 2+200 | 78.3 | ||
| Ad-p53+5-Aza-Cdr+TSA (VP+mg/L+ug/L) | 1010+0.5+50 | 38.4 | 0.24 |
| 1010+1+100 | 43.7 | 0.46 | |
| 1010+2+200 | 48.4 | 0.50 |
Effect of 5-Aza-Cdr and Ad-p53 on Hep-2 Transplanted Tumor Growth in Nude Mice
After successfully making the model (Figure 1), we used 5-Aza-Cdr and/or Ad-p53 to treat implanted tumors, then observed every group’s tumor volume. All mice were sacrificed after 20 d, then we took out the tumors (Figure 2). These groups were significantly different from each other (P<0.05), except the control group and blank group Table 8. The tumor grew slowest in the p53 group but fastest in both control group and blank group. And the tumor growth speed of combination group was slower than that of epigenetic group. The inhibition rates of tumor growth were calculated as[9]: Inhibition rate=(1−average tumor weight in the experimental group/average tumor weight of control group) ×100%. From this, we found that the inhibition rate of p53 group was 50.55%, which was superior to that of combination group (39.83%). And the inhibition rate of epigenetic group was the lowest (14.94%).
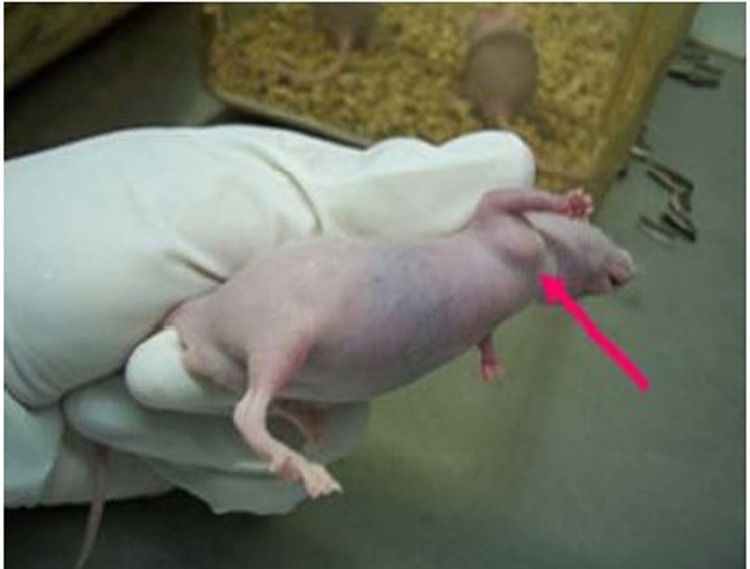
Figure 1.
Obvious tumor at right axilla of nude mouse.
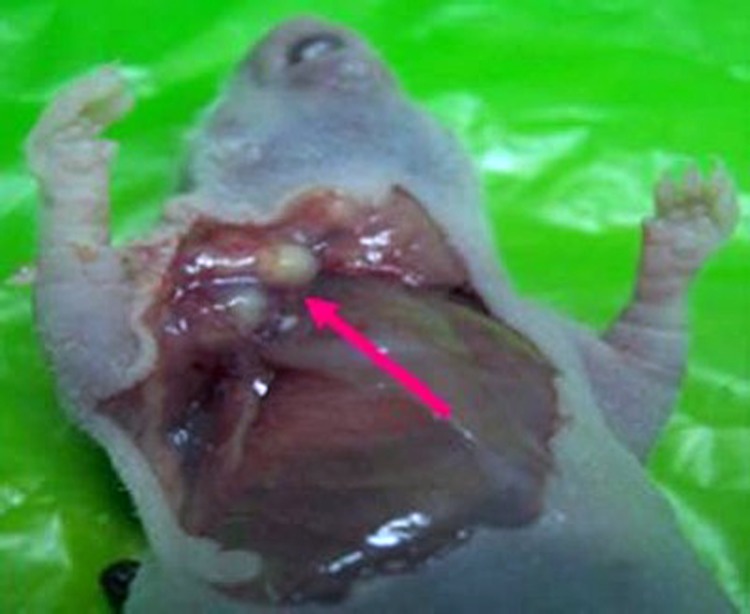
Figure 2.
