Abstract
Objective
Metaplastic meningioma is a rare subtype of benign meningiomas, classified as WHO grade I with well prognosis. Here we presented our experiences on 15 cases of metaplastic meningioma, to investigate the clinicopathological features, therapies and prognosis of these cases.
Methods
15 patients underwent surgical treatment for intracranial metaplastic meningioma between 2001 and 2010 at Neurosurgery Department of Huashan Hospital, Shanghai, China. The clinical data, radiological manifestation, treatment strategy, pathological findings and prognosis of all patients were analyzed retrospectively.
Results
Among the 15 cases (10 males and 5 females), the age ranged from 22 to 74 years old (the mean age was 50.67-year old). The clinical manifestations include headache, dizziness, seizure attack, vision decrease, and weakness of bilateral lower limbs. All the patients received surgical treatment, combined with radiotherapy in some cases. In the follow-up period, recurrence occurred in 2 cases, of which 1 patient died of other system complications.
Conclusions
Metaplastic meningiomas are characterized by focal or widespread mesenchymal differentiation with formation of bone, cartilage, fat, and xanthomatous tissue elements. Surgical removal is the optimal therapy, and the overall prognosis is well. But recurrence may occur in some cases, thus radiotherapy is necessary for such kind of patients.
Key Words: Metaplastic meningioma, follow-up, mesenchymal differentiation
Introduction
Meningiomas are relatively common tumors derived from arachnoidal cells and most frequently occur in association with intracranial meninges. They make up about 20% of primary brain and spinal cord tumors (1). The World Health Organization (WHO) classification of the tumors of the central nervous system defines various types of meningiomas. Most meningiomas are slowly growing tumors (WHO grade I), including some rare subtypes such as microcystic meningioma, secretory meningioma and metaplastic meningioma. Brain invasive (WHO grade II), atypical (WHO grade II), and anaplastic (WHO grade III) meningiomas are considerably more aggressive (2,3).
Metaplastic meningioma is a rare subtype of WHO grade I tumor, being characterized by focal or widespread mesenchymal differentiation with formation of bone, cartilage, fat, and xanthomatous tissue elements in pathology (4). In the study, we studied the clinical, radiological and histopathologic features of 15 cases of metaplastic meningioma in the Neurosurgery Department of Huashan Hospital, Shanghai, China. In particular, we focused on the immunomarker of the tumors, such as epithelial membrane antigen (EMA), vimentin and glial fibrillary acidic protein (GFAP). And, we evaluated the relationship of prognosis with histopathologic features. To the best of our knowledge, this is very large cases of metaplastic meningioma review in the existing literature.
Patients and clinical data
According to the WHO 2007 tumor grading system, 15 patients were diagnosed as metaplastic meningioma at the Department of Neurosurgery, Huashan Hospital of Fudan University, Shanghai, China from 2001 to 2010. The clinical data, radiologic manifestation, treatment, histopathologic features and prognosis were retrospectively analyzed.
As shown in Table 1, the 15 patients (10 males and 5 females) had a mean age of 50.67 (Mean±SD) years old (from 22 to 74-year old). All of these 15 patients underwent operations in our department. 14 of them were at initial presentation, and one patient had recurrent tumor at admission. He was previously undergone meningioma resection (No. 10 case). In the follow-up period, one patient got recurrent tumor, and he took gamma-knife therapy first and underwent operation later, but died eventually due to other systems’ complications (No. 2 case).
Table 1. List of the 15 metaplastic meningioma cases.
