Abstract
Background:
Skin prick test (SPT) is the most effective diagnostic test to detect IgE mediated type I allergic reactions like allergic rhinitis, atopic asthma, acute urticaria, food allergy etc. SPTs are done to know allergic sensitivity and applied for devising immunotherapy as the therapeutic modality.
Materials and Methods:
This prospective study was conducted in the department of Immunology and Molecular medicine at SKIMS. A total of 400 patients suffering from allergic rhinitis, asthma and urticaria were recruited in this study. SPT was performed with panel of allergens including house dust mite, pollens, fungi, dusts, cockroach, sheep wool and dog epithelia. Allergen immunotherapy was given to allergic rhinitis and asthmatic patients as therapeutic modality.
Results:
In our study, age of patients ranged from 6 to 65 years. Majority of patients were in the age group of 20-30 years (72%) with Male to female ratio of 1:1.5. Of the 400 patients, 248 (62%) had urticaria, 108 (27%) patients had allergic rhinitis and 44 (11%) patients had asthma. SPT reaction was positive in 38 (86.4%) with allergic asthma, 74 (68.5%) patients with allergic rhinitis and 4 (1.6%) patient with urticaria, respectively. Allergen immunotherapy was effective in 58% patients with allergic rhinitis and 42% allergic asthma.
Conclusion:
Identifiable aeroallergen could be detected in 86.4% allergic asthma and 68.5% allergic rhinitis patients by SPT alone. Pollens were the most prevalent causative allergen. There was significant relief in the severity of symptoms, medication intake with the help of allergen immunotherapy.
Keywords: Allergic rhinitis, asthma, Kashmir, skin prick test
Introduction
What was known?
Skin prick test is the most effective diagnostic test to detect IgE mediated type I allergic reactions like allergic rhinitis, atopic asthma, acute urticaria, food allergy etc.
Allergy refers to immediate (type I) hypersensitivity to environmental antigens. It is characterized by wheal and flare reactions to skin testing with common environmental antigens, usually with appropriate clinical history. Atopy is demonstration of allergy and familial aggregation of this trait.
The pathophysiology of allergy response has been explained by conceptualization sometimes called the Th1-Th2 paradigm of CD4 T helper cells. Antigen presenting cells display dipeptide antigens, either allergen or infectious, in their cell surfaces for recognition by native T cells. Native T cells differentiate into Th1 or Th2 cells depending on the nature of antigen, Th1 cells secrete IFN-γ, while Th2 cells secrete IL-4, IL-5 and IL-13, Th2 cells promote allergic inflammation through the production of cytokines including IL-4, IL-5 and IL-13, IL-4 and IL-13 include B lymphocyte to differentiate into IgE producing plasma cells. IL-5 play role in eosinophilopoiesis and resistance to apoptosis. A Th1 response results in activation of macrophages and natural killer cells by elaboration TNF-α and IL-2 cytokines plays a role in complement binding and opsonization. Th1 and Th2 cells have been observed to synergistically promote inflammation and airways hyper-responsiveness. It is found allergic disorders viz; Allergic rhinitis, allergic asthma and urticaria are associated with IL-4, IL-5, IgE production, and INF-γ production is associated with airway hyper-reactivity and skin test reactivity.
