Abstract
Objective
To analyze the differences in clinicopathologic characteristics and prognosis between mucinous gastric carcinoma (MGC) and signet-ring cell carcinoma (SRCC).
Methods
Clinicopathologic and prognostic data of 1,637 patients with histologically confirmed MGC or SRCC who received surgical operations in the Department of Gastroenterological Surgery, Beijing Cancer Hospital between December 2004 and December 2009 were retrospectively collected and analyzed. The clinicopathological features were analyzed statistically using χ2 test. Survival was analyzed using the Kaplan-Meier method and multivariate analysis of Cox proportional hazards regression model (backward, stepwise).
Results
A total of 181 patients with gastric cancer (74 MGC, 107 SRCC) were included. MGC, when compared with SRCC, was featured by senile patients, stage III and IV, upper third stomach, large tumor size, positive lymph node metastasis, and positive lymphatic vascular invasion (P<0.05). The overall 5-year survival rate showed no difference between the two groups (48.8% vs. 44.8%, P>0.05). However, the survival rate for MGC patients was significant lower than that for SRCC patients when compared among the age <60 years, negative distant metastasis, and tumor localized at upper third stomach (P<0.05). Multivariate Cox proportional hazards models revealed that distant metastasis was a significant independent prognostic indicator in MGC group, and lymph node metastasis and distant metastasis was significant independent prognostic indicators in SRCC group.
Conclusions
While compared with SRCC, MGC is associated with a more aggressive tumor biologic behavior. There is no statistically significant difference in distant metastasis, an independent prognostic indicator for both MGC and SRCC, which might be the reason for no significant difference of the overall survival rate between the patients with MGC and SRCC.
Key Words: Mucinous gastric carcinoma, signet-ring cell carcinoma, clinicopathology, prognosis
Introduction
Mucinous gastric carcinoma (MGC) and signet-ring cell carcinoma (SRCC) are mucin-producing cancers, characterized by the presence of intracellular mucin or extracellular mucin pool. A diagnosis of MGC was made when more than half of the tumor area contained extracellular mucin pools, and SRCC was diagnosed when adenocarcinoma was seen with a predominant component (>50%) of isolated tumor cells contained mucin (1). In gastric cancer, MGC was reported to be correlated with a worse prognosis than nonmucinous gastric carcinoma (NMGC) (2-4). Nevertheless, SRCC was associated with a favorable prognosis than non-signet ring cell carcinoma (NSRC) (5-8). According to the above conclusion, it seems like that MGC is correlated with a more aggressive cancer behavior and a worse prognosis than SRCC.
To clarify the histologic features and surgical outcome of patients between MGC and SRCC, we compared 74 MGC and 107 SRCC to verify if they are different on clinicopathological features and prognosis. The study included the followings: (I) clinicopathologic findings of MGC and SRCC; (II) survival rate of MGC and SRCC; and (III) the clinicopathological features influencing the prognosis of patients with MGC and SRCC, respectively.
Materials and methods
Patients
We studied a consecutive series of 1,637 patients who had undergone gastrectomy for cure of adenocarcinoma of the stomach at the Department of Gastroenterological Surgery, Beijing Cancer Hospital, from December 2004 to December 2009. There were 74 patients with MGC and 107 with SRCC. All patients had absence of preoperative chemotherapy or radiotherapy. A diagnosis of SRCC was made when adenocarcinoma was seen with a predominant component (>50%) of isolated tumor cells contained mucin, and MGC was diagnosed when more than half of the tumor area contained extracellular mucin pools (1). All resected specimens were fixed in 10% formalin solution and embedded in paraffin. Microscopic examinations were carried out using serial hematoxylin and eosin stained tissue sections taken through tumor centers.