| Case No. | Gender/age | Symptom and duration | Tumor location | Surgical removal | Recurrence (Y/N) |
|---|---|---|---|---|---|
| 1 | Male/40 | Bilateral vision blur for 1 month | Right parietal-occipital, parafalcine | Sympson I | N |
| 2 | Male/70 | Headache for 2 years | Left ventricular | Sympson I | Y |
| 3 | Female/72 | Numbness and weakness of bilateral lower limbs for 2 years | T4-5 | Sympson I | N |
| 4 | Male/41 | Headache for 1 year | Right ventricular | Sympson I | N |
| 5 | Female/48 | Bilateral visual acuity decrease for 2 years |
Petroclival | Sympson III | / |
| 6 | Female/60 | Epilepsy twice within 6 months | Right petroclival | Sympson I | N |
| 7 | Male/34 | Epilepsy twice within 4 months | Right frontal, parasagittal | Sympson I | N |
| 8 | Female/51 | Numbness and weakness of bilateral lower limbs for 4 months | T7-9 | Sympson I | N |
| 9 | Male/47 | Found intracranial lesion by checkup for 4 months | Right CPA | Sympson I | N |
| 10 | Male/42 | Post-operation of right sphenoid ridge meningioma for 6 years | Right sphenoid ridge, sellar region | Sympson II | N |
| 11 | Male/51 | Epilepsy for 2 years | Right middle cranial fossa | Sympson II | N |
| 12 | Female/74 | Numbness and weakness of bilateral lower limbs for 5 months | T7 | Sympson I | / |
| 13 | Male/22 | Headache for 3 months | Sinus region | Sympson III | / |
| 14 | Male/68 | Diplopia for 20 days | Right frontal | Sympson I | N |
| 15 | Male/40 | Absence epilepsy three times within half a month | Right sphenoid ridge | Sympson II | Y |
Results
Clinical characteristics
The 15 patients (10 males and 5 females) had a mean age of 50.67 (x̄±s) years old (from 22 to 74-year-old), as Table 1 showed. Among the 15 patients, the major preoperative signs and symptoms were headache (3/15), dizziness (1/15), seizure attack (4/15), vision blur and decrease (3/15), weakness of bilateral lower limbs (3/15), and one case was found by health checkup. The average duration between the onset of symptoms and admission was 13.88 months (from 2 weeks to 6 years). The locations were mainly convexity (3/15), spinal cord (3/15), sphenoid wing and sellar region (2/15), intraventricular (2/15), cerebellopontine angle (CPA) (1/15), sinus region (1/15), petroclival (2/15), and cranial fossa (1/15).
Radiological findings
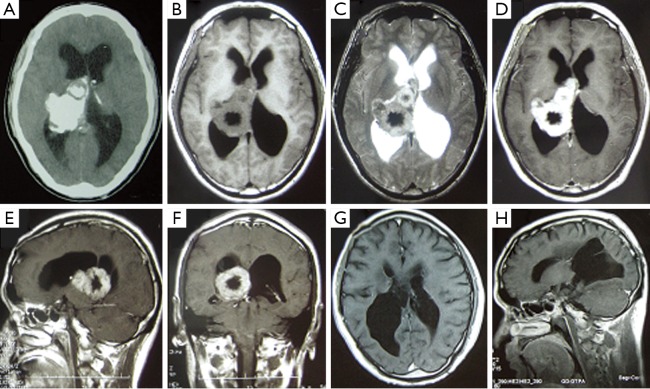
All the 15 cases had routine brain computed tomography (CT) and magnetic resonance imaging (MRI) examination before operation. The tumors ranged from 1.5 to 36 cm3 in volume, with a mean volume of 17.61 cm3 (Mean±SD). In terms of radiological images, 4 tumors contained calcification in brain CT scan (Figure 1A). 8 tumors were isointense and 7 tumors were hypointense on T1-weighted MRI images (Figure 1B), 2 of which had obvious peritumoral brain edema; 10 tumors were hyperintense and 5 tumors showed mixed intense on T2-weighted MRI images (Figure 1C); 12 tumors exhibited homogenous enhancement and 3 tumors were heterogeneously enhanced by contrast MRI (Figure 1D-F).
Figure 1.
