Abstract
Several radiotracers have been used for assessing cell death, whether by necrosis or apoptosis. 99mTc glucarate, which has initially been reported to be concentrating/accumulating in myocardial infarction or zones of cerebral injury, has also shown some tumor-seeking properties in a few preliminary studies. Under International Atomic Energy Agency (IAEA)'s coordinated research program, we report here the standardization, quality control, and clinical evaluation (detection, evaluation of response, and comparison with 18F Fluorodeoxyglucose) of this tracer in well-characterized lung cancer and head neck malignancies in a single-arm prospective observational study. Forty-seven patients (29 inoperable lung carcinoma and 18 head and neck malignancies) were prospectively enrolled and underwent 99mTc glucarate imaging [whole body planar and single-photon emission computed tomography of the region of interest] 4-5 hours after injection of 20 mCi of the radiopharmaceutical. Excellent 99mTc glucarate concentration was noted in the target lesion in lung cancer and head and neck malignancies. The sensitivity was found to be better in lung cancer. Avid concentration of tracer was seen in the metastatic sites. During response evaluation, the glucarate concentration correlated well with the clinical and other radiological findings. 99mTc glucarate showed avid concentration of tracer in the tumor, suggesting it to be a potential tumor imaging agent which can be used for detection and assessment of therapeutic response in malignancy.
Keywords: Tumor viability, 99mTc glucarate, SPECT imaging
Introduction
There is a continuous search for effective and specific radiotracers to evaluate tumor characteristics such as proliferation, aggressiveness, and the effectiveness of anti-cancer treatments. Several radiotracers have been tried for determining cell death, whether by necrosis or apoptosis.[1] In routine clinical practice, however, evaluation of reduction of cell viability has remained the cornerstone of oncological practice, and more recently, 18F FDG has been used for this purpose.[2] 99mTc glucarate was initially described as a tracer accumulating in myocardial infarction and zones of cerebral injury.[3,4] Glucarate is a 6-carbon dicarboxylic acid similar to glucose. Several studies demonstrated that necrotic cell death can be modulated for therapeutic purposes; it may be suppressed to prevent damage to normal tissue or activated to induce damage of tumor tissue. For this reason, recently developed new anti-cancer molecules are designed to disrupt the fine balance between necrosis and apoptosis and promote the programmed cell death necrotic pathway rather than apoptotic pathway.[5,6] In mammals, d-glucaric acid (glucarate) is a 6-carbon dicarboxylic acid sugar and a natural catabolite of glucuronic acid metabolism. All mammals excrete glucaric acid as a physiologic end product. It is the dicarboxylic acid, which may act as a bifunctional ligand with 99mTc. Experimental studies have shown favorable targeting potential of 99mTc glucarate in severe ischemia or early necrosis of the heart and brain, as well as its potential as a tumor agent.[7]
Necrosis implies a broad spectrum of morphological changes that follows the cellular death in the tissue, derived mainly from progressive action of enzymes on cells which suffer a kind of lesion such as trauma, bacterial infection, or acute hypoxia. Two processes take place at the same time: enzymatic cell digestion and protein denaturalization. Apoptosis is characterized by cell shrinkage, chromatin condensation and margination, nuclear pycnosis, fragmentation, and membrane blebbing into membrane-enclosed vesicles. In the patient, both apoptotic and most necrotic cells are mostly processed by a phagocytic process.[8]
Glucarate has been found to concentrate in necrotic cells but not in apoptotic cells, as per reports in the literature. However, till date, very limited data are available regarding utility of 99mTc glucarate in tumor cells, although being a glucose analog, it is thought to be concentrating in tumor cells which metabolize glucose.
For developing countries, 99mTc-based radiopharmaceuticals and single-photon emission computed tomography (SPECT) systems continue to play an important role in the management of oncology patients. There is a pressing need for a suitable SPECT radiotracer for assessing tumor viability. An agent which is technetium based, clinically proven, and inexpensive could serve millions of cancer patients, especially in developing countries where facilities for positron emission tomography (PET) are not available. 99mTc glucarate, being a glucose analog, appears to be a suitable tracer for this purpose. However, due to lack of commercial interest, it has not undergone formal clinical trials. Labeled with technetium pertechnetate (99mTcO4), this has been used as a tumor imaging agent in experimental studies.
Under International Atomic Energy Agency (IAEA) coordinated research program, we report here the standardization, quality control, and clinical evaluation (detection, response evaluation, and comparison with 18F FDG) of this tracer in well-characterized lung cancer and head and neck malignancies in a prospective single-arm observational study.