The patients were evaluated with respect to age, gender, tumor location, tumor size, depth of invasion, lymph node metastasis, distant metastasis, lymphatic vascular invasion, Borrmann type, and TNM stage according to the Union International Cancer Control classification (9). Tumor size was the maximal tumor diameter reported on gross assessment of the tumor on the original pathology report. Surgical procedures were defined as curative when no grossly visible tumor tissue remained after resection, and resection margins were histologically normal (R0 resection). Any procedure that did not satisfy these conditions (R1 or R2 resection) was defined as non-curative. All patients received regular follow-up with physical examinations, laboratory tests, chest X-ray, computed tomography, ultrasonography, and endoscopy. The patient survival was evaluated according to census register certificates or outpatient records.
Statistical analysis
Data were analyzed statistically using χ2 test. Survival was analyzed using the Kaplan-Meier method. A multivariate analysis of Cox proportional hazards regression model (backward, stepwise) was created to assess the influence of each variable on survival. A P-value of <0.05 was considered statistically significant.
Results
Clinicopathological features
A total of 181 patients were studies, including 124 men and 57 women (mean age 58 years, range, 25-82 years). Table 1 shows the clinicopathological features of 74 patients with MGC and 107 patients with SRCC. MGC, when compared with SRCC, was featured by senile patients (59.5% vs. 40.2%), stage III and IV (68.9% vs. 50.5%), upper third stomach (36.5% vs. 12.1%), large tumor size (66.2% vs. 45.8%), positive lymph node metastasis (83.8% vs. 60.7%), and positive lymphatic vascular invasion (51.4% vs. 29.0%).
Table 1. Clinicopathological features in patients with MGC and SRCC.
| Variables | MGC (n=74) | SRCC (n=107) | P |
|---|---|---|---|
| Age (mean, years) | |||
| <60 | 30 (40.5%) | 64 (59.8%) | 0.011 |
| ≥60 | 44 (59.5%) | 43 (40.2%) | |
| Gender | |||
| Male | 56 (75.7%) | 68 (63.6%) | 0.084 |
| Female | 18 (24.3%) | 39 (36.4%) | |
| TNM Stage | |||
| I + II | 23 (31.1%) | 53 (49.5%) | 0.013 |
| III + IV | 51 (68.9%) | 54 (50.5%) | |
| Tumor location | |||
| Upper third stomach | 27 (36.5%) | 13 (12.1%) | <0.001 |
| Middle third stomach | 13 (17.6%) | 29 (27.1%) | |
| Lower third stomach | 25 (33.8%) | 59 (55.1%) | |
| Whole stomach | 9 (12.2%) | 6 (5.6%) | |
| Tumor size | |||
| <5 cm | 25 (33.8%) | 58 (54.2%) | 0.007 |
| ≥5 cm | 49 (66.2%) | 49 (45.8%) | |
| Depth of invasion | |||
| T1 + T2 | 26 (35.1%) | 53 (49.5%) | 0.055 |
| T3 + T3 | 48 (64.9%) | 54 (50.5%) | |
| Lymph node metastasis | |||
| Negative | 12 (16.2%) | 42 (39.3%) | 0.001 |
| Positive | 62 (83.8%) | 65 (60.7%) | |
| Distant metastasis | |||
| Negative | 48 (64.9%) | 68 (63.6%) | 0.856 |
| Positive | 26 (35.1%) | 39 (36.4%) | |
| Lymphatic vascular invasion | |||
| Negative | 36 (48.6%) | 76 (71.0%) | 0.002 |
| Positive | 38 (51.4%) | 31 (29.0%) | |
| Borrmann type | |||
| I + II | 21 (28.4%) | 20 (18.7%) | 0.126 |
| III + IV | 53 (71.6%) | 87 (81.3%) |
Overall survival analysis
The 1-year, 3-year and 5-year survival rates of MGC patients were 85.1%, 59.5% and 53.9%, respectively. The 1-year, 3-year and 5-year survival rates of SRCC patients were 71.4%, 51.7% and 45.4%, respectively. In all registered patients, there was no significant difference in the overall survival rate between patients with MGC and SRCC (χ2=1.882, P>0.05) (Figure 1).
Figure 1.