A variety of clinical allergy markers have been utilized, specific and total IgE measured by skin or serological testing, assess sensitization as well as exposure to environmental antigens and frequently are used to determine the prevalence of allergic responsiveness. Skin test reactivity depends on at least three separate factors: (1) an intact immune system; (2) the presence of IgE sensitized mass cells that release mediators when exposed to antigen; (3) and skin that can respond to histamine with the development of inflammatory response including erythema and induration. Although these manifestations of an allergic response depend on prior exposure to environmental antigen, they do not measure or take into account the level of exposure in the environment. Hence for assessing the presence of allergen, specific IgE antibodies, allergy skin testing is preferred over blood allergy test, it is more sensitive and specific, simpler to use and less expensive.[1]
Total serum IgE, although used in epidemiological studies, has relative limited value in diagnosis of atopic diseases, with exception of allergic bronchopulmonary aspergillosis. These are intended to provide qualitative test result, giving a yes, or no answer in patients with suspected allergic sensitization, one such method has sensitivity of about 70.8% and a positive predictive value of 72.6% according to large study.[2] A low total IgE level is not adequate to rule out sensitization to commonly inhaled allergens.[3]
Allergic asthma is a heterogeneous disease with interplay between genetic and environment factors, asthma is chronic inflammatory disorder causes recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night and early in morning. These episodes are usually associated with airflow obstruction that is often reversible either spontaneously or with treatment. Patient with asthma commonly suffer from other atopic disease, particularly Allergic rhinitis which may be found in over 80% of asthmatic patients and atopic dermatitis (eczema). Atopy may be found in 40-50% of population in affluent countries with only a proportion of atopic individual becoming asthmatic.[4] This observation suggests that some other environmental or genetic factors predispose to the development of asthma in atopic individuals. The allergens that lead to sensitization are usually proteins that have protease activity, and commonest allergens are derived from house dust mite, cat and dog fur, cockroaches, grass and tree pollens. A minority of asthmatic patients (approximately 10%) have negative skin test to common inhalant allergens and normal serum concentration of IgE. Asthma and rhinitis commonly coexist and share epidemiological, histological, physiological and immunopathogenic characteristics.[4–20] Nasal inflammation may significantly influence the lower airways, and vice-versa; uncontrolled allergic rhinitis may lead to worsening of coexisting asthma.[9–20]
Allergic rhinitis has characteristic symptoms of watery nasal discharge, sneezing, itchy nose, and stuffy nose. It is due to allergic reaction to aeroallergens including dust mites, pollens, animal danders, and moulds. Similar symptoms can be due to non-allergic rhinitis which consists of a group of rhinitis due to diversities of causes and the diagnosis is usually based on either identification of known non allergic causes or by exclusion of allergy. Chronic rhinitis is common worldwide and according to epidemiological studies it is estimated to affect 10-40 % of the population.[5–15] However, most of these epidemiologic surveys were based on questionnaires without further clinical nasal examination and allergic test to establish allergic rhinitis as cause of symptoms.
Urticaria presenting as well circumscribed wheal with erythematous raised serpiginous borders with blanched centers that may coalesce to become giant wheals. These lesions increase in frequency after adolescence, with highest incidence occurring in third decade of life. Most cases of chronic urticaria are idiopathic, while urticaria occurring during appropriate season in patients with seasonal respiratory allergy or as a result of exposure to animals or molds is attributed to inhalation or physical contact with pollens, animal danders and mold spore, respectively. Urticaria secondary to inhalation are relatively uncommon compared to urticaria elicited by ingestion of fresh fruits, selfish, fish, milk products, chocolates, legumes including peanuts and various drugs.
Desensitization or hyposensitization is treatment in which patient is gradually vaccinated with progressively large doses of allergen in question. This can either reduce the sensitivity or eliminate hypersensitivity. It relies on IgG antibody production to block excessive IgE production seen in atopy. In a sense, person builds up immunity to increasing amounts of the allergen in question. Studies have demonstrated long-term efficacy and protective effect of immunotherapy in reducing the development of new allergy.[21–24] Meta-analysis have also confirmed efficacy of treatment in Allergic rhinitis in children and in asthma. A review by Mayo clinic in Rochester confirmed the safety and efficacy of allergen immunotherapy for allergic rhinitis and conjuctivitis, allergic forms of asthma and stinging insect based on various studies.[25] Additionally, national and international guidelines confirm efficacy of injection immunotherapy in rhinitis and asthma as well as safety provided that recommendation are followed.[26]
The present study therefore aimed to identify the various aeroallergens by skin prick test (SPT) that give rise to Allergic asthma, Allergic rhinitis and Urticaria in our Kashmiri population; and to look for effectiveness of allergen immunotherapy given to SPT positive allergic diseases, in our patients.
