Abstract
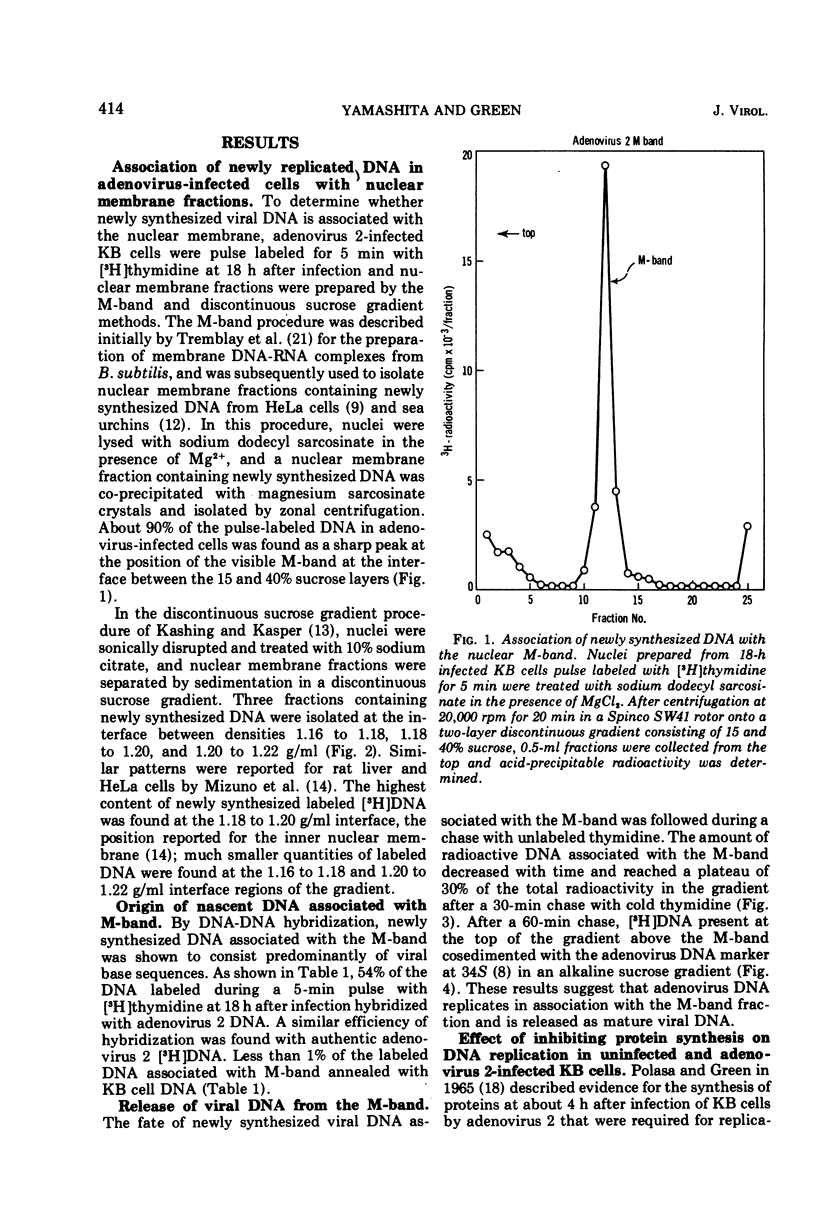
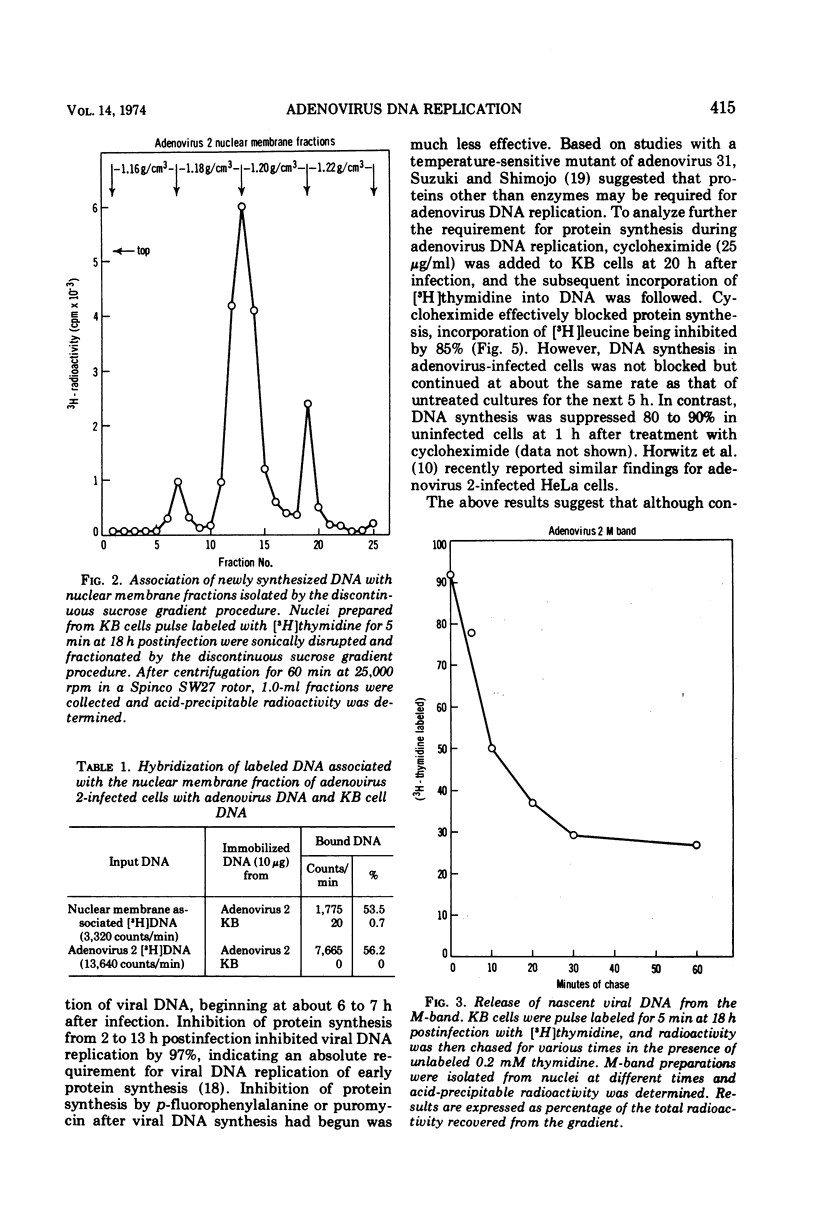
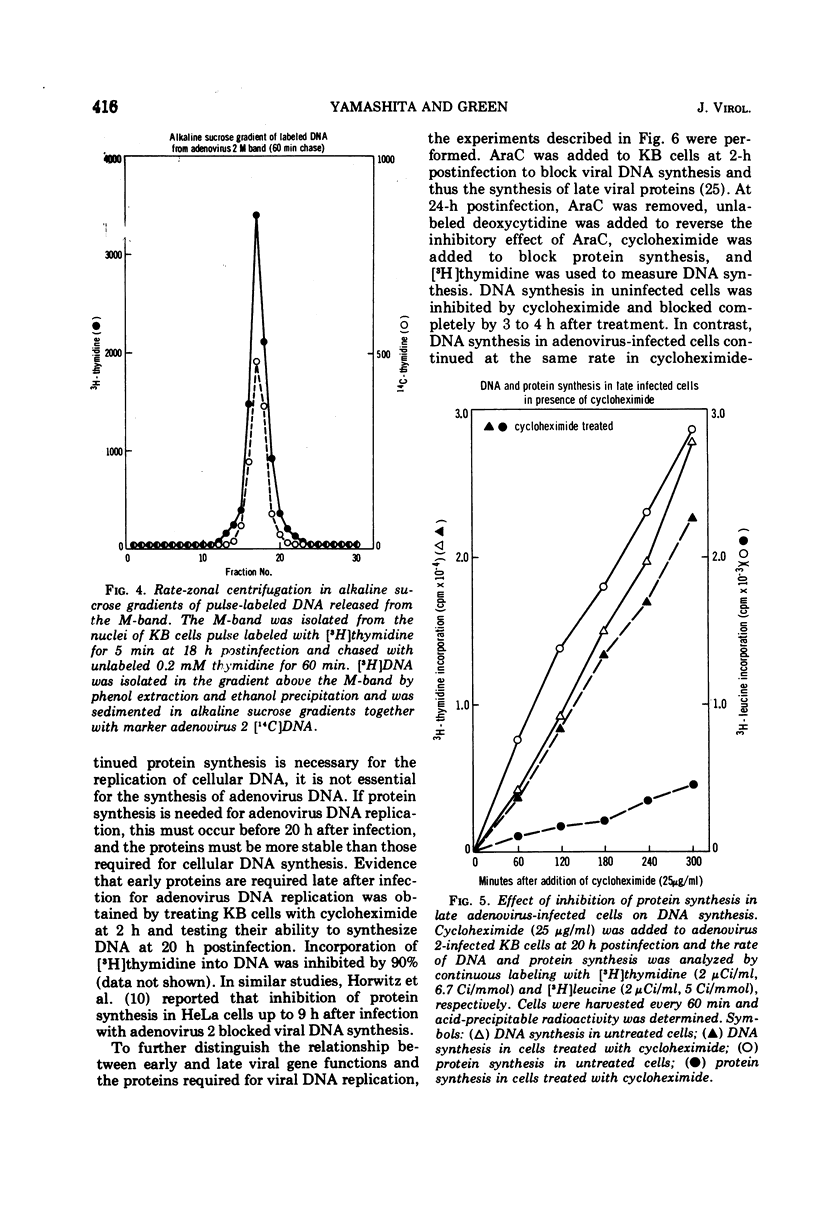
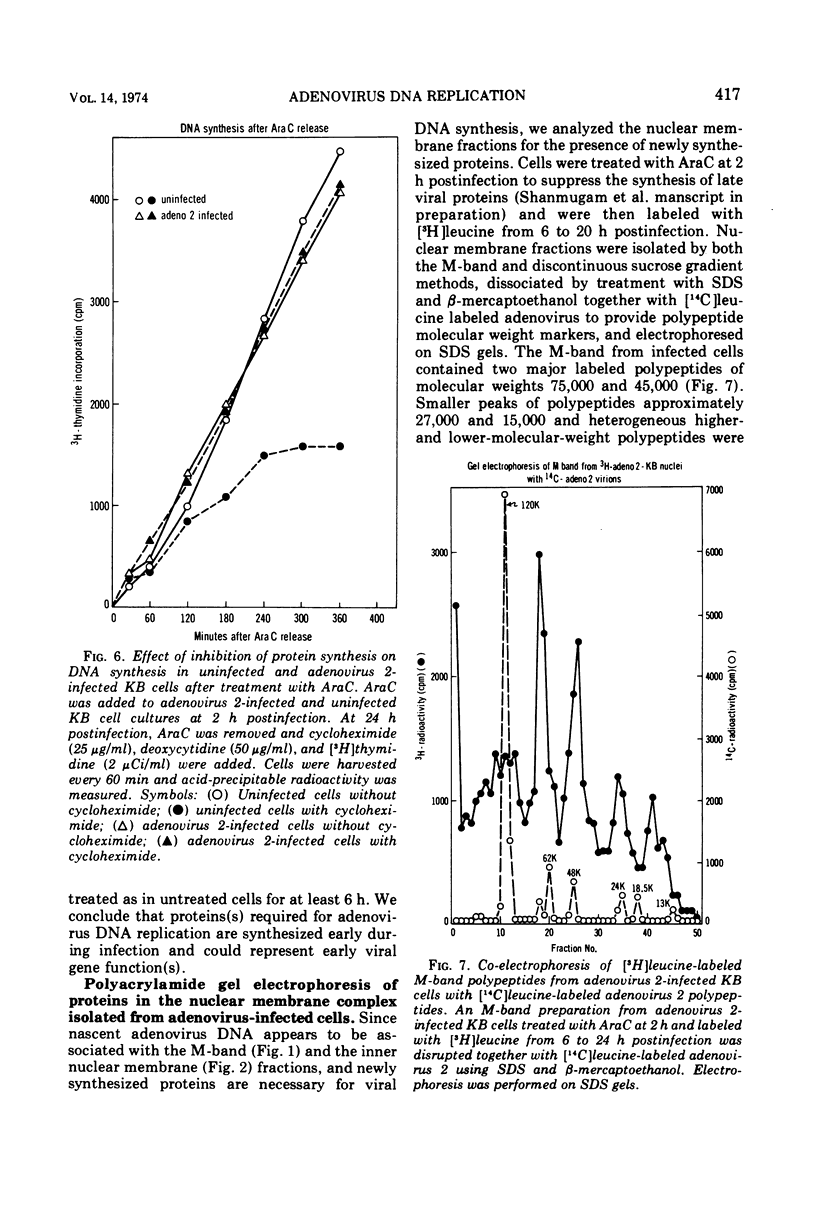
Nuclear membrane fractions were prepared by two procedures from KB cells pulse labeled with [3H]thymidine for 5 min late after infection with adenovirus 2: (i) the M-band technique, which yields a sharp peak containing most of the newly synthesized viral DNA, and (ii) the discontinuous sucrose gradient method, which yields three membrane fractions, one which bands at the interface between sucrose layers at density 1.18 and 1.20 g/ml and contains most of the newly synthesized viral DNA. Studies using cycloheximide to inhibit protein synthesis showed that proteins whose synthesis begins early after infection and occurs in the absence of viral DNA replication are required for viral DNA synthesis late after infection. To study the nature of these proteins, nuclear membrane fractions were isolated from cells labeled with [3H]leucine from 6 to 24 h postinfection in the presence of arabinosyl cytosine to block viral DNA replication, and were analyzed by electrophoresis in sodium dodecyl sulfate polyacrylamide gels. Two proteins of molecular weights 75,000 and 45,000 were the