No. 4 case of giant right ventricular metaplastic meningioma. A. Axial head CT scan revealing a giant right ventricular mass with high density and calcification in case 4; B. The tumor was mostly isointense in TI-MRI, but part of it was hypointense; C. The tumor was a little hyperintense in T2-MRI; D-F. After the administration of contrast, the lesion demonstrated obvious enhancement, but part of it wasn’t enhanced; G-H. Follow-up MRI obtained after operation showed no tumor recurrence
Treatment and prognosis
All 15 patients were treated surgically. The Simpson Classification was used to evaluate the extent of surgical resection. 10 patients were achieved Simpson Grade I resection, 3 patients were Simpson Grade II resection, and 2 patients were Simpson Grade III resection. In the follow-up period, three patients were lost. One patient had recurrent tumor 1 year later, and received gamma-knife therapy first and operation later. The patient was died finally in 2004 due to other systems’ complications (No. 2 case). Another patient got recurrence 6 months later, and received gamma-knife therapy. He was kept in follow-up without any uncomfortable signs (No. 15 case).
Histopathological features
The tissue samples taken from the operation were stained with hematoxylin and eosin (Figure 2A). Tumor cells displayed typical features, for instance, focal or widespread mesenchymal differentiation with formation of bone, cartilage, fat, and xanthomatous tissue elements. Among the 15 meningiomas, 9 tumors contained bone or cartilage tissue, 2 tumors formed fat-like tissue, 2 tumors exhibited xanthomatous tissue elements, and 2 tumors contained smooth muscle actin (SMA) positive tissues.
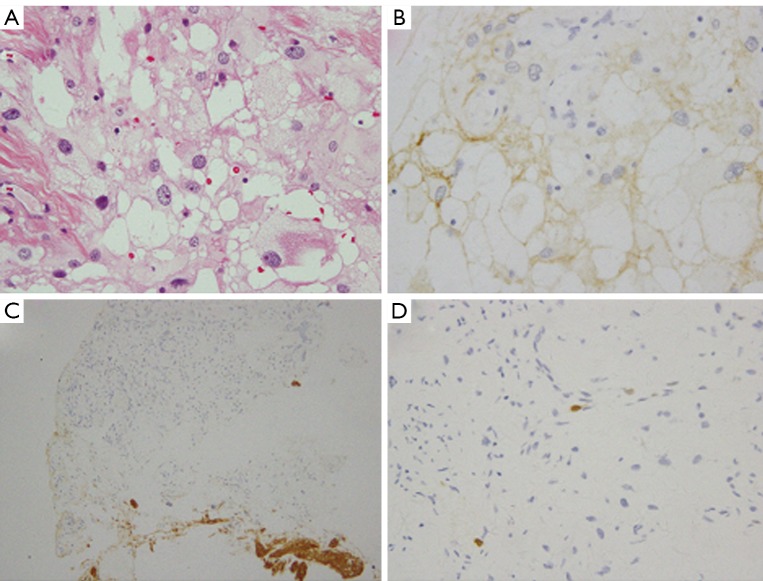
Figure 2.
Histopathological findings of case 10. A. Tumor tissue contained large fat-like proliferation with conventional meningothelial neoplastic cells. Tumor cells have round nuclei and fat vacuole in cytoplasm in case 10 (H&E, original magnification ×40); B. The tumor cells were positive for EMA (original magnification ×40); C. The tumor cells were negative for GFAP (original magnification ×20); D. MIB-1 labeling index was less than 1% (original magnification ×40)
By immunohistochemical staining, tumors were positive for vimentin and epithelial membrane antigen (EMA) in all 15 cases (Figure 2B). 14 of the 15 tumors were negative for glial fibrillary acidic protein (GFAP) (Figure 2C), and one was GFAP (-/+). Two tumors were S-100 (-/+), two tumors were CD34 (+), and two tumors were positive for SMA (Figure 3). The MIB-1 labeling index varied from 0.1% to 3% (Table 2, Figure 2D). It was interesting that the original pathology of No. 10 case was meningothelial meningioma, while the recurrent tumor was metaplastic meningioma containing fat-like tissue.
Figure 3.