Materials and Methods
Data were prospectively collected from 400 consecutive patients who had SPTs to identify aeroallergen for allergic disorders viz, allergic rhinitis, allergic asthma and Urticaria in the Department of Immunology and Molecular Medicine, Sher-i-Kashmir Institution of Medical Science, Srinagar, Kashmir over the period January 2007 to March 2011 were included. The diagnosis was based on clinical history and physical examination. Patients with chronic sinusitis or other infective causes for chronic rhinitis were excluded. Patients with similar nasal symptoms, due to atrophic rhinitis, nasal poylposis, nasal tumours, or other known causes of non-allergic rhinitis including occupational rhinitis, aspirin sensitivity, endocrine disease, pregnancy and drug induced rhinitis were excluded. Similarly wheezy bronchitis, bronchopulmonary aspergillosis, bronchectasis, drugs, viral infections, exercise and occupational induced asthma were also excluded. Urticaria caused by drugs, CINH esterase deficiency and mastocytosis were all excluded.
For at least one week, no patients were taking medications (antihistamines, steroids and other drugs) considered liable to affect the SPT. Patients who had active skin disorders or dermatographia were considered not suitable for SPTs. The tests were performed according to standard methods with allergens, histamine-positive and negative controls purchased from Creative Drug Industries (Mumbai, INDIA). The skin prick reaction was read at 20 minutes and consider positive when the reaction wheal diameter was at least 3mm larger than negative control. The medical history and visual analogue symptom scores of these patients were also evaluated (On a scale of 0-6; 0 = no symptom, and 6 = maximum severity).
The statistics were performed by using Statistical Package for the Social Sciences (Windows version 13), chi square and t test were performed as appropriate.
Results
In our study, age of patients ranged from 6 to 65 years. Majority of patients were in the age group of 20-30 years (72%) with male to female ratio of 1:1.5. Of the 400 patients, 248 (62%) had urticaria, 108 (27%) patients had allergic rhinitis and 44 (11%) patients had asthma. SPT reaction was positive in 38 (86.4%) with allergic asthma, 74 (68.5%) patients with allergic rhinitis and 4 (1.6%) patients with urticaria, respectively. The commonest allergen was pollens (52%) followed by house dust mite (44%), dusts (2%) (Cotton, grain and paper), (1.5%) patients to cockroach and (0.5%) to fungi. The results of SPTs are shown in Tables 1–3.
Table 1.
Results of skin prick tests in Allergic asthma
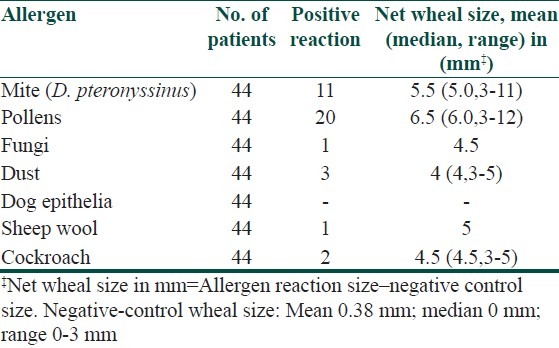
Table 3.
Result of skin prick test in Urticaria
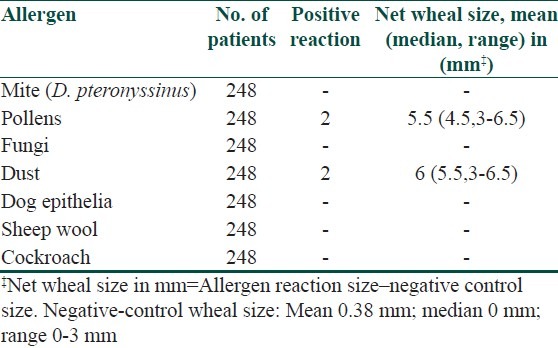
Table 2.
Results of skin prick tests in Allergic rhinitis
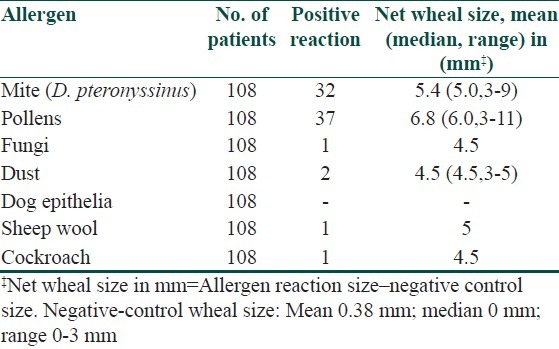
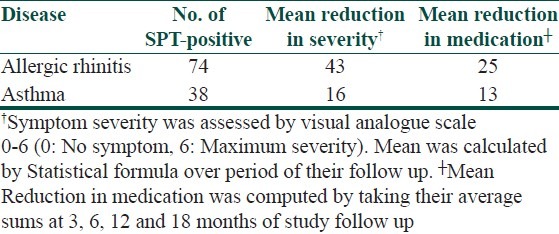
Regarding the 44 patients with asthma, 38 (86.4%) tested positive to selected routine panel of antigen by SPT. Out of 38 patients, 20 (54%) were positive to pollens mainly to Parthenium hysterophorus (36%), followed by Amaranthus spinosus (32%) and Cyanodon dactylon (24%). Rest 11 (32%) asthma patients were positive to D. pteronyssinus, 3 (8%) to Dust (Grain and house dust), 2 (5.3%) to cockroach, 1 (1.5%) to sheep wool and 1 (1.5%) to fungi.