major labeled polypeptides in the nuclear membrane fractions prepared from infected cells both by the M-band and the discontinuous sucrose gradient methods. These two proteins were not found in nuclear membrane fractions from uninfected cells. It is suggested that the 75,000 and 45,000 proteins may be early viral gene products that may play a role in the viral DNA replication.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comings D. E., Okada T. A. DNA replication and the nuclear membrane. J Mol Biol. 1973 Apr 25;75(4):609–618. doi: 10.1016/0022-2836(73)90295-7. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Mak S., Green M. A method for determining the fraction of the viral genome transcribed during infection and its application to adenovirus-infected cells. Proc Natl Acad Sci U S A. 1968 Jul;60(3):959–966. doi: 10.1073/pnas.60.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka F., Yamada M. Localization of the replication point of mammalian cell DNA at the membrane. Biochem Biophys Res Commun. 1971 Feb 19;42(4):647–653. doi: 10.1016/0006-291x(71)90537-7. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Brayton C., Baum S. G. Synthesis of type 2 adenovirus DNA in the presence of cycloheximide. J Virol. 1973 Apr;11(4):544–551. doi: 10.1128/jvi.11.4.544-551.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Tsai A., Deich R. A. DNA replication sites within nuclei of mammalian cells. Nature. 1973 Jan 5;241(5384):32–36. doi: 10.1038/241032a0. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Nauta R., Gilbert S., Hobart P., Firshein W. DNA synthesis in developing sea urchins: role of a DNA-nuclear membrane complex. Nat New Biol. 1973 Mar 7;242(114):5–8. doi: 10.1038/newbio242005a0. [DOI] [PubMed] [Google Scholar]
- Kashnig D. M., Kasper C. B. Isolation, morphology, and composition of the nuclear membrane from rat liver. J Biol Chem. 1969 Jul 25;244(14):3786–3792. [PubMed] [Google Scholar]
- Mizuno N. S., Stoops C. E., Sinha A. A. DNA synthesis associated with the inner membrane of the nuclear envelope. Nat New Biol. 1971 Jan 6;229(1):22–24. doi: 10.1038/newbio229022a0. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Green M. Adenovirus DNA. IV. Topology of adenovirus genomes. J Mol Biol. 1973 Mar 15;74(4):735–738. doi: 10.1016/0022-2836(73)90061-2. [DOI] [PubMed] [Google Scholar]