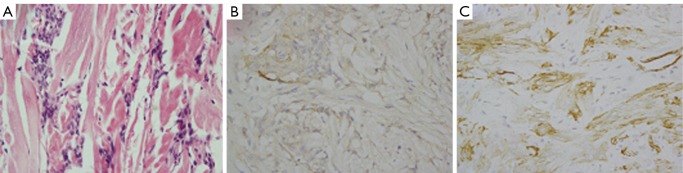
Histopathological features of SMA positive meningioma in case 2. A. Tumor tissue contained much smooth muscle-like tissues in case 2 (H&E, original magnification ×40); B. The tumor cells were positive for EMA (original magnification ×40); C. The tumor cells were positive for SMA (original magnification ×40)
Table 2. Pathology of the 15 metaplastic meningiomas.
| Case No. | Pathology subtype | EMA | vimentin | GFAP | MIB-1 (%) |
|---|---|---|---|---|---|
| 1 | smooth muscle | + | + | - | 1 |
| 2 | smooth muscle | + | + | - | 1 |
| 3 | osseous | + | + | - | <1 |
| 4 | osseous | + | + | - | <1 |
| 5 | osseous | + | + | - | <1 |
| 6 | osseous | + | + | - | <1 |
| 7 | osseous | + | + | - | 0.1 |
| 8 | osseous | + | + | - | <1 |
| 9 | osseous | + | + | - | <1 |
| 10 | lipomatous | + | + | - | <1 |
| 11 | lipomatous | + | + | - | 1 |
| 12 | osseous | + | + | - | <1 |
| 13 | xanthomatous | + | + | - | <1 |
| 14 | xanthomatous | + | + | -/+ | <1 |
| 15 | osseous | + | + | - | 3 |
Statistical analysis
Student’s t-test was used to compare the difference of MIB-1 labeling index among the 15 patients. Distributions of time to progression and time to recurrence were estimated using the Kaplan-Meier. Data were presented as x̄±s, and the significance level was 0.05. All analyses were performed using Statistical Package for Social Sciences (SPSS 16).
Discussion
Meningiomas are neoplasms derived from meningothelial cells and show histological diversity. The World Health Organization (WHO) recognizes a metaplastic type of meningioma as grade I tumor, which is characterized by mesenchymal elements including osseous, cartilaginous, lipomatous, xanthomatous and myxoid tissue (4). Currently, the biological behavior of intracranial metaplastic meningioma is poorly understood, partly due to a limited number of reported cases. In this paper, we retrospectively analyzed the clinical, radiological manifestation, histological and immunohistochemical characteristics of 15 metaplastic meningiomas observed in our department.
Clinical and radiological features
In our report of the 15 cases, there were 10 males and 5 females. The clinical symptoms of metaplastic meningioma were usually various, which depend on the location and volume of the tumors. Dizziness, headache, epilepsy and neurological deficits were presented. The tumor sites were mainly convexity, spinal cord, sphenoid wing and sellar region, intraventricular, CPA, sinus region, petroclival, and cranial fossa (Table 1).
In terms of radiological images, 4 tumors contained calcification in brain CT scan. 8 tumors were isointense and 7 tumors were hypointense on T1-weighted MRI images, 2 of which had obvious peritumoral brain edema; 10 tumors were hyperintense and 5 tumors were mixed intense on T2-weighted MRI images; 12 tumors were homogenous enhancement and 3 tumors were heterogeneously enhanced by contrast MRI (Figure 1).
Histopathological findings
Immunohistochemical staining is helpful in diagnosing metaplastic meningioma. Metaplastic meningioma shows positive reactivity for vimentin and EMA, similarly to other meningioma subtypes, and it couldn’t be immunoreactive to GFAP (Table 2, Figure 2). Their staining for vimentin and EMA reflects their dual mesenchymal and epithelial properties.
The metaplastic tumor type encompasses a broad range of tumor subtypes depending on the mesenchymal differentiation involved. Myxoid, osseous, cartilaginous, lipomatous, and xanthomatous subtypes are categorized in this group (2,5). These meningeal tumors are referred to as “metaplastic” because their transformed neoplastic cells demonstrate the full histological characteristics of the cells they mimic (6,7).