Anatomical tumor tissue.
Table 8. Comparison among tumor volume of different groups.
| Group |
P |
Tumor volume (mm3) | ||||
|---|---|---|---|---|---|---|
| Ad-p53 | Ad-p53+5-Aza-Cdr | 5-Aza-Cdr | Control | Blank | ||
| Ad-p53 | / | 0.001 | 0 | 0 | 0 | 120.0887±24.3976 |
| Ad-p53+5-Aza-Cdr | 0.001 | / | 0 | 0 | 0 | 146.1111±22.4860 |
| 5-Aza-Cdr | 0 | 0 | / | 0.001 | 0.001 | 206.5502±40.1077 |
| Control | 0 | 0 | 0.001 | / | 0.997 | 242.8406±57.0162 |
| Blank | 0 | 0 | 0.001 | 0.997 | / | 247.8339±54.0919 |
DISCUSSION
Epigenetic modification is an important form of gene-expression regulation, including DNA methy-lation and histone deacetylation. This abnormal modification inhibits gene transcription, which leads to antioncogene inactivation. Luckily, gene can be silenced by DNA methylation and histone deacety-lation, that is to say − could be re-expressed after treatment with demethylating reagent and acetylating reagent[10, 11].
5-Aza-Cdr is the most important DNMT inhibitor. It is a pyrimidine analogue and can bind to DNMT in a covalent complex to specifically inhibit the activity of DNMT and the growth of tumor cells[12, 13]. TSA derives from metabolites of streptomyces. It was first used as an antifungal agent. Since Japanese scholar Yoshida first discovered it could inhibit non-competitively the activity of HDAC in breast carcinoma cells of mouse, Nowadays, as a HDAC inhibitor, it has been mostly studied. It had the characteristic of a HDAC inhibitor and could inhibit reversibly the activity of HDAC, induce cell cycle arrest, differentiation and apoptosis and inhibit the growth of tumor cells[14,15]. It also showed HDAC inhibitors may also contribute to increased DNA demethylation while increasing the histone acetylation levels, so there is close relationship between DNA methylation and histone acety-lation[16,17]. Thus, the combination of 5-Aza-Cdr and TSA could fully exert the synergistic effect, reduce the methylation levels in the promoter region, achieve the re-expression of the silent tumor-suppressor genes and realize the purpose of tumor therapy finally[18]. In recent years, as the representative drugs of DNMT inhibitor and HDAC inhibitor, 5-Aza-Cdr and TSA have been a hot spot for tumor treatment research. Related study showed that silenced O6-methylguanine DNA methyltransferase (MGMT) gene was re-expressed after treatment with 5-Aza-Cdr in laryngeal cancer Hep-2 cell line. Some researchers also used 5-Aza-Cdr acting on laryngeal cancer in nude mice and the result showed the median tumor volume of nude mice decreased obviously, compared with the control group.
Shaker, et al. showed that the combination of TSA with 5-Aza-Cdr in the treatment of leukemia could reduce side effects and showed a synergistic effect.
In our experiments, we used 0.5 mg/L, 1.0 mg/L and 2.0 mg/L 5-Aza-Cdr acting on Hep-2 cells. And it inhibited the growth of Hep-2 cells, at the concentration of 0.5 mg/L, and the inhibitory effect increased with the augment of concentration and time (P<0.05). After we used 50 ng/ml, 100 ng/ml, 200 ng/ml TSA on Hep-2 cells, we also found the similar inhibitory effect. According to the Q value, the two drugs in combination have an antagonistic effect at lower initial concentration, but the effect becomes weaker and weaker with the increment of the drug concentration and prolongation of the exposed time. When they reach certain concentration and time, the antagonistic effect eventually disappeared and then gradually tended toward an additive effect to each other. It might show that the two drugs’ combination was antagonistic to each other at lower concentration and shorter exposed time, only when the concentration and time increased to a certain degree, they could be synergic and additive. So these two drugs could be combined to use.
