Abstract
Selumetinib (AZD6244, ARRY-142886) is a potent, selective, uncompetitive inhibitor of MEK 1 / 2, part of the RAF/MEK/ERK protein kinase signal cascade, which is responsible for tumor. This pilot study was used to explore if 18F-fluoro-l-thymidine (FLT), a thymidine analogue positron emission tomography (PET) tracer and a surrogate marker for proliferation, can be used as an early predictor of response for patients with solid cancers treated with Selumetinib. FLT-PET scans were performed in four patients at baseline and after 2 weeks of treatment with Selumetinib. FLT uptake in tumors was analyzed qualitatively and quantitatively by measuring standard uptake value (SUV) max in regions of interest (ROI). Results were compared to computed tomography (CT) scans (baseline and after 8 weeks), which were evaluated using the response evaluation criteria in solid tumors (RECIST) 1.0 criteria. One patient with melanoma showed both a qualitative and quantitative decrease in FLT uptake associated with a decrease in sum of longest diameter of 12% RECIST on CT evaluation. Another patient who had colorectal carcinoma (CRC) showed a significant increase in FLT uptake with initially stable, but eventually progressive disease on CT. The other two patients (one with melanoma and one with CRC) showed no significant changes in FLT uptake, whereas CT evaluation showed progressive disease. This is the first report describing changes in FLT-PET in patients receiving Selumetinib. In two patients, changes in FLT uptake as early as after 2 weeks of treatment were consistent with CT results after 8 weeks. Biomarkers to predict and evaluate treatment the outcome of targeted therapies are highly warranted. These initial results need further investigation.
Keywords: F-18 FLT, PET-CT, Selumetinib, treatment response
Introduction
The intracellular Ras-regulated Raf/MEK/ERK protein kinase signal cascade is a key pathway involved in cellular proliferation and survival. A strong correlation between deregulation of this pathway and uncontrolled cell proliferation has been demonstrated.[1] Selumetinib (AZD6244, ARRY-142886) is a potent, selective, uncompetitive inhibitor of MEK ½, developed as targeted therapy to treat solid cancers. A favorable toxicity, pharmacokinetic, and pharmacodynamic profile has been observed in phase I studies and phase II studies focusing on melanoma and colorectal cancer (CRC).[2–4] The development of targeted agents requires identification and better understanding of positive predictive biomarkers of clinical response to these agents. Currently, the CT Response Evaluation Criteria in Solid Tumors (RECIST) are mostly used to evaluate response.[5] These RECIST guidelines are based on the sum of one-dimensional measurements of the greatest diameter of the tumor and/or metastases.[5,6] However, treatment with targeted therapies is cytostatic rather than cytotoxic, and can result in necrosis and cavitation without a change in lesion (or tumor) size, leading to an underestimation of therapeutic efficacy. Imaging techniques able to predict treatment outcome in an early phase of treatment are warranted. Molecular imaging may enable alternative evaluation procedures for these new drugs and enable the early change to an alternative therapy if no functional response is indicated. Recently the functional imaging technique of positron emission tomography (PET) using the radiopharmaceutical tracer 18F-fluorodeoxyglucose (FDG) has been found to be a useful method for response monitoring in various malignancies.[7–11] However, FDG is a tracer for glucose metabolism, which does not always reflect proliferation activity. The PET agent 18F-fluoro-l-thymidine (FLT) has been introduced for imaging of cell proliferation. FLT is a thymidine analogue, which is retained in proliferating cells through the activity of the enzyme thymidine kinase-1, which is expressed during the DNA synthesis phase of the cell cycle.[12] FLT-PET has been applied for the assessment of proliferation rate in different tumors.[13–18] In this pilot study, we assessed the effect of Selumetinib on tumor cell proliferation in patients with a variety of solid tumors by FLT-PET-CT and determined whether changes in FLT uptake can potentially be used as an early predictive biomarker for treatment response.
Materials and Methods
Patients
This single-institution study was conducted in conjunction with Phase I clinical trial of the capsule formulation of Selumetinib (NCT00463814) in patients with solid tumors.[2] In this study, patients with advanced solid cancer refractory to standard therapies or for whom no conventional therapies exists were treated with oral Selumetinib twice daily in a dose escalation schedule. As part of this study protocol, an evaluation CT scan was performed after every two cycles (i.e. at 8 weekly intervals of treatment with Selumetinib).