For instance, with lipomatous metaplastic meningioma, the adipocytes resemble true fat cells with their signet-ring appearance (Figure 2) (8-15). The myxoid appearance in the myxoid type is attributable to the excessive presence of hyaluronic acid and chondroitin sulfate (16-19). There have been several reported cases of metaplastic meningioma in the literature with the majority being osseous types (20,21). In our 15 cases, most metaplastic meningiomas are also osseous subtypes (Figure 4). Additionally, there were several cases about xanthomatous subtype (22).
Figure 4.
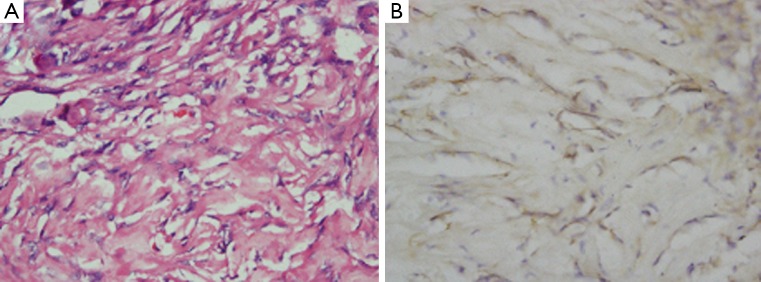
Histopathological findings of metaplastic meningioma with osseous tissues. A. Tumor tissue contained areas of calcification and ossification in case 4 (H&E, original magnification ×40); B. The tumor cells were positive for EMA (original magnification ×40)
In our 15 cases, two tumors were positive for SMA (Figure 3). Meningiomas cells with SMA expression suggested its muscular differentiation (or dedifferentiation) (23). Metaplastic meningioma is histologically unique. But it should be differentiated from other CNS tumors, such as other benign meningiomas, high grade gliomas or metastases.
It was interesting that the original pathology of No. 10 case was meningothelial meningioma, while the recurrent tumor was metaplastic meningioma containing fat-like tissue. Researchers proposed that lipomatous component ought to be considered as an advanced lipidisation of neoplastic meningothelial cells rather than true metaplastic transformation of meningothelial cells into mature fat tissue (12). Meningothelial features are often retained even in cells that look remarkably mesenchymal (4).
Treatment and natural history
All the 15 patients were treated surgically in our report. 10 patients were achieved Simpson Grade I resection, 3 patients were Simpson Grade II resection, and 2 patients were Simpson Grade III resection. In the follow-up period, three patients were lost. No. 2 case had recurrent tumor 1 year later, and received gamma-knife therapy first and operation later. The patient was died finally due to other systems’ complications. Another patient got recurrence 6 months later, and received gamma-knife therapy. He was kept in follow-up without any uncomfortable signs (No. 15 case).
The current literature indicates that metaplastic meningioma grows like other grade I meningiomas with similar recurrence rates (24). However, there are no known retrospective cohort studies with long-term follow-up that help to shed light on the natural course of this disease and its anticipated prognosis. Given its low histological grade, this lesion is believed to carry a good prognosis with little chance for recurrence.
Acknowledgements
The study was supported by National Natural Science Foundation of China (81200936, 30872675, 30901549), Shanghai Committee of Science and Technology (12JC1401800), and 2011 Shanghai Medical College Young Scientist Fund of Fudan University (11L-24).
Disclosure: The authors declare no conflict of interest.