A large number of experiments elementarily showed that wild-type p53 gene has a strong potential in the treatment of certain tumors[19,20]. And related clinical trials also indicated that Ad-p53 used alone or combinated is believed to be effective in treatment of laryngocarcinoma and other head and neck tumors. Han, et al. have proved the safety of its clinical applications from the phase I clinical trial on Ad-p53 plus surgical operation for middle or advanced-stage laryngocarcinoma. Clinical tests also have proven that Ad-p53 was well-tolerated, and did not increase the toxicity when combined with cytotoxic agents[21-23].
In addition, Ad-p53 has been recently approved by the State Food and Drug Administration of China as the first gene therapy product for head and neck squamous cell carcinoma (HNSCC)[REMOVED HYPERLINK FIELD][24]. It is adenovirus type 5 carrying p53 gene, which can regulate cell cycle, induce apoptosis, inhibit tumor angiogenesis, and increase the sensitivity of cancer to chemotherapy or radiotherapy[25,26]. That also confirmed by Roth, et al.[27]. The possible mechanism of Ad-p53 includes[28-31]: (1) blocking tumor cell proliferation cycle and inducing apoptosis to control tumor growth; (2) wild-type p53 gene transfection can be used to improve the effect of radiotherapy or/and chemotherapy; (3) inhibiting the expression of vascular endothelial growth factor (VEGF), and inducing tumor cell apoptosis; (4) inducing the specific antitumor immunity; and (5) killing tumor cells by regulating immune systems and bystander effect.
5-Aza-Cdr has showed anti-tumor activity to some extent in animal models and cell culture systems. In our experiment, we used 1010 VP Ad-p53 acting alone or in combination with epigenetic drugs on Hep-2 cells in vivo and in vitro. It was found that Ad-p53 could obviously inhibit Hep-2 cells either in vitro or in vivo, compared with the control group. However, we observed that the inhibitory effect became weaker in the combination group of Ad-p53 and epigenetic drugs (5-Aza-Cdr or TSA). From combination group, all of the Q values were less than 0.85, the maximum one is only 0.5 and this shows antagonistic effect.
Mechanism of tumorigenesis is very complicated, it may be impractical to cure the tumor with one or two agents. From our experiment, there may be some antagonistic mechanism between Ad-p53 and epigenetic drugs (5-Aza-Cdr or TSA). As for its precisely effect mechanisms, it is very necessary for us to make an further inquiry. In a word, our work is just an attempt and we hope to find out a new target for treating tumors in the near future.
REFERENCES
- 1.Sandoval J, Esteller M.Cancer epigenomics: beyond genomics. Curr Opin Genet Dev 2012; 22:50-5 [DOI] [PubMed] [Google Scholar]
- 2.Li X, Liu J, Zhou R, et al. Gene silencing of MIR22 in acute lymphoblastic leukaemia involves histone modifications independent of promoter DNA methylation. Br J Haematol 2010; 148:69-79 [DOI] [PubMed] [Google Scholar]
- 3.Majid S, Dar AA, Shahryari V, et al. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer 2010; 116:66-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akkiprik M, Sonmez O, Gulluoglu BM, et al. Analysis of p53 gene polymorphisms and protein over-expression in patients with breast cancer. Pathol Oncol Res 2009; 15:359-68 [DOI] [PubMed] [Google Scholar]
- 5.Malkin D.Li-Fraumeni syndrome. Genes Cancer 2011; 2:475-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng X, Sun YX, Qu W, et al. Biotinylated transferrin/avidin/biotinylated disulfide containing PEI bioconjugates mediated p53 gene delivery system for tumor targeted transfection. Biomaterials 2010; 31:4771-80 [DOI] [PubMed] [Google Scholar]
- 7.Jin ZJ. Additivity effect of drug combination. Zhong Guo Yao Li Xue Bao 1980; 1:70-6 [PubMed] [Google Scholar]
- 8.Verma B, Neethling FA, Caseltine S, et al. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol 2010; 184:2156-65 [DOI] [PubMed] [Google Scholar]