Materials and Methods
The study comprised two aspects and was a part of multicentric IAEA coordinated research project, “standardization of hospital prepared radiopharmaceuticals for nuclear oncology.”
Preparation and quality control of 99mTc glucarate
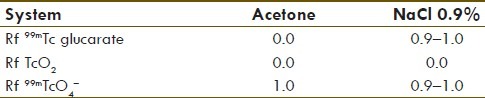
The Tc-99mglucarate (GLA) kit consisted of monopotassium glucarate (12 mg), sodium bicarbonate (NaHCO3) 16.8 mg, 0.1 M acetic acid (40 μl), stannous chloride (0.1 mg), and ascorbic acid (1 mg). Glucarate kits were prepared in a clean room, under a flow laminar cabinet in the Radiopharmacy Department, Nuclear Research Centre, Faculty of Sciences, Universidad de la República Montevideo, Uruguay. IAEA operational guidance on hospital radiopharmacy operation level 3a was followed. All the procedures were in agreement and all records of each batch were registered. Chromatographic controls were performed in Whatman No. 1 paper, and acetone and NaCl 0.9% were used as mobile phases. The possible impurities were reduced hydrolyzed technetium (TcO2) and that obtained from the elution that was not reduced (99mTcO4-). The Rf values of the impurities and the radiopharmaceutical were as follows:
The lyophilized kits were formulated by adding 1 ml of 99mTcO4 containing 10–30 mCi of radioactivity obtained from a 99mo/99mTc generator. The pH of the solution was adjusted to 8. After shaking well, the formulation was incubated at room temperature for 20 min. Radiochemical purity of this formulation was expected to be superior to 95%. This was validated by in vivo bio-distribution studies where each patient served as his/her own control.
Clinical protocol
The clinical evaluation protocol comprising the study population, patient preparation, and inclusion/exclusion criteria was finalized and approved by the IAEA during the 2nd research coordination meeting of this coordinated multicentric research project (CRP) at the headquarters in Vienna in April 2006. A brief description of the approved clinical protocol is given below:
Patient preparation: No specific patient preparation was recommended.
Inclusion criteria: Malignancy proven by histopathologic examination, curative surgery not possible, alternative single or multimodality treatment possible, life expectancy more than 3 months, has not received chemotherapy or External beam radiotherapy (EBRT) in the preceding 4 weeks, and able to sign informed consent.
Imaging protocol
After formulation of the glucarate kit (by the method given above), 20–25 mCi of 99mTc glucarate was injected intravenously. Imaging was performed 3–4 h post injection. It was performed with a Siemens dual head SPECT gamma camera (MULTI SPECT) with Low energy high resolution (LEHR) collimator. Whole body imaging was performed in all patients, followed by a high count local view imaging of the region of interest (e.g. chest for lung cancer; skull base to upper mediastinum for head and neck malignancies). SPECT was acquired in 64 × 64 matrix for lung and 128 × 128 matrix for head and neck malignancies by step and shoot method, 30 s per frame, non-circular orbit, and 32 projections over 180°. Reconstruction was done using Butterworth filter with appropriate frequency cut-off. The SPECT study was obtained within 1 week of the standard morphological imaging used for staging, e.g., computed tomography (CT) or magnetic resonance imaging (MRI). CT/MRI was reported using standard procedures by diagnostic radiologists blinded to the findings of the functional study. Glucarate scans were reported qualitatively according to the following grades based on the intensity of tracer uptake. CT/MRI image was used for point-to-point comparison for localization of the abnormality. No quantitative parameters were calculated. PET-CT was performed by a Siemens Biograph (High REZ) with a 40-slice CT, at least 1 h after injection of 8-10 mCi of 18F FDG from base skull to mid-thigh. Non-contrast CT scan was performed for attenuation correction and localization.
99mTc glucarate avidity in the documented proven lesions on CT scan was graded as per the following visual scale of 0–3:
0, no concentration;
1, minimal concentration; slightly more than the background;
2, interpretable concentration; and
3, intense concentration.
18F FDG PET-CT scans were also analyzed qualitatively.
Before initiation of the protocol, it was approved by the institutional review board (IRB) of Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India.
Results
Forty-seven patients, consisting of 29 inoperable cases (males =27, females =2; age range: 41–70 years) of lung carcinoma planned for systemic treatment and 18 cases (males =13, females =5; age range: 30–70 years) of head and neck cancer were prospectively enrolled and underwent GLA imaging as per the approved protocol. Based on the aim of the study, the evaluation process was carried out in three major groups of patients: Group I for the detection of tumor, Group II for the detection of metastatic sites, and Group III for the evaluation of response to treatment. In addition, another group included a pilot study of 11 patients of lung cancer who underwent both 18F FDG PET and 99mTc glucarate studies for comparison of the findings. The distribution of tumor sites and histopathologic patterns are shown in Tables 1 and 2, respectively.