References
- 1.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006;5:1045-54 [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Cavenee WK. eds. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC, 2000. [Google Scholar]
- 3.Perry A, Louis DN, Scheithauer BW, et al. In: Louis DN, Ohgaki H, Wiestler OD, et al. eds. The WHO Classification of Tumors of the Nervous System. Lyon: IARC, 2007:163-72. [Google Scholar]
- 4.Mawrin C, Perry A.Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010;99:379-91 [DOI] [PubMed] [Google Scholar]
- 5.Roncaroli F, Scheithauer BW, Laeng RH, et al. Lipomatous meningioma: a clinicopathologic study of 18 cases with special reference to the issue of metaplasia. Am J Surg Pathol 2001;25:769-75 [DOI] [PubMed] [Google Scholar]
- 6.Rosai J, Akerman M, Dal Cin P, et al. Combined morphologic and karyotypic study of 59 atypical lipomatous tumors. Evaluation of their relationship and differential diagnosis with other adipose tissue tumors (a report of the CHAMP Study Group). Am J Surg Pathol 1996;20:1182-9 [DOI] [PubMed] [Google Scholar]
- 7.Scheithauer BW. Tumors of the meninges: proposed modifications of the World Health Organization classification. Acta Neuropathol 1990;80:343-54 [DOI] [PubMed] [Google Scholar]
- 8.Colnat-Coulbois S, Kremer S, Weinbreck N, et al. Lipomatous meningioma: report of 2 cases and review of the literature. Surg Neurol 2008;69:398-402; discussion 402 [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal AK, Mehrotra A, Kumar B, et al. Lipomatous meningioma: a study of five cases with brief review of literature. Neurol India 2011;59:87-91 [DOI] [PubMed] [Google Scholar]
- 10.Ohba S, Yoshida K, Akiyama T, et al. Lipomatous meningioma. J Clin Neurosci 2007;14:1003-6 [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Sharma KC. Lipomatous metaplasia occurring within meningiomas--two case reports. Indian J Pathol Microbiol 2005;48:477-8 [PubMed] [Google Scholar]
- 12.Matyja E, Naganska E, Zabek M, et al. Meningioma with the unique coexistence of secretory and lipomatous components: a case report with immunohistochemical and ultrastructural study. Clin Neuropathol 2005;24:257-61 [PubMed] [Google Scholar]
- 13.Withers T, Klevansky A, Weinstein SR. Lipomeningioma: case report and review of the literature. J Clin Neurosci 2003;10:712-4 [DOI] [PubMed] [Google Scholar]
- 14.Bolat F, Kayaselcuk F, Aydin MV, et al. Lipidized or lipomatous meningioma, which is more appropriate? A case report. Neurol Res 2003;25:764-6 [DOI] [PubMed] [Google Scholar]
- 15.Mariniello G, Spaziante R, Del Basso De Caro ML, et al. An unusual case of lipoblastic meningioma of the falx cerebri. Clin Neurol Neurosurg 2000;102:180-5 [DOI] [PubMed] [Google Scholar]
- 16.Krisht KM, Altay T, Couldwell WT. Myxoid meningioma: a rare metaplastic meningioma variant in a patient presenting with intratumoral hemorrhage. J Neurosurg 2012;116:861-5 [DOI] [PubMed] [Google Scholar]
- 17.Dulai MS, Khan AM, Edwards MS, et al. Intraventricular metaplastic meningioma in a child: case report and review of the literature. Neuropathology 2009;29:708-12 [DOI] [PubMed] [Google Scholar]
- 18.Bégin LR. Myxoid meningioma. Ultrastruct Pathol 1990;14:367-74 [DOI] [PubMed] [Google Scholar]
- 19.Harrison JD, Rose PE. Myxoid meningioma: histochemistry and electron microscopy. Acta Neuropathol 1985;68:80-2 [DOI] [PubMed] [Google Scholar]
- 20.Barresi V, Caffo M, Ieni A, et al. Osteoblastic meningiomas: clinico-pathological and immunohistochemical features of an uncommon variant. J Neurooncol 2011;105:225-32 [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Petersson F.Intracerebral metaplastic meningioma with prominent ossification and extensive calcification. Rare Tumors 2011;3:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikota H, Nakazato Y.A case of metaplastic meningioma with extensive xanthomatous change. Neuropathology 2008;28:422-6 [DOI] [PubMed] [Google Scholar]
- 23.Zhang FL, Gao J, Huang FP, et al. The significances of smooth muscle actin positive expression in meningeomas. Chinese Journal of Neuro-oncology 2007;5:108-11 [Google Scholar]
- 24.Johnson MD, Stevenson CB, Thompson RC, et al. December 2006: 31-year-old woman with hemiparesis. Brain Pathol 2007;17:255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]