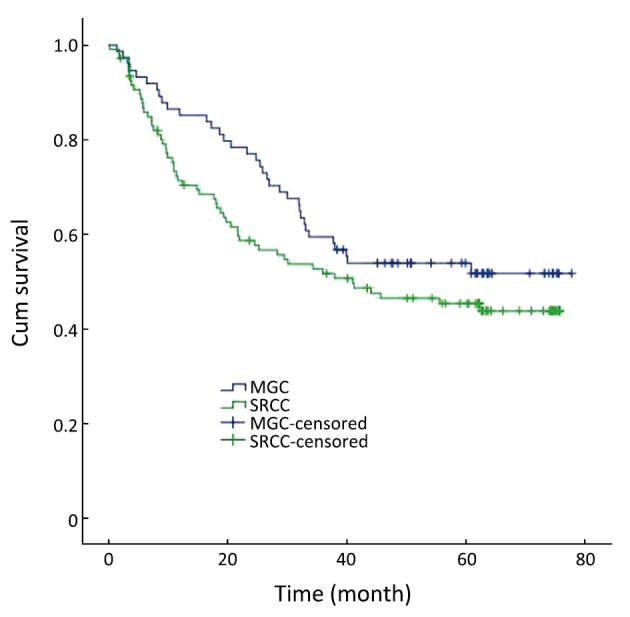
Survival curves for patients with MGC and SRCC. The difference of overall survival rates in patients was not statistically significant (P>0.05)
Stratified survival analysis in MGC and SRCC patients
The survival analysis was stratified by all above clinicopathological features including age, gender, TNM stage, tumor location, tumor size, depth of invasion, lymph node metastasis, distant metastasis, lymphatic vascular invasion and Borrmann type in MGC and SRCC patients, respectively. There was no significant difference in the stratified survival analyses, except the groups of age <60 years, without distant metastasis and tumor localized at upper third stomach.
When the age is <60 years, the 1-year, 3-year and 5-year survival rates were 86.7%, 70.0% and 66.5% in MGC patients, and 62.7%, 44.1% and 38.7% in SRCC patients, respectively. The overall survival rate was lower for SRCC than MGC when the age is less than 60 years (χ2=5.539, P=0.019) (Figure 2).
Figure 2.
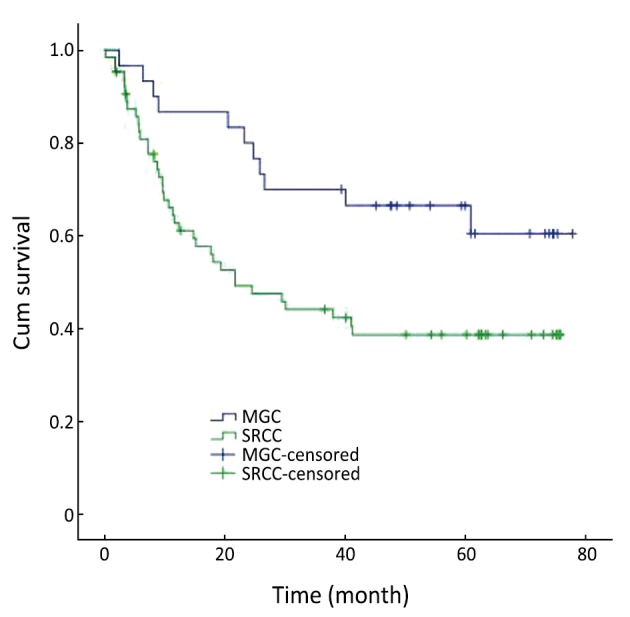
Survival curves for patients with MGC and SRCC below 60 years of age
In the group without distant metastasis, the 1-year, 3-year and 5-year survival rates were 97.9%, 68.7% and 64.5% in MGC patients, and 62.7%, 44.1% and 38.7% in SRCC patients, respectively. The overall survival rate was lower for SRCC than MGC in the group without distant metastasis (χ2=3.936, P=0.047) (Figure 3).
Figure 3.