Table 1.
Distribution of head and neck cancer according to subsite
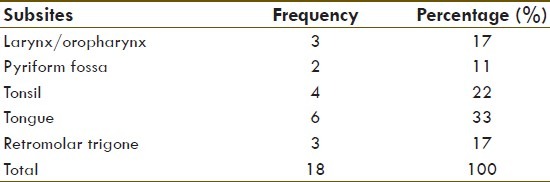
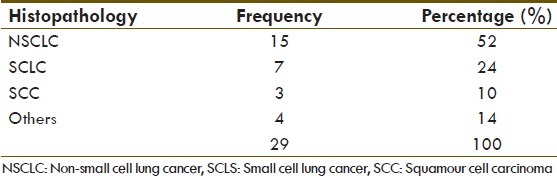
Table 2.
Distribution of lung cancer according to histopathology
Detection of primary tumor (Group I)
Twenty-nine patients of lung cancer and 18 patients of head and neck malignancies were enrolled. All patients had a measurable lesion in contrast-enhanced CT (CECT)/MRI scans and underwent biopsy. Abnormal area of increased tracer concentration graded from 0 to 4 other than the normal bio-distribution of the radiotracer was considered to be positive. The findings were correlated with the results of other imaging modalities and histopathology [Tables 3 and 4]. The sensitivity of detection of primary tumor in lung, and head and neck malignancies were 93% and 83%, respectively [Figures 1–5].
Table 3.
Correlation between GLA positivity and histopathology in the evaluation of suspected lung cancer (one false negative in primary lesion)

Table 4.
Correlation between 99mTc glucarate positivity and histopathology in the evaluation of suspected head and neck malignancy

Figure 1.
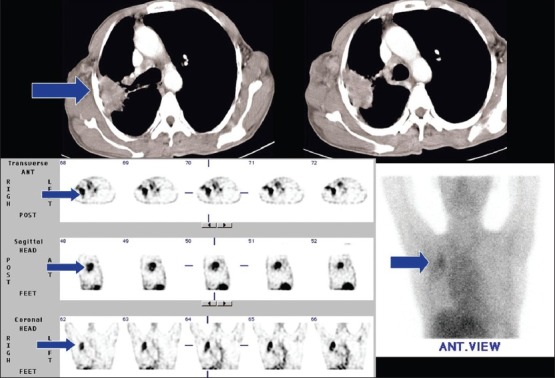
A 65-year-old male, a case of small cell lung carcinoma. Planar and SPECT 99mTc-glucarate study shows avid tracer concentration in the right upper lobe lung lesion, better seen in the SPECT study
Figure 5.
A 51-year-old male, a case of SCLC. Pre and post 3 cycles of chemotherapy. Scan shows good response to treatment
Figure 3.
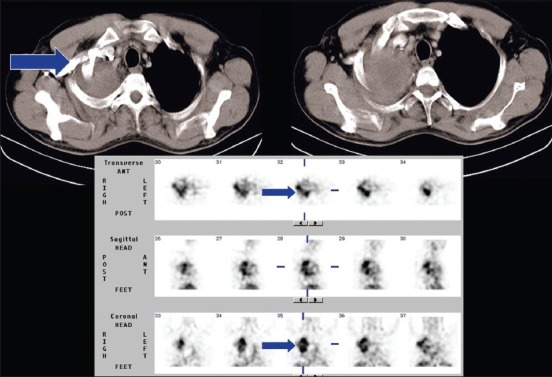
A 50-year-old male with SCC involving right lung upper lobe with extension into the chest wall. 99mTc-glucarate SPECT scintigraphy shows avid tracer concentration in the primary lesion
Figure 4.