Of the 108 Allergic rhinitis patients, 74 (68.5%) had positive reactions to an allergens of routine panel, 37 (48%) were positive to pollens, 32 (46%) patients had positive reaction to D. pteronyssinus, 2 (2.5%) patients to dust(house dust and grain dust), 1 (1.5%) each positive to fungi, sheep wool and cockroach, respectively.
Similarly, out of 248 urticaria patients, just 4 (1.6%) patients had positive reaction. Out of the four patients who were SPT positive, 2 (50%) of them were positive to saw dust and rest 2 (50%) were to pollens (Parthenium hysterophorus).
Out of 400 patients with various allergic disorders, 38 (86.4%) of allergic asthma, 74 (67.5%) of allergic rhinitis and only four (1.6%) of urticaria had SPT positivity. All patients who tested positive to panel allergens included 0.5% to a single allergens, 11% to two allergen, 34% to three allergens, 38.5% to four allergens, 9% to five allergens and the rest (7%) were sensitive to more than five allergens.
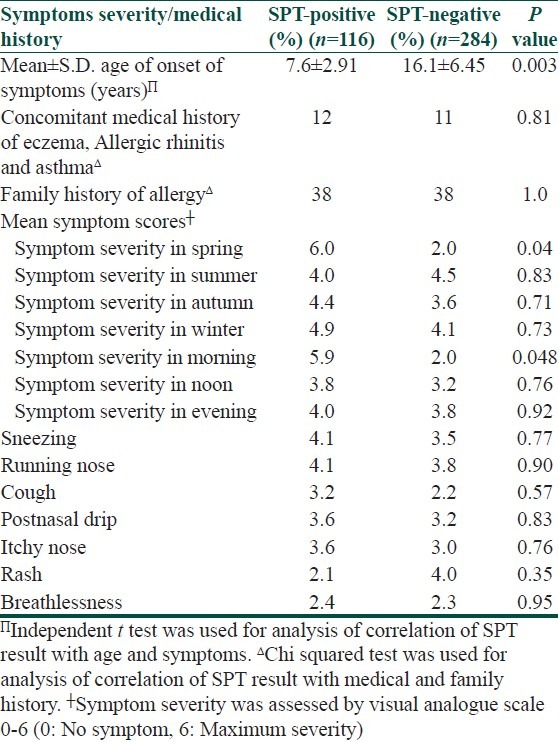
The medical history and symptoms severity of SPT positive and negative patients are compared in Table 4. SPT positive patients had earlier age of symptom onset and more likely history of allergic march. i.e. patient who develop asthma have had eczema in past, followed by allergic rhinitis. The SPT-positive patients in case of allergic asthma and allergic rhinitis had more severe symptoms in the morning compared to those who were SPT-negative. All patients of asthma and Allergic rhinitis had more severe symptoms in the morning than at noon, especially in months of spring seasons.
Table 4.
Comparison of symptoms severity and medical history of skin prick test (SPT) - positive and SPT-negative patients
Immunotherapy was given to the SPT-positive Allergic rhinitis and asthma patients; it was effective in reducing severity of symptoms in 43 (58%) patients of allergic rhinitis and 16 (42%) patients of asthma. Of the SPT-positive, 25 (34%) patients of allergic rhinitis and 13 (32%) patients of asthma got reduced medication during their maintenance therapy, usually after a period of one year [Figure 1]. Table 5 shows outcome of immunotherapy given to SPT-positive allergic rhinitis and asthma patients in our study
Figure 1.