- POLASA H., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. 8. ANALYSIS OF PROTEIN SYNTHESIS. Virology. 1965 Jan;25:68–79. doi: 10.1016/0042-6822(65)90253-9. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H. A temperature-sensitive mutant of adenovirus 31, defective in viral deoxyribonucleic acid replication. Virology. 1971 Feb;43(2):488–494. doi: 10.1016/0042-6822(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Tockstein G., Polasa H., Piña M., Green M. A simple purification procedure for adenovirus type 12T and tumor antigens and some of their properties. Virology. 1968 Nov;36(3):377–386. doi: 10.1016/0042-6822(68)90162-1. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Wise G. E., Prescott D. M. Initiation and continuation of DNA replication are not associated with the nuclear envelope in mammalian cells. Proc Natl Acad Sci U S A. 1973 Mar;70(3):714–717. doi: 10.1073/pnas.70.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Shimojo H. Replication of adenovirus 12 deoxyribonucleic acid in association with the nuclear membrane. Jpn J Microbiol. 1973 Sep;17(5):419–423. doi: 10.1111/j.1348-0421.1973.tb00793.x. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., van Kesteren L. W., van Bruggen E. F. Structural properties of adenovirus DNA's. Biochim Biophys Acta. 1969 Jun 17;182(2):530–541. doi: 10.1016/0005-2787(69)90205-6. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]