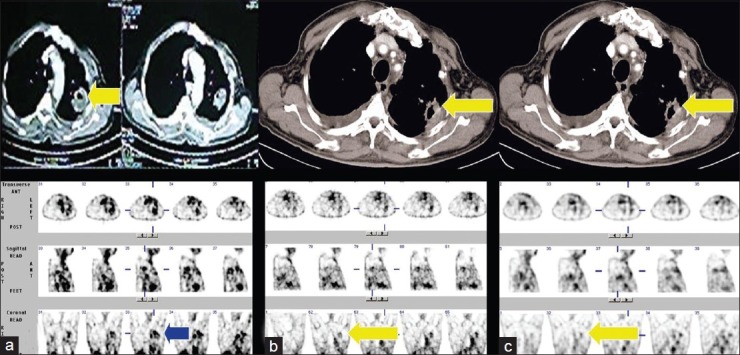
A 79-year-old male with a squamous cell carcinoma in the left lung upper lobe. There is avid concentration of 99mTc-glucarate in the primary lesion in the pre-treatment/baseline study. (a) Significant regression in the size of the mass lesion with corresponding decreased radiotracer uptake is noted post 3 cycles of chemotherapy. (b) Further decrease in radiotracer uptake is seen post 6 cycles of chemotherapy,(c) with evidence of stable disease in corresponding CT scan
Evaluation of response (Group II)
Eleven patients of lung cancer underwent 29 studies (including baseline study) for evaluation of response. Nine patients underwent interim evaluation post 3 cycles of chemotherapy and 2 patients after 6 cycles of chemotherapy. Subsequent follow-up GLA imaging was performed in 7 / 11 patients. On evaluating by Response Evaluation Criteria In Solid Tumors (RECIST) criteria, CECT scan showed partial response (PR) in 16 evaluations out of which GLA findings were concordant in 14. This was shown by regression in tracer uptake. Functional complete response was seen in two cases signified by absence of tracer uptake. Residual masses were seen in CECT in both of them. CECT showed progression in two patients, out of which two had concordant findings [Table 5, Figures 6–8].
Table 5.
Correlation between CT scan and GLA scintigraphy in evaluation of response to treatment in lung cancer

Figure 6.
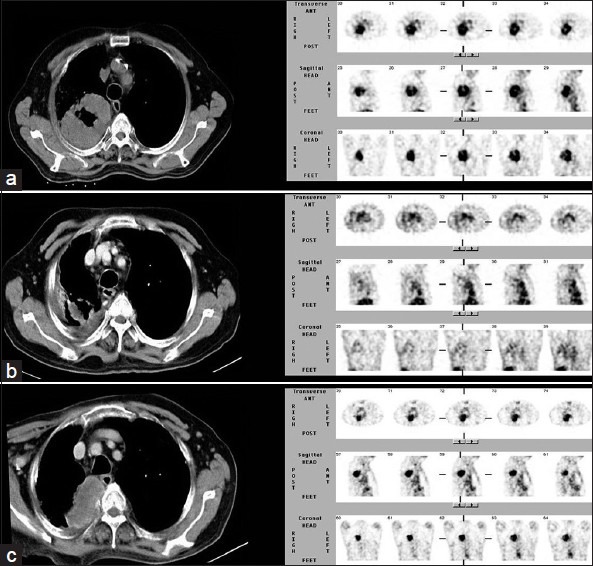
A 78-year-old male, a case of NSCLC. Baseline scan (a) shows avid tracer concentration in the primary lesion in right lung. Post 3 cycles of chemotherapy, the lesion shows complete functional response with residual lung changes in CT. (b) On followup, a recurrent lesion (c) is seen after 8 months
Figure 8.
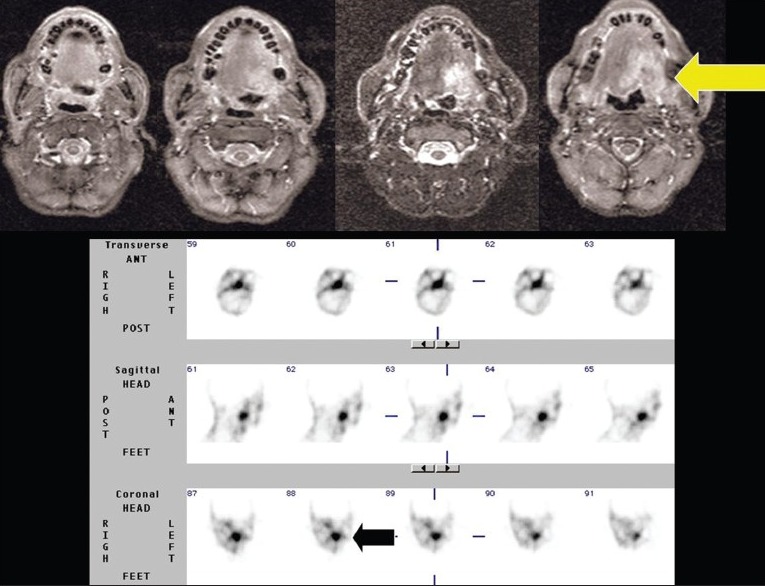
A 57-year-old old male, a case of carcinoma of the base of tongue. Avid 99mTc-glucarate concentration is seen in the tumor corresponding to the lesion in MRI in the baseline study
Figure 7.