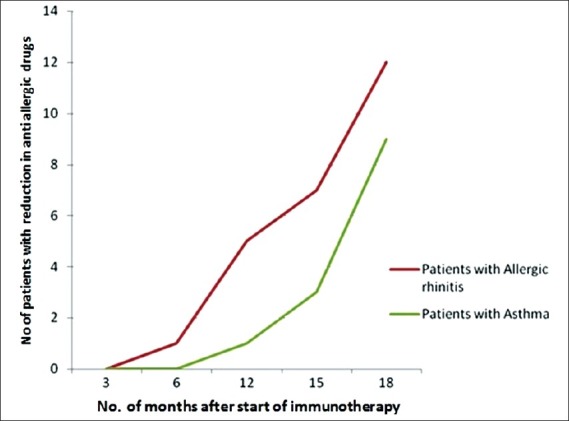
Graph showing the reduction in the dosage of anti-allergic drugs vis a vis months from the start of immunotherapy
Table 5.
Immunotherapy effectiveness in SPT-positive patients
Discussion
The symptoms of allergic disorders recognized in our study were rhinorrhoea, sneezing, itchy nose, post nasal drip, cough, breathlessness and rash, other respiratory and skin diseases can give rise to similar symptoms. The latter non allergic respiratory and skin diseases should be ruled out by careful history taking and clinical examination. Moreover diagnosis of Allergic rhinitis, asthma and Urticaria can only be made after investigations to confirm the presence of an allergic reaction. The SPT is the recommended initial investigation for this purpose.[5]
We have shown that 86.4% of allergic asthma, 67.5% of allergic rhinitis and only 1.6% of urticaria patients reacted to aeroallergens identifiable by SPT alone. Of those patients who had no identifiable aeroallergens, they had been clinically classified as non-allergic rhinitis, intrinsic asthma and idiopathic urticaria, respectively. Thus, the clinical terms of all these ailments should be interpreted cautiously to mean patients without identifiable allergen rather than non -allergic in aetiology. Similarly, SPT-negative patients are often labeled as non-allergic, may be misnomer, which literally means a different pathophysiological cause of symptoms (not related to IgE - mediated allergy). For example SPT-negative allergic rhinitis patient, since a presumed vasomotor aetiology can be there, but it cannot be tested, besides we had used routine panel of aeroallergens can be positive to some other; Thus it seems preferable to substitute the label idiopathic rhinitis (meaning aetiology not yet identified) in place of vasomotor rhinitis.[5] In this paper, we therefore use terms idiopathic rhinitis, intrinsic asthma and idiopathic urticaria, respectively for SPT- negative patients.
These idiopathic rhinitis, intrinsic asthma and idiopathic urticaria patients with negative SPTs might nevertheless be suffering from allergic causes not detected by the SPTs used. One possible reason could relate to intrinsic limitations of SPTs themselves (depending on the available allergens and their specificity and affinity for the circulating IgE).[16] Moreover, SPTs may not identify patients with low-level IgE hypersensitivity reactions (triggering smaller than 3-mm size wheals).
The most common aeroallergen in our population is the pollens. Of all SPT-positive patients, 54% of asthmatics, 48% having allergic rhinitis and 2.5% having urticaria, were sensitive to one or more species of pollens. The reason could be inhabitants are living in close proximity of farmlands, meadows and forest areas. The surroundings in our part of world are highly enriched with natural flora. The patterns of aeroallergens in the environment differ widely in different localities and seasonal changes (particularly when they affect pollen) are important.
The second most common aeroallergen in our study is house dust mite. 46% Allergic rhinitis and 32% asthma patients were sensitive to (D. pteronyssinus) house dust mite species. Blomia tropicalis, which is commonly found in tropical regions, it is rarely found locally. So we did not include it in our working protocol. Nevertheless, Blomia tropicalis should always be included as a routine SPT allergen in these tropical countries.