- 9.Huang HY, Niu JL, Lu YH. Multidrug resistance reversal effect of DMC derived from buds of Cleistocalyx operculatus in human hepatocellular tumor xenograft model. J Sci Food Agric 2012; 92:135-40 [DOI] [PubMed] [Google Scholar]
- 10.Allegrucci C, Rushton MD, Dixon JE, et al. Epigenetic reprogramming of breast cancer cells with oocyte extracts. Mol Cancer 2011; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyer JA, Chu A, Brar S, et al. Aberrant epigenetic silencing is triggered by a transient reduction in gene expression. PLoS One 2009; 4:e4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SI, Aumsuwan P, Khan IA, et al. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol 2012; 25:61-73 [DOI] [PubMed] [Google Scholar]
- 13.Pandey M, Shukla S, Gupta S.Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer 2010; 126:2520-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tambunan US, Wulandari EK. Identification of a better Homo sapiens Class II HDAC inhibitor through binding energy calculations and descriptor analysis. BMC Bioinformatics 2010; 11(Suppl 7):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francisco R, Pérez-Perarnau A, Cortés C, et al. Histone deacetylase inhibition induces apoptosis and autophagy in human neuroblastoma cells. Cancer Lett 2012; 318:42-52 [DOI] [PubMed] [Google Scholar]
- 16.Brooks WH, Le Dantec C, Pers JO, et al. Epigenetics and autoimmunity. J Autoimmun 2010; 34:J207-19 [DOI] [PubMed] [Google Scholar]
- 17.Mani S, Herceg Z.DNA demethylating agents and epigenetic therapy of cancer. Adv Genet 2010; 70:327-40 [DOI] [PubMed] [Google Scholar]
- 18.Li GD, Fang JX. From bench to bedside: Targeting epigenetics for cancer therapy. Clin Oncol Cancer Res 2011; 8:191-201 [Google Scholar]
- 19.Shao Y, Liu Y, Shao C, et al. Enhanced tumor suppression in vitro and in vivo by co-expression of survivin-specific siRNA and wild-type p53 protein. Cancer Gene Ther 2010; 17:844-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol 2011; 2011:978312. [DOI] [PMC free article] [PubMed]
- 21.Cillessen SA, Meijer CJ, Notoya M, et al. Molecular targeted therapies for diffuse large B-cell lymphoma based on apoptosis profiles. J Pathol 2010; 220:509-20 [DOI] [PubMed] [Google Scholar]
- 22.Guan YS, Liu Y, Zou Q, et al. Adenovirus-mediated wild-type p53 gene transfer in combination with bronchial arterial infusion for treatment of advanced non-small-cell lung cancer, one year follow-up. J Zhejiang Univ Sci B 2009; 10:331-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian G, Liu J, Zhou JS, et al. Multiple hepatic arterial injections of recombinant adenovirus p53 and 5-fluorouracil after transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a pilot phase II trial. Anticancer Drugs 2009; 20:389-95 [DOI] [PubMed] [Google Scholar]
- 24.Guan YS, Liu Y, He Q, et al. p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: One-year follow-up. World J Gastroenterol 2011; 17:2143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu P, Yang X, Huang Y, et al. Antitumor activity of a combination of rAd2p53 adenoviral gene therapy and radiotherapy in esophageal carcinoma. Cell Biochem Biophys 2011; 59:147-52 [DOI] [PubMed] [Google Scholar]
- 26.Cho HS, Park JH, Kim YJ.Epigenomics: novel aspect of genomic regulation. J Biochem Mol Biol 2007;40:151-5 [DOI] [PubMed] [Google Scholar]
- 27.Roth JA, Swisher SG, Meyn RE. p53 tumor suppressor gene therapy for cancer. Oncology 1999; 10(Spuppl 5):148-54. [PubMed]
- 28.Lu C, El-Deiry WS. Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis 2009; 14:597-606 [DOI] [PubMed] [Google Scholar]
- 29.Chen GX, Zheng LH, Liu SY, et al. rAd-p53 enhances the sensitivity of human gastric cancer cells to chemotherapy. World J Gastroenterol 2011; 17:4289-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang ZX, Wang D, Wang G, et al. Clinical study of recombinant adenovirus-p53 combined with fractionated stereotactic radiotherapy for hepatocellular carcinoma. J Cancer Res Clin Oncol 2010; 136:625-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achison M, Hupp TR. Hypoxia attenuates the p53 response to cellular damage. Oncogene 2003; 22:3431-40 [DOI] [PubMed] [Google Scholar]