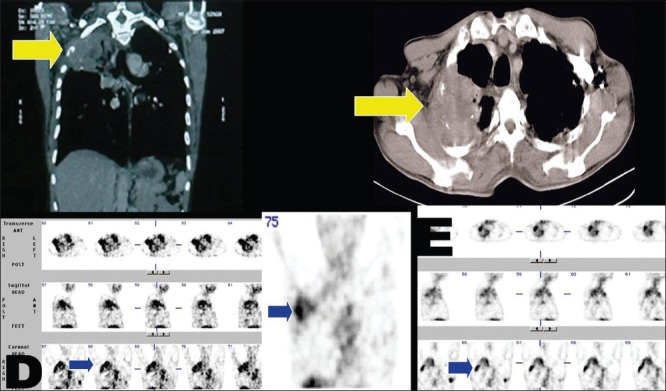
A 53-year-old male, a case of NSCLC. There is avid 99mTc-glucarate concentration in the mass in right lung, rib, and axillary nodal metastasis in the baseline/pre-treatment study (D). There is a significant regression in the size of the primary lesion in lung and axillary nodal metastasis post 3 cycles of chemotherapy (E), with corresponding clinical/symptomatic improvement
Evaluation of metastatic sites (Group III)
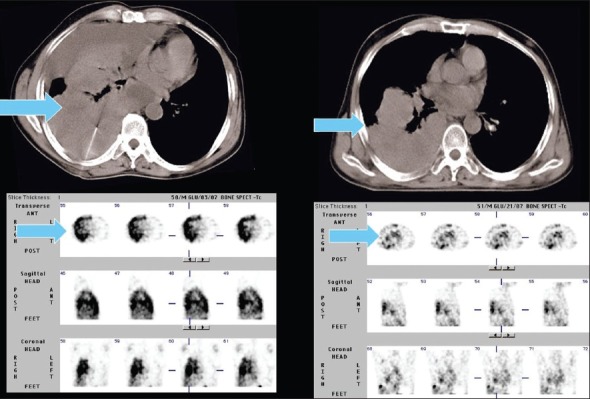
Eleven patients of lung carcinoma had radiological evidence of metastases at 17 sites, comprising lymph nodes, lung, bones, and liver. Excellent sensitivity was noted in the detection of bone metastases in 7 / 7 cases (100%). GLA concentration was grade 3 in all the sites. 5 / 8 sites of lymph nodal and 1 / 1 site of contralateral lung metastases showed positive GLA uptake [Table 6, Figures 2 and 9]. No GLA concentration was seen in one case of liver metastasis and rest of the patients who did not have any clinical or radiological evidence of distant metastasis.
Table 6.
Correlation between radiological evidence of metastatic disease and glucarate concentration
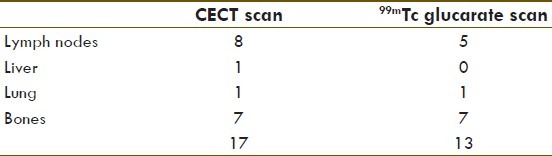
Figure 2.
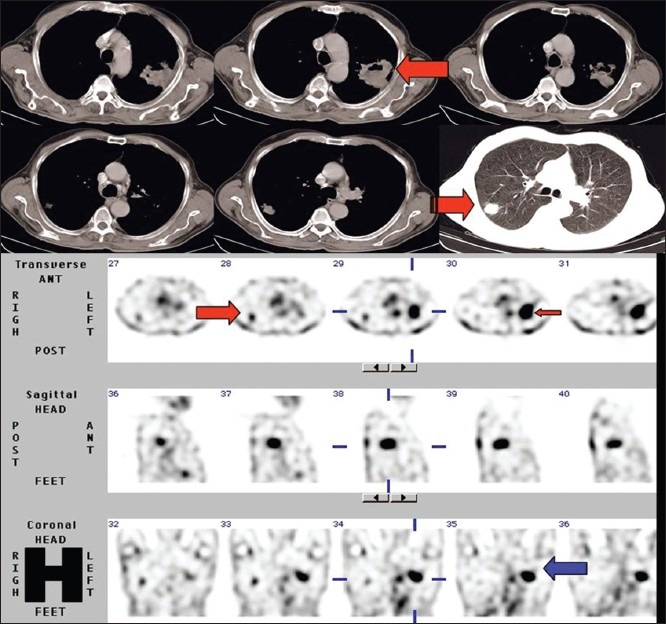
A 60-year-old male, a case of NSCLC. There is avid 99mTc-glucarate concentration in the mass lesion in the left lung corresponding to the primary lesion seen on the CT scan. The metastatic lesion in contralateral lung, seen better in the lung window in the CT scan, also shows avid glucarate concentration
Figure 9.