Dusts (cotton, grain, saw dust and paper), cockroach and fungi allergens affected significant percentages of our patients. Duc et al.,[27] has also found total house dust to be the most common allergen in patients of rhinitis with bronchial asthma followed by grass pollens, HDM, and animal dander. There were a few patients who were sensitive to sheep wool, as they used to deal with sheep husbandry. Neither of patients was tested positive for dog or cat animal allergy, likely possibility could be as there is no custom of keeping such animals as pet. Usually our people avoid coming in contact with dogs and cats. In the study by Prasad et al.,[28] 12% patients of nasobronchial allergy showed markedly positive skin reaction to various dusts. Most common dusts identified by this study were house dust (25%), wheat dust (12.5%), cotton dust (6.3%) and paper dust (4.2%). In a similar study by Acharya et al.,[29] house dust followed by wheat dust, cotton dust and paper dust were found to be common among patients of nasobronchial allergy.
SPT-positive patients were more likely to have earlier age of onset of the disease. They also had severe symptoms on presentation. It is well documented that Allergic rhinitis is closely related to asthma;both conditions together are often considered to be single disease affecting the whole respiratory tract.[6] SPT-negative patients can be regarded as either having low level IgE-mediated (below reaction threshold of the SPT) or due to non IgE-mediated pathophysiological causes. Such patients had weaker IgE –mediated skin reactions than SPT-positive patients. The extent of reaction in the skin also reflected the degree of IgE-mediated allergic reactivity in other body organs including the eyes, nose and lungs, which might account for difference in symptom severity between SPT-positive and negative patients. Irrespective of underlying aetiology, SPT-negative patients were older at time of disease onset, were less likely to have severe symptoms in the morning and in the spring.
Allergen immunotherapy was given to SPT-positive Allergic rhinitis and asthma; it proved effective in reducing severity of symptoms determined by visual analogue scale in 58% patients of Allergic rhinitis and 42% patients of asthma. Similarly 34% patients of Allergic rhinitis and 32% patients of asthma, remain drug free during their maintenance therapy, usually after period of one year. To explain the immunological mechanisms underlying the clinical improvement, intensive research has concentrated upon the specific antibody response in serum with respect to class and subclass distribution.[26,30] Studies showed that the allergen-specific IgE levels rise temporarily during initial phase of SIT, but fall back to pre-treatment levels during maintenance therapy.[31] Subsequently, it was demonstrated that SIT also induces allergen-specific IgG (mostly IgG1 and IgG4) and in few reported cases IgA levels.[32] These findings led to the hypotheses that these IgG antibodies contribute to the immunosuppressive effects of SIT by blocking IgE-facilitated antigen presentation[33,34] and that such antibodies act as blocking antibodies by engaging low-affinity Fc receptors for Ig (e.g., FcγRII) expressed by B lymphocytes, basophils, and mast cells. The poor response was seen in multiple antigen SPT-positive patients and in those with chronic persistent disease. In SPT-positive Urticaria patients, allergen immunotherapy was withheld, in view of their less number and literature did not mention much role of this kind of therapy. In 2006 a double blind, randomized, placebo controlled study showed that patients with seasonal Allergic rhinitis poorly controlled with medical management experienced a 27% reduction in symptoms and 32% reduction in medication scores after one year of allergen immunotherapy.[23] Similarly a meta-analysis reviewing immunotherapy in Allergic asthma revealed that treated subjects were almost three times more likely to have improved asthma symptoms and lung function. They were two times more likely to decreased medication requirement.[24] Another review found that with immunotherapy the number needed to treat to prevent worsened asthma symptoms is four and number needed to treat in order to avoid medication increase is five.[25]
Conclusion
In conclusion, 86.4% having allergic asthma, 67.5% having allergic rhinitis and only 1.6% of urticaria patients had identifiable aeroallergen detected by SPTs alone. The most common aetiologic allergen in our part of world were Pollens followed by house dust mite (D. pteronyssinus). Dusts and cockroach were also common allergens, whereas sheep, fungi, dog and cat were uncommon. SPT-positive patients were more likely to have earlier age of onset of symptoms, more severe symptoms in the months of spring and in the morning. Allergen immunotherapy was effective in reducing severe symptoms in 58% patients of SPT-positive allergic rhinitis and 42% SPT-positive allergic asthma patients. Moreover immunotherapy showed reduction in medication of 34% SPT-positive allergic rhinitis and 32% SPT-positive allergic asthma patients during their maintenance therapy, usually after period of one year. The role of SPTs in diagnosis and guiding immunotherapy in allergic disorders is immense; this information may be useful to clinicians managing patients suffering from such disorders.