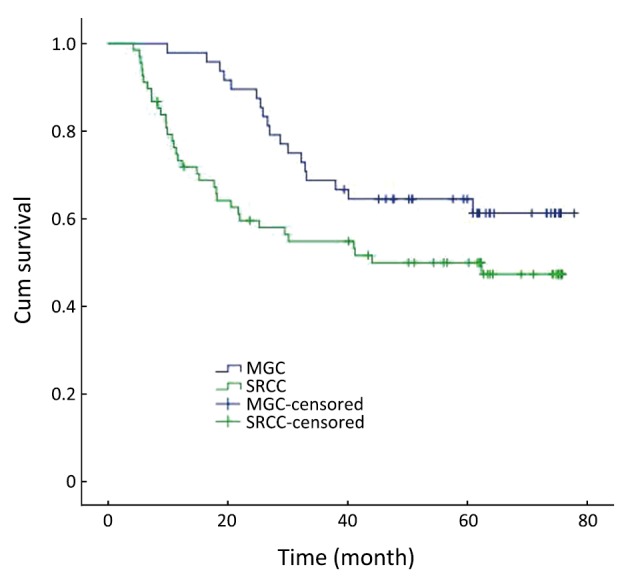
Survival curves for patients with MGC and SRCC without distant metastasis
In the group with tumor localized at the upper third stomach, the 1-year, 3-year and 5-year survival rates were 92.6%, 66.7% and 63.0% in MGC patients, and 61.5%, 35.9% and 26.9% in SRCC patients, respectively. The overall survival rate was lower for SRCC than MGC in the group without distant metastasis (χ2=4.967, P=0.026) (Figure 4).
Figure 4.
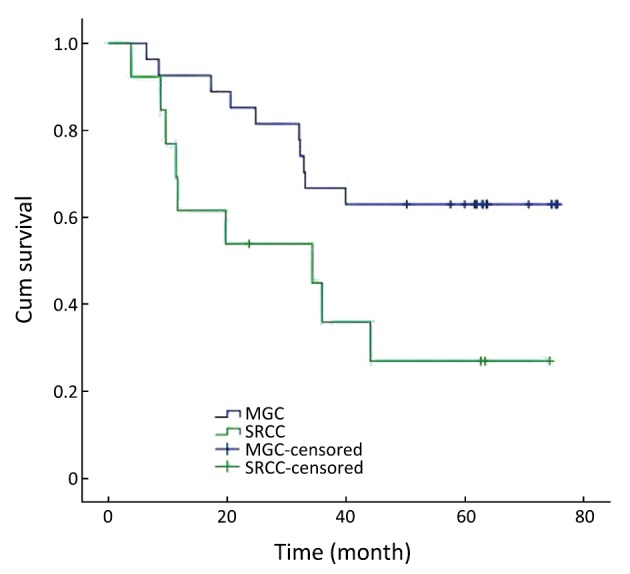
Survival curves for patients with MGC and SRCC at the upper third stomach
Multivariate analysis of Cox proportional hazards regression model
Multivariate Cox proportional hazards models used those variables associated with survival in MGC and SRCC groups, respectively, in our study, including depth of invasion, lymph node metastasis, distant metastasis, tumor location, tumor size, lymphatic vascular invasion, and Borrmann type. Table 2 reveals distant metastasis (P=0.001) was a significant independent prognostic indicator in MGC group. Table 3 reveals lymph node metastasis (P=0.019) and distant metastasis (P=0.039) were significant independent prognostic indicators in SRCC group.