Patients participating in the FLT-PET side study had to have at least one tumor deposit of at least 2 cm outside the liver and axial skeleton. All patients gave written informed consent and both studies were approved by the local ethical committee.
FLT-PET
FLT-PET scans were performed at baseline and after 2 weeks of treatment. The scans were performed on an integrated PET-CT scanner (Siemens/Biograph) using a static whole body protocol (hips to base of skull) 1 h after administration of 250 MBq FLT.
The FLT-PET images were analyzed both visually for any tumor targeting and quantitatively for changes in FLT uptake. Quantitative assessment was realized by drawing CT-derived 3D regions of interest (ROI) over the tumors, with both a threshold of 50% and 70% of the maximum FLT-activity via an automatic algorithm. Standardized uptake values (SUVs) were calculated using the concentration of FLT in the volume of interest (VOI) as measured by PET, divided by the injected dose per kg body weight as a normalization factor.
Evaluation
FLT-PET scans were analyzed by two nuclear medicine physicians. Only lesions outside of the liver and axial skeleton, with a diameter of 2 cm or more, which were also measured on CT, were evaluated. Based on two recent studies by de Langen et al. and Wahl et al., any changes in SUVmax greater than 30% were considered as significant and medically relevant, independent of day-to-day variability.[19,20] CT scans were analyzed using the RECIST 1.0 guidelines.[6] Quantitative (mean SUVmax of measured lesions) FLT-PET results were compared with the results of the radiological evaluation with CT-scan based on RECIST 1.0 criteria.
Results
In four patients, both baseline and follow-up FLT-PET-CTs were performed. Two patients had metastasized melanoma and two patients had advanced/metastatic CRC. One patient with melanoma showed both a qualitative and quantitative decrease in FLT uptake, correlating with a decrease in sum of diameters of 12% applying RECIST to CT evaluation [Figures 1 and 2]. Another patient who had CRC showed a significant increase in FLT uptake with initially (at 8 weeks) stable disease, but eventually progressive disease on CT. The other two patients (one with melanoma and one with CRC) showed no significant changes in FLT uptake, whereas CT evaluation showed progressive disease [Table 1].
Figure 1.
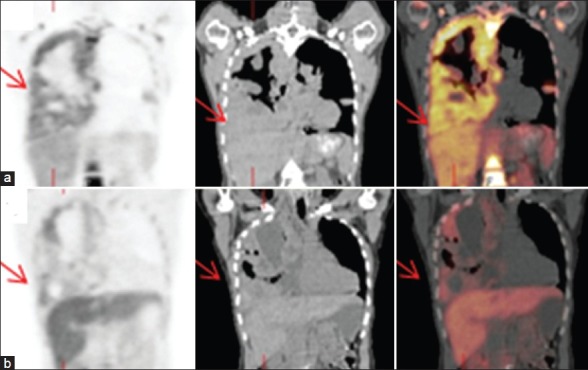
F-18 FLT PET/CT scan. Coronal images: (a) Before treatment and (b) after treatment. A female patient with pleural metastases of a melanoma, showing decreased FLT uptake in the pleural metastases after treatment with Selumetinib for 2 weeks (Patient No. 1) [Table 1]
Figure 2.
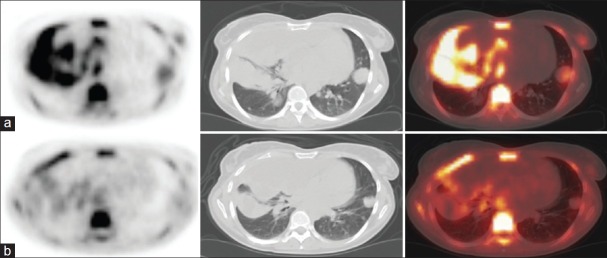
F-18 FLT PET/CT scan. Transaxial images: (a) Before treatment and (b) after treatment. A female patient with pleural metastases of a melanoma, showing decreased FLT uptake in the pleural metastases after treatment with Selumetinib for 2 weeks (Patient No. 1) [Table 1]
Table 1.