What is new?
1. 86.4% allergic asthma, 67.5% allergic rhinitis and only 1.6% of urticaria patients had identifiable aeroallergen detected by SPTs alone.
2. Allergen immunotherapy was effective in reducing severe symptoms in 58% patients with SPT-positive allergic rhinitis and 42% SPT-positive Allergic asthma patients.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Tess RM, Klein JS, Frigas E. Allergy skin testing. Mayo Clin Proc. 1995;70:783–4. doi: 10.4065/70.8.783. [DOI] [PubMed] [Google Scholar]
- 2.Vidal C, Gude F, Boquete O, Fernández-Merino MC, Meijide LM, Rey J, et al. Evaluation of phadiatop test in Diagnosis of allergic sensitization in a general adult population. J Investig Allergol Clin Immunol. 2005;15:124–30. [PubMed] [Google Scholar]
- 3.Kerkhof M, Dubois AE, Portma DS, Schouten JP, de Monchy JG. Role and Interpretation of total serum IgE measurement in the diagnosis of Allergic airway disease in adults. Allergy. 2003;58:905–11. doi: 10.1034/j.1398-9995.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 4.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 5.van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus statement on the treatment of Allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:116–34. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Bousquest J, van Cauwenberge P, Bachert C, Canonica GW, Demoly P, Durham SR, et al. Requirements medications commonly used in the treatment of Allergic rhinitis. Academy of Allergy and Clinical Immunology (EAACI), Allergic rhinitis and Its Impact on Asthma (ARIA) Allergy. 2003;58:192–7. doi: 10.1034/j.1398-9995.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 7.Worldwide variation in prevalence of symptoms of asthma, allergic rhino-conjunctivitis, and atopic eczema: ISSAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 8.Weiland SK, Bjorksten B, Brunekreef B, Cookson WO, Von Mutius E, Strachan DP. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and methods. Eur Respir J. 2004;24:406–12. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 9.Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW. ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int J Tuberc Lung Dis. 2005;9:10–6. [PubMed] [Google Scholar]
- 10.Zhao T, Wang HJ, Chen Y, Xiao M, Duo L, Liu G, et al. Prevalence of childhood asthma, Allergic rhinitis and eczema in Urumqi and Beijing. J Paediatr Child Health. 2000;36:128–33. doi: 10.1046/j.1440-1754.2000.00457.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee SL, Wong W, Lau YL. Increasing prevalence of Allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood) Pediatr Allergy Immunol. 2004;15:72–8. doi: 10.1046/j.0905-6157.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 12.Leung R, Ho P. Asthma, allergy, and atopy in three south-east Asian populations. Thorax. 1994;49:1205–10. doi: 10.1136/thx.49.12.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–9. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 14.Lau YK, Karlberg J. Prevalence and risk factors of childhood asthma, rhinitis and eczema in Hong Kong. J Paediatr Child Health. 1998;34:47–52. doi: 10.1046/j.1440-1754.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 15.Leung R, Wong G, Lay J, Ho A, Chan JK, Choy D, et al. Prevalence of asthma and allergy in Hong Kong school children: An ISAAC study. Eur Respir J. 1997;10:354–60. doi: 10.1183/09031936.97.10020354. [DOI] [PubMed] [Google Scholar]
- 16.Pierson-Mullany LK, Jackola DR, Blumenthal MN, Rosenberg A. Evidence of affinity threshold for IgE-allergen binding in the percutaneous skin test reaction. Clin Exp Allergy. 2002;32:107–16. doi: 10.1046/j.0022-0477.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 17.Kidon MI, Chiang WC, Liew LK, Lim SH, See Y, Goh A, et al. Sensitization to dust mites in children with Allergic rhinitis in Singapore: Does it matter if you scratch while you sneeze. Clin Exp Allergy. 2005;35:434–40. doi: 10.1111/j.1365-2222.2005.02208.x. [DOI] [PubMed] [Google Scholar]