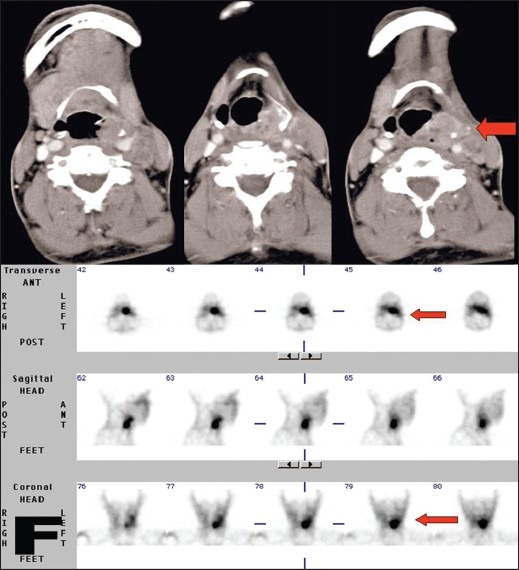
A 53-year-old male, a case of carcinoma of pyriform fossa and base of tongue. Avid 99mTc-glucarate concentration is seen in the tumor, corresponding to the lesion in CT scan, in the baseline study
Comparison between 99mTc glucarate and 18F FDG PET scan (Group IV)
Eleven patients were enrolled who underwent a scheduled 18F FDG PET-CT for staging of lung cancer. 99mTc glucarate study was done within 48 h of FDG PET/CT scans in these patients. The metabolically active primary lesion showed positive glucarate uptake in 7 (64%) cases. Due to technical reasons, only planar regional images could be acquired in this group. SPECT was not evaluated [Table 7, Figure 10].
Table 7.
Comparison between 18F FDG PET-CT scan and 99mTc glucarate scan in lung cancer
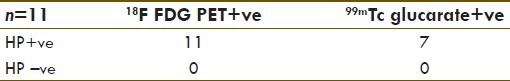
Figure 10.
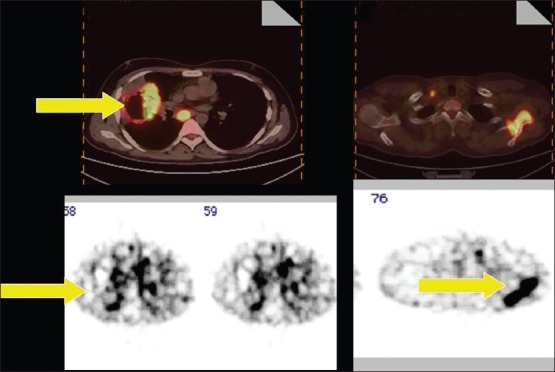
A 31-year-old male, a case of NSCLC. Both 18-F FDG PET-CT and 99mTc-glucarate study show tracer concentration in the primary lesion and bone metastasis
Discussion
There is a general paucity of clinical data on tumor imaging with 99mTc glucarate in the medical literature. Historically 99mTc glucarate was used to study myocardial infarction as an infarct-avid imaging agent for very early detection of myocardial infarction. The mechanism of 99mTc glucarate uptake and its retention in infarcted myocardium has been postulated to be through a mechanism of binding to nuclear histones which are exposed by cellular and nuclear membrane breaks caused by irreversible myocyte injury.[8,9] Approximately 83% of the nuclear uptake of glucarate appears to be associated with nuclear histones.[10] Most of the data on this aspect have been on animal models. Okada et al.[11] studied the detection of infarct in a well-characterized canine model and found enhanced tracer uptake and retention in necrotic myocardium as early as 130 min after coronary occlusion. Excellent gamma camera images of infarct could be obtained within 30 min of tracer injection. In a group of 28 patients presenting with acute chest pain to the emergency, Mariani et al.[12] reported that 99mTc glucarate does concentrate in myocardial infarction, but the sensitivity of detection is remarkably time dependent and detection is only possible if injection was performed within 9 h of onset of infarction. Recently, Okada et al. reported the kinetics of glucarate to differentiate normal, stunned, hibernating, and nonviable myocardium in a rat model where they demonstrated that glucarate uptake strongly correlated with myocardial necrosis and minimal uptake was seen in stunned and hibernating myocardium as in normal controls.[13] Based on the fact that this radiotracer concentrated in injured cells and a continuing search for tumor imaging agents in solid tumors, 99mTc glucarate has been proposed as a tumor imaging agent in few studies. It was postulated initially that glucarate behaves as an analog of glucose, similar to 18F FDG. This was supported by the evidence that normal bio-distribution of glucarate was affected by the administration of insulin.[14] Ballinger et al.[15] reported the accumulation of glucarate in in vitro cell cultures and in vivo tumor models. They found that this radiopharmaceutical was taken up and retained in a variety of cells in vitro. The accumulation enhanced under hypoxic conditions in most of the cell lines. It was seen that glucarate showed high tumor to muscle and tumor to blood ratios after injection in animal tumor models. Liu et al.[16] subsequently demonstrated that MCF7 human breast tumor xenografts can be detected by 99mTc glucarate in vivo imaging with early unequivocal visualization and longer retention and can localize drug-resistant breast tumors.