Table 2. Cox proportional-hazard analysis of death for MGC.
| Variables | P | Relative Risk | 95% CI |
|
|---|---|---|---|---|
| lower | upper | |||
| Depth of invasion | ||||
| T1 + T2 vs. T3 +T4 | 0.372 | 1.467 | 0.633 | 3.404 |
| Lymph node metastasis | ||||
| Negative vs. positive | 0.289 | 1.847 | 0.594 | 5.748 |
| Distant metastasis | ||||
| Negative vs. positive | 0.001 | 3.891 | 1.718 | 8.772 |
| Tumor location | ||||
| Upper third stomach vs. middle third stomach | 0.614 | 0.738 | 0.227 | 2.403 |
| Middle third stomach vs. lower third stomach | 0.287 | 2.053 | 0.547 | 7.706 |
| Lower third stomach vs. whole stomach | 0.826 | 0.876 | 0.268 | 2.862 |
| Tumor size | ||||
| <5 cm vs. ≥5 cm | 0.520 | 1.298 | 0.586 | 2.874 |
| Lymphatic vascular invasion | ||||
| Negative vs. positive | 0.386 | 0.688 | 0.295 | 1.603 |
| Borrmann type | ||||
| I + II vs. III + IV | 0.369 | 0.651 | 0.256 | 1.659 |
95% CI, 95% confidence interval
Table 3. Cox proportional-hazard analysis of death for SRCC.
| Variables | P | Relative Risk | 95% CI |
|
|---|---|---|---|---|
| lower | upper | |||
| Depth of invasion | ||||
| T1 + T2 vs. T3 +T4 | 0.539 | 1.258 | 0.605 | 2.618 |
| Lymph node metastasis | ||||
| Negative vs. positive | 0.019 | 2.604 | 1.167 | 5.812 |
| Distant metastasis | ||||
| Negative vs. positive | 0.039 | 2.273 | 1.042 | 4.950 |
| Tumor location | ||||
| Upper third stomach vs. middle third stomach | 0.476 | 1.635 | 0.424 | 6.305 |
| Middle third stomach vs. lower third stomach | 0.865 | 0.893 | 0.241 | 3.311 |
| Lower third stomach vs. whole stomach | 0.761 | 0.825 | 0.239 | 2.848 |
| Tumor size | ||||
| <5 cm vs. ≥5 cm | 0.821 | 0.918 | 0.436 | 1.930 |
| Lymphatic vascular invasion | ||||
| Negative vs. positive | 0.760 | 0.901 | 0.463 | 1.754 |
| Borrmann type | ||||
| I + II vs. III + IV | 0.161 | 0.581 | 0.272 | 1.242 |
95% CI, 95% confidence interval
Discussion
Our data shows that, when compared with SRCC, MGC was featured by senile patients, stage III and IV, upper third stomach, large tumor size, positive lymph node metastasis, and positive lymphatic vascular invasion (P<0.05). Although the overall 5-year survival rate showed no difference between the two groups, the survival rate for MGC patients was significant lower than that for SRCC patients when compared among the age <60 years, negative distant metastasis, and tumor localized at upper third stomach (P<0.05). Our data also showed that distant metastasis is an independent prognostic indicator for both MGC and SRCC. However, there is no statistical significance between MGC and SRCC. That might be the reason that there is no significant difference in the overall survival between them.
Tumor depth of invasion significantly correlates with both lymphovascular invasion and lymph nodes metastasis (10). Lymphatic vascular invasion was reported to be an independent risk factor for survival in gastric cancer (11). Patients with lymph node metastases had a higher recurrence rate than those who were lymph node-negative, and had a higher rate of adverse prognoses (12). Our study also showed that lymph node metastasis and distant metastasis are the independent prognostic factors for SRCC.
Goseki (13) and Martin (14) histological grading system classified gastric cancer according to mucin component and tubular differentiation. These cannot be applied to the current study because all our patients had mucin-rich cancer. SRCC does not form glandular tubules, resulting in the accumulate of mucin in the cytoplasm (13). On the contrary, the glandular tubules in MGC can drain the mucus out of the cancer cells, resulting in the pooling of cancer cells in the mucin (13), which may lead to the loss of cancer cell adhesion. It is well known that abnormal expression of some cell adhesion molecules, such as E-cadherin-catenin complex, is associated with loss of differentiation and increased invasive capacity both in vivo (15) and in vitro (16). Loss of E-cadherin expression occurs during epithelial-to-mesenchymal transition (EMT), which facilitates migration and invasion of epithelial tumor cells. The loss of E-cadherin promotes metastasis (17-19). In gastric cancer, expression loss of E-cadherin was reported to be higher in MGC than in SRCC (20). As a result, loss of cell adhesion might play an important role in the aggressive behavior and poor prognosis in MGC. Similar to the above findings, we found that MGC tends to be with lymph node metastasis and lymphatic vascular invasion. Moreover, the survival rate for MGC patients was significantly lower than that for SRCC patients when there is no distant metastasis, and distant metastasis is the independent prognostic factor for MGC.