- 18.Puccio FA, Lynch NR, Noya O, Noda A, Hagel I, López E, et al. Importance of including Blomia tropicalis in the routine diagnosis of Venezuelan patients with persistent allergic symptom. Allergy. 2004;59:753–7. doi: 10.1111/j.1398-9995.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 19.Wickens K, de Bruyne J, Calvo M, Choon-Kook S, Jayaraj G, Lai CK, et al. The determinants of dust mite allergen and its relationship to the prevalence of symptoms of asthma in the Asia-Pacific region. Paediatr Allergy Immunol. 2004;15:55–61. doi: 10.1046/j.0905-6157.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun BQ, Wu A, Chan A, Chik S, Wong D, Zhong NS. House dust mite allergen (Derp 1 and Blot 5) levels in asthmatics, home in Hong Kong. Chin Med Sci J. 2004;19:185–8. [PubMed] [Google Scholar]
- 21.Ross RN, Nelson HS, Finegold Effectiveness of specific immunotherapy in treatment of Allergic rhinitis an analysis of randomized, prospective, single or double-blind, placebo-controlled studies. Clin Ther. 2001;22:342–50. doi: 10.1016/S0149-2918(00)80038-7. [DOI] [PubMed] [Google Scholar]
- 22.Rank MA, Li JT. Allergen Immunotherapy. Mayo Clin Proc. 2007;82:1119–23. doi: 10.4065/82.9.1119. [DOI] [PubMed] [Google Scholar]
- 23.Passalacqua G, Durham SR. Allergic rhinitis and its impact on asthma Update; Allergen immunotherapy. J Allergy Clin Immunology. 2007;119:881–91. doi: 10.1016/j.jaci.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 24.Frew AJ, Powell RJ, Corrigan CJ, Durham SR UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with subcutaneous allergen extract in treatment resistant seasonal allergic rhino-conjunctivitis. J Allergy Clin Immunol. 2006;117:319–25. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Ross RN, Nelson HS, Finegold I. Effectiveness of immunotherapy in treatment of asthma meta-analysis of prospective randomized, double-blind placebo controlled studies. Clin Ther. 2000;22:329–41. doi: 10.1016/S0149-2918(00)80037-5. [DOI] [PubMed] [Google Scholar]
- 26.Abramson MJ, Poy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Sys Review. 2003;4:CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- 27.Duc J, Kolly M, Pecoud A. Frequency of respiratory allergens involved in rhinitis and bronchial asthma in adults. Schwetz Med Wochenschr. 1986;116:1205–10. [PubMed] [Google Scholar]
- 28.Prasad R, Verma SK, Dua R, Kant S, Kushwaha RA, Agarwal SP. A study of skin sensitivity to various allergens by skin prick test in patients of nasobronchial allergy. Lung India. 2009;26:70–3. doi: 10.4103/0970-2113.53228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acharya PJ. Skin test response to some inhalant allergens in patients of nasobronchial allergy from Andhra Pradesh. Aspects Allergy Appl Immunol. 1980;8:34–6. [Google Scholar]
- 30.Djurup R. The subclass nature and clinical significance of the IgG antibody response in patients undergoing allergen-specific immunotherapy. Allergy. 1985;40:469–86. doi: 10.1111/j.1398-9995.1985.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 31.Creticos PS, Van Metre TE, Mardiney MR, Rosenberg GL, Norman PS, Adkinson NF., Jr Dose response of IgE and IgG antibodies during ragweed immunotherapy. J Allergy Clin Immunol. 1984;73:94–104. doi: 10.1016/0091-6749(84)90490-1. [DOI] [PubMed] [Google Scholar]
- 32.Gehlhar K, Schlaak M, Becker W, Bufe A. Monitoring allergen immunotherapy of pollen-allergic patients: The ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 33.Jakobsen CG, Bodtger U, Poulsen LK, Roggen EL. Vaccination for birch pollen allergy: Comparison of the affinities of specific immunoglobulins E, G1 and G4 measured by surface plasmon resonance. Clin Exp Allergy. 2005;35:193–8. doi: 10.1111/j.1365-2222.2005.02160.x. [DOI] [PubMed] [Google Scholar]
- 34.Wachholz PA, Durham SR. Induction of ‘blocking’ IgG antibodies during immunotherapy. Clin Exp Allergy. 2003;33:1171–4. doi: 10.1046/j.1365-2222.2003.01765.x. [DOI] [PubMed] [Google Scholar]