We found reports in literature mostly with respect to the use of 99mTc glucarate as a tumor imaging agent in experimental studies. Evidence of a formal clinical trial is lacking. Gambini et al.[17] have demonstrated its tumor-seeking properties in a preliminary study. They found avid tracer concentration in head and neck tumors with good tumor to background ratio and concluded that glucarate could be a potential radiotracer for the evaluation of these malignancies. In our study, lung cancer has been studied in more detail. We validated the tumor-seeking properties of the radiopharmaceutical and its ability to evaluate therapeutic response. Results of glucarate concentration in lung cancer have been better than those in the head and neck malignancies, with the viable tumor showing avid tracer concentration with no uptake in necrotic areas. Bone and lymph nodal metastasis was also detected in this group of patients. We concur that the mechanism of uptake in the tumors could be multi-factorial and could result from high metabolic rate of tumors, hypoxia, necrosis, or apoptotic process that could dynamically coexist in different proportions in malignant tumors All of these are expected to play various roles in this mechanism. Perek et al.[18] demonstrated that 99mTc glucarate is able to distinguish apoptotic cells from necrotic cells with in vitro and in vivo studies in leukemic tumor cell line U937. The specificity of glucarate as a marker of necrosis is an interesting property of this tracer with several potential applications in oncology, including monitoring the efficacy of treatment. It is likely that in acutely injured cells, the loss of membrane integrity allows entry and intracellular diffusion of the small-sized 99mTc glucarate where its negative charge attracts and binds to the positively charged histones. Myocardium injured by apoptosis has not demonstrated increased uptake of glucarate, indicating that in dying and dead cells which do not have membrane breaks and exposed histones, no such binding occurs. In the head and neck malignancies, our study has shown avid tracer uptake in detection of primary head and neck malignancies, with excellent target to background ratios. However, our false-negative rates were high in head and neck malignancies. We presume the reason for this to be more technical rather than related to radiopharmaceutical properties. Tumor heterogeneity could be a contributing factor. However, the resolution of the gamma camera and more importantly absence of SPECT-CT fusion could be a potential cause.
On the whole, this study had some novel and valuable findings. It has provided the “proof of concept” that glucarate can be successfully labeled with 99mTcO4 with a favorable normal bio-distribution. It has excellent tumor concentrating properties and accumulates in viable tumor in vivo. There is initial evidence to suggest that 99mTc glucarate has some properties of being a glucose analog and can be used for assessment of therapeutic response in patients of lung cancer. Our observations have shown a trend toward a longer disease-free interval/time to progression in a patient who had significant functional response validated by near-total regression of radiotracer uptake post treatment. This, however, needs to be confirmed in future studies. Thus, it can be used as an agent for assessing tumor viability in places/countries where facilities for PET are not available.
There were few limitations in this study. Due to sample selection bias, the specificity of this new tracer could not be well established. The concentration of this radiopharmaceutical could not be established in conditions mimicking tumors or other benign conditions which may co-exist in these groups of patients. Small-sized functionally active lesions could have been detected better with an integrated SPECT–CT system. Non-availability of SPECT in the later phase (during pilot study of PET and glucarate) of the study was also a limitation.
Conclusion
99mTc glucarate is an easy to prepare radiopharmaceutical agent which can be formulated in a hospital radiopharmacy. It shows avid concentration of the tracer in the tumor in lung cancer and head neck malignancies. The results obtained so far suggest that 99mTc glucarate is a potential tumor imaging agent with high sensitivity which can be used for detection and assessment of therapeutic response in malignancy. A clinical trial involving a larger number of patients for comparing with 18F FDG glucose should be done in future.