Changes in mean SUVmax compared to CT changes according to RECIST after Selumetinib therapy in four patients with metastatic melanoma or metastatic colorectal carcinoma
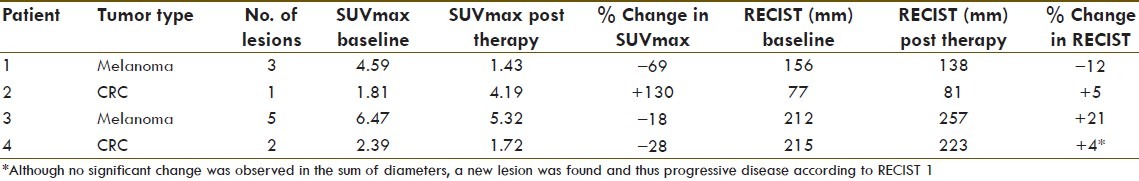
Discussion
In the new era of targeted therapies, there is a need for early identification of therapy responding and non-responding patients, in order to be able to change therapy, thereby working toward personalized medicine. Response monitoring for targeted therapies demands more specific diagnostic modalities than conventional imaging alone. Differentiation between vital tumor and fibrosis or necrosis is not possible by using morphological features as in RECIST. Furthermore, with functional and molecular imaging techniques, one could prevent a patient from unnecessarily suffering from drug side effects by differentiating responders from non-responders in an early stage of treatment.
As mentioned before, PET with FDG already has an established role in monitoring various anti-cancer therapies such as targeted therapies in malignant gastrointestinal stromal tumors.[10] This has led to the creation of the recently developed PET Response Criteria in Solid Tumors (PERCIST).[20]
Beyond FDG, more specific imaging biomarkers for PET have been developed of which a reasonable amount is already in clinical stage like 18F-16α-17-fluoroestradiol (18F-FES), 18F-galacto-RGD, and 18F-FLT, which was used in this study.[21]
However, PET is not the only imaging modality with potential in early response monitoring in clinical oncology. Different magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) techniques for functional and molecular imaging have been developed.
One of them is dynamic contrast-enhanced MRI (DCE-MRI) in which the kinetics of contrast agent inflow into the tumor after intravenous injection of the agent is followed.[22] Since tumor angiogenesis is associated with an increase in vessel permeability, this can be measured using DCE-MRI techniques.[23] Morgan et al. found that in patients treated with an anti-angiogenic vascular endothelial growth factor receptor tyrosine kinase inhibitor, there were significantly greater reductions in a pharmacokinetic parameter that was related to vessel permeability in patients who showed a positive response to treatment than in those who had progressive disease.[24]
Furthermore, multimodality imaging has potential role in clinical response monitoring, whereas PET/CT and single-photon emission computed tomography (SPECT) CT are already widely used for the evaluation of cancer.[25] However, the major drawback of these techniques is that they are combined by software and not acquired simultaneously. Recently, Judenhover et al. have performed simultaneously FLT-PET and MRI in a mouse model for colon carcinoma. This system was able to image three functional imaging techniques, PET, functional MRI, and MRS with morphological MRI.[26]
No fully statistically powered human studies on FLT-PET for cancer therapy response monitoring have been performed. Nevertheless, a possible beneficial role in therapeutic response of various solid tumors with different types of therapy have been shown,[27–29] as well as the potential of using quantitative parameters for FLT uptake such as SUV.[19]
This is the first report of 18F-FLT-PET to assess the effect of the MEK inhibitor Selumetinib. In this limited study, FLT-PET-CT as an early predictor of response on Selumetinib is interesting. Further investigation of FLT-PET as a biomarker of early treatment response is needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Kolch W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1 / 2 inhibitor AZD6244 (ARRY-142886): A phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 3.Bennouna J, Lang I, Valladares-Ayerbes M, Boer K, Adenis A, Escudero P, et al. A Phase II, open-label, randomised study to assess the efficacy and safety of the MEK1 / 2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–8. doi: 10.1007/s10637-010-9392-8. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, et al. Phase II, open-label, randomized trial of the MEK1 / 2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18:555–67. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–93. doi: 10.1007/s00259-007-0426-1. [DOI] [PubMed] [Google Scholar]