In conclusion, while compared with SRCC, MGC is associated with a more aggressive tumor biologic behavior. There is no statistically significant difference in distant metastasis, an independent prognostic indicator for both MGC and SRCC, which might be the reason for no significant difference of the overall survival rate between the patients with MGC and SRCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma, ed. 1st. Tokyo: Kanehara, 1995. [Google Scholar]
- 2.Yasuda K, Adachi Y, Shiraiahi N, et al. Pathology and prognosis of mucinous gastric carcinoma. J Surg Oncol 2001;76:272-7 [DOI] [PubMed] [Google Scholar]
- 3.Lim SW, Kim DY, Kim YJ, et al. Clinicopathologic features of mucinous gastric carcinoma. Dig Surg 2002;19:286-90 [DOI] [PubMed] [Google Scholar]
- 4.Yin C, Li D, Sun Z, et al. Clinicopathologic features and prognosis analysis of mucinous gastric carcinoma. Med Oncol 2012;29:864-70 [DOI] [PubMed] [Google Scholar]
- 5.Otsuji E, Yamaguchi T, Sawai K, et al. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol 1998;67:216-20 [DOI] [PubMed] [Google Scholar]
- 6.Kim DY, Park YK, Joo JK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 2004;74:1060-4 [DOI] [PubMed] [Google Scholar]
- 7.Jiang CG, Wang ZN, Sun Z, et al. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: Results from a Chinese mono-institutional study. J Surg Oncol 2011;103:700-3 [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Zhu G, Zhang H, et al. Clinicopathologic feathers of gastric carcinoma with signet ring cell histology. J Gastrointest Surg 2010;14:601-6 [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80;1803-4. [DOI] [PubMed] [Google Scholar]
- 10.Dicken BJ, Saunders LD, Jhangri GS, et al. Gastric cancer: establishing predictors of biologic behavior with use of population based data. Ann Surg Oncol 2004;11:629-35 [DOI] [PubMed] [Google Scholar]
- 11.Dicken BJ, Graham K, Hamilton SM, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg 2006;243:64-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DY, Seo KW, Joo JK, et al. Prognostic factors in patients with node-negative gastric carcinoma: a comparison with node-positive gastric carcinoma. World J Gastroenterol 2006;12:1182-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goseki N, Takizawa T, Koike M.Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut 1992;33:606-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin IG, Dixon MF, Sue-Ling H, et al. Goseki histological grading of gastric cancer is an important predictor of outcome. Gut 1994;35:758-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Kitajima Y, Edakuni G, et al. Abnormal expression of E-cadherin and beta-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg 2002;89:236-44 [DOI] [PubMed] [Google Scholar]
- 16.Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991;113:173-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savagner P.Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays 2001;23:912-23 [DOI] [PubMed] [Google Scholar]
- 18.Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol 2000;60:1091-9 [DOI] [PubMed] [Google Scholar]
- 19.Yonemura Y, Ninomiya I, Kaji M, et al. Decreased E-cadherin expression correlates with poor survival in patients with gastric cancer. Anal Cell Pathol 1995;8:177-90 [PubMed] [Google Scholar]
- 20.Sugihara H, Hattori T, Fukuda M, et al. Cell proliferation and differentiation in intramucosal and advanced signet ring cell carcinomas of the human stomach. Virchows Arch A Pathol Anat Histopathol 1987;411:117-27 [DOI] [PubMed] [Google Scholar]