Footnotes
Source of Support: This work is a part of International Atomic Energy Agency's (IAEA) co-ordinated research project (CRP) entitled ‘Standardization of Hospital Prepared Radiopharmaceuticals for Nuclear Oncology (IAEA CRP E1.30.28) contract no.12832/R0. Initial part of this work was presented in the Lancet Asia Medical Forum 2007 in Singapore
Conflict of Interest: None declared
References
- 1.Imam SK. Molecular nuclear imaging: The radiopharmaceuticals (review) Cancer Biother Radiopharm. 2005;20:163–72. doi: 10.1089/cbr.2005.20.163. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger JR, Proulx A, Ruddy TD. Stable kit formation of technetium- 99m-glucarate. Radiat Appl Instrum Part A. 1991;42:405–6. [Google Scholar]
- 4.Yaoita H, Uehara T, Brownell AL, Rabito CA, Ahmad M, Khaw BA, et al. Localization of technetium-99m-glucarate in zones of acute cerebral injury. J Nucl Med. 1991;32:272–8. [PubMed] [Google Scholar]
- 5.Schimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chemother Pharmacol. 1999;43:165–72. doi: 10.1007/s002800050879. [DOI] [PubMed] [Google Scholar]
- 6.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: A specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 7.Schultz DR, Harrington WJ., Jr Apoptosis: Programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32:345–69. doi: 10.1053/sarh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 8.Narula J, Petrov A, Pak KY, Lister BC, Khaw BA. Very early noninvasive detection of acute experimental nonreperfused myocardial infarction with 99mTc-labeled glucarate. Circulation. 1997;95:1577–84. doi: 10.1161/01.cir.95.6.1577. [DOI] [PubMed] [Google Scholar]
- 9.Khaw BA, Silva JD, Petrov A, Hartner W. Indium 111 antimyosin and Tc-99m glucaric acid for noninvasive identification of oncotic and apoptotic myocardial necrosis. J Nucl Cardiol. 2002;9:471–81. doi: 10.1067/mnc.2002.124479. [DOI] [PubMed] [Google Scholar]
- 10.Rammohan RP, Haider N, Vural I, Pak CK, Narula J, Khaw BA. Subnuclear localization of 99mTc Glucarate in necrotic myocardium. J Nucl Med. 1996;37:175P. [Google Scholar]
- 11.Okada DR, Johnson G, Liu Z, Hocherman SD, Khaw BA, Okada RD. Early detection of infarct in reperfused canine myocardium using 99mTc-glucarate. J Nucl Med. 2004;45:655–64. [PubMed] [Google Scholar]
- 12.Mariani G, Villa G, Rossettin PF, Spallarossa P, Bezante GP, Brunelli C, et al. Detection of acute myocardial infarction by 99mTc-labeled D-glucaric acid imaging in patients with acute chest pain. J Nucl Med. 1999;40:1832–9. [PubMed] [Google Scholar]
- 13.Okada DR, Liu Z, Johnson G, 3rd, Beju D, Khaw BA, Okada RD. 99mTc-glucarate kinetics differentiate normal, stunned, hibernating, and nonviable myocardium in a perfused rat heart model. Eur J Nucl Med Mol Imaging. 2010;37:1909–17. doi: 10.1007/s00259-010-1495-0. [DOI] [PubMed] [Google Scholar]
- 14.TenCate CI, Fischman AJ, Wilkinson RA. 99mTc Glucarate: A glucose analog (abstract) Eur J Nucl Med. 1990;16:451. [Google Scholar]
- 15.Ballinger JR, Hsue V, Rauth AM. Accumulation of technetium- 99mglucarate: In vitro cell cultures and in vivo tumour models. Nucl Med Commun. 2003;24:597–606. doi: 10.1097/00006231-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Stevenson GD, Barrett HH, Kastis GA, Bettan M, Furenlid LR, et al. 99mTc glucarate high-resolution imaging of drug sensitive and drug resistant human breast cancer xenografts in SCID mice. Nucl Med Commun. 2004;25:711–20. doi: 10.1097/01.mnm.0000130243.06821.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambini JP, Nuñez M, Cabral P, Lafferranderie M, Noble J, Corchs E, et al. Evaluation of patients with head and neck cancer by means of 99mTc-glucarate. J Nucl Med Technol. 2009;37:229–32. doi: 10.2967/jnmt.109.062927. [DOI] [PubMed] [Google Scholar]
- 18.Perek N, Sabido O, Le Jeune N, Prevot N, Vergnon JM, Clotagatide A, et al. Could 99mTc-glucarate be used to evaluate tumour necrosis? Eur J Nucl Med Mol Imaging. 2008;35:1290–8. doi: 10.1007/s00259-007-0689-6. [DOI] [PubMed] [Google Scholar]