- 8.De Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–8. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 9.De Geus-Oei LF, van Laarhoven HW, Visser EP, Hermsen R, van Hoorn BA, Kamm YJ, et al. Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol. 2008;19:348–52. doi: 10.1093/annonc/mdm470. [DOI] [PubMed] [Google Scholar]
- 10.Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- 11.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 12.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 13.Apisarnthanarax S, Alauddin MM, Mourtada F, Ariga H, Raju U, Mawlawi O, et al. Early detection of chemoradioresponse in esophageal carcinoma by 3’-deoxy-3’- 3H-fluorothymidine using preclinical tumor models. Clin Cancer Res. 2006;12:4590–7. doi: 10.1158/1078-0432.CCR-05-2720. [DOI] [PubMed] [Google Scholar]
- 14.Buck AK, Bommer M, Stilgenbauer S, Juweid M, Glatting G, Schirrmeister H, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–61. doi: 10.1158/0008-5472.CAN-06-1955. [DOI] [PubMed] [Google Scholar]
- 15.Francis DL, Visvikis D, Costa DC, Arulampalam TH, Ownsend C, Luthra SK, et al. Potential impact of [18F]3’- deoxy-3’- fluorothymidine versus [18F]fluoro-2-deoxy-D-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging. 2003;30:988–94. doi: 10.1007/s00259-003-1187-0. [DOI] [PubMed] [Google Scholar]
- 16.Seitz U, Wagner M, Neumaier B, Wawra E, Glatting G, Leder G, et al. Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3’- [(18)F]fluoro-3’- deoxythymidine ([(18)F]FLT) in pancreatic cancer cell lines. Eur J Nucl Med Mol Imaging. 2002;29:1174–81. doi: 10.1007/s00259-002-0851-0. [DOI] [PubMed] [Google Scholar]
- 17.Van Westreenen HL, Cobben DC, Jager PL, van Dullemen HM, Wesseling J, Elsinga PH, et al. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. J Nucl Med. 2005;46:400–4. [PubMed] [Google Scholar]
- 18.Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3‘deoxy-3’- [(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: Correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–23. [PubMed] [Google Scholar]
- 19.De Langen AJ, Klabbers B, Lubberink M, Boellaard R, Spreeuwenberg MD, Slotman BJ, et al. Reproducibility of quantitative 18F-3’- deoxy-3’- fluorothymidine measurements using positron emission tomography. Eur J Nucl Med Mol Imaging. 2009;36:389–95. doi: 10.1007/s00259-008-0960-5. [DOI] [PubMed] [Google Scholar]
- 20.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seaman ME, Contino G, Bardeesy N, Kelly KA. Molecular imaging agents: Impact on diagnosis and therapeutics in oncology. Expert Rev Mol Med. 2010;12:e20. doi: 10.1017/S1462399410001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: Issues and recommendations. Br J Cancer. 2005;92:1599–610. doi: 10.1038/sj.bjc.6602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald DM, Choyke PL. Imaging of angiogenesis: From microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 24.Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: Results from two phase I studies. J Clin Oncol. 2003;21:3955–64. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 25.Bockisch A, Freudenberg LS, Schmidt D, Kuwert T. Hybrid imaging by SPECT/CT and PET/CT: Proven outcomes in cancer imaging. Semin Nucl Med. 2009;39:276–89. doi: 10.1053/j.semnuclmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat Med. 2008;14:459–65. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–21. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann K, Wieder HA, Buck AK, Schöffel M, Krause BJ, Fend F, et al. Early response assessment using 3’- deoxy-3’- [18F]fluorothymidine-positron emission tomography in high-grade non-Hodgkin's lymphoma. Clin Cancer Res. 2007;13:3552–8. doi: 10.1158/1078-0432.CCR-06-3025. [DOI] [PubMed] [Google Scholar]
- 29.Sohn HJ, Yang YJ, Ryu JS, Oh SJ, Im KC, Moon DH, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res. 2008;14:7423–9. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]


