Abstract
In single photon emission computed tomography (SPECT), the collimator is a crucial element of the imaging chain and controls the noise resolution tradeoff of the collected data. The current study is an evaluation of the effects of different thicknesses of a low-energy high-resolution (LEHR) collimator on tomographic spatial resolution in SPECT. In the present study, the SIMIND Monte Carlo program was used to simulate a SPECT equipped with an LEHR collimator. A point source of 99mTc and an acrylic cylindrical Jaszczak phantom, with cold spheres and rods, and a human anthropomorphic torso phantom (4D-NCAT phantom) were used. Simulated planar images and reconstructed tomographic images were evaluated both qualitatively and quantitatively. According to the tabulated calculated detector parameters, contribution of Compton scattering, photoelectric reactions, and also peak to Compton (P/C) area in the obtained energy spectrums (from scanning of the sources with 11 collimator thicknesses, ranging from 2.400 to 2.410 cm), we concluded the thickness of 2.405 cm as the proper LEHR parallel hole collimator thickness. The image quality analyses by structural similarity index (SSIM) algorithm and also by visual inspection showed suitable quality images obtained with a collimator thickness of 2.405 cm. There was a suitable quality and also performance parameters’ analysis results for the projections and reconstructed images prepared with a 2.405 cm LEHR collimator thickness compared with the other collimator thicknesses.
Keywords: Image quality, low-energy high-resolution collimator, resolution, simulating medical imaging nuclear detectors, single photon emission computed tomography
Introduction
In Single Photon Emission Computed Tomography (SPECT) cameras, the system spatial resolution mainly depends on the geometry of the collimation design.[1] Recent developments in pinhole SPECT imaging for small animal applications have reached a spatial resolution on the order of 0.6 mm.[2]
There are some approaches for scatter correction in SPECT,[3–8] but optimization of the collimator, as a prime order for scatter inhibition, could affect other image quality parameters and also accurate quantification in SPECT.[7–12] Monte Carlo techniques are becoming very popular for the development of nuclear medicine apparatus.[11,13] A study on characterization of scatter and penetration using Monte Carlo simulation has shown that in 131 I SPECT, object scatter as well as collimator scatter and penetration have a significant contribution on the image.[14]
The purpose of the current study was to evaluate the effects of the collimator thickness, fine tuning of a low-energy high-resolution (LEHR) collimator with a thickness of 4.05 cm, on the tomographic spatial resolution by image quality analysis by visual inspection and using structural similarity index (SSIM), a method for measuring the similarity between two images.[15] The SSIM index is a full reference metric, in other words, the measuring of image quality based on an initial uncompressed or distortion-free image as reference. SSIM is designed to improve on traditional methods like peak signal-to-noise ratio (PSNR) and mean squared error (MSE), which have proved to be inconsistent with human eye perception.[16]
Materials and Methods
Imaging system
A dual-headed variable angle scintillation gamma camera (Siemens Medical Solutions USA, Inc., USA), equipped with two rectangular detectors with a field of view (FOV) of 53.3 × 38.7 cm2 and 9.5-mm-thick NaI (Tl) crystals, was used in this study and also for the Monte Carlo simulations. The camera consists of two removable LEHR collimators. The parameters of LEHR collimator, used for low-energy sources such as 99mTc, for experiment and simulation are shown in Table 1. The NaI (Tl) crystal specifications are as follows: Planar, 9.5 mm in thickness, 59.1 × 44.5 cm2 in area, light yield 40K photons/MeV, and a peak emission spectrum at 415 nm.[17,18] Emitted scintillation light photons are collected by a matrix composed of 59 photomultiplier tubes (PMT), 53 with a diameter of 7.6 cm and 6 with 5.1 cm. The photocathode is of the bialkali type with a quantum efficiency of approximately 30% for the wavelength of maximum NaI (Tl) emission.[19] A light guide ensures maximum collection of light and optical grease results in good optical coupling between the scintillating crystal and PMTs.
Table 1.
Physical specifications of an SI-LEHR collimator

Monte carlo simulation
The SIMIND Monte Carlo program was used to simulate the SPECT camera and phantom studies.[20] When I-131 is used, various structures attached to back of the crystal contribute in backscattering of the emitted photons, and also, this is true to some extent with 99mTc. To assess the effect of these parts, a single 6-cm slab of Pyrex[21] was substituted and simulated. For details on the gamma camera simulation, refer to Bahreyni Toossi et al.[22]
Phantoms
For this study, we have simulated a 99mTc point source of 37 MBq, an acrylic cylindrical Jaszczak Deluxe phantom,[23,24] with spherical inserts measuring 9.5, 12.7, 15.9, 19.1, 25.4, and 31.0 mm in diameter and insert of rods measuring 4.8, 6.4, 7.9, 9.5, 11.1, and 12.7 mm in diameter, filled with water and 370 MBq activity of 99mTc; and also a four-dimensional (4-D) NURBS-based cardiac-torso (NCAT) phantom[25] with normal organs and also with hot and cold lesions on liver, lung, and myocardium. As the SIMIND-simulated SPECT projections are noise free, for realization, we add noise according to the administered dose.
Image acquisition
In this work, we have studied the following parameters of collimator: (i) Energy resolution; (ii) spatial resolution; and (iii) image contrast as a function of 11 different collimator thicknesses ranging from 2.400 to 2.410 cm using the established and published quality control tests.[26–32] The specifications of the collimator, also used in simulation, are shown in Table 1. A 130–151 keV energy window was centered on the 99mTc photo peak.[31] Spatial and energy resolutions were determined by placing the point source at the center of the FOV, at 10 and 25 cm from lower collimator surface, respectively, for both simulation and experiment. An energy pulse-height distribution was acquired for 107 photons/projection with various thicknesses of the collimator. A study of the SPECT reconstructed spatial resolution was also carried out both experimentally and by SIMIND simulation. SPECT projections along the axis of rotation were acquired of the Jaszczak Deluxe Phantom,[33] which was positioned 15 cm from the collimator surface. Spatial resolution was obtained from viewing the smallest visible and recognizable rods. The images were reconstructed in matrices of 128 × 128 pixels, with a pixel size of 0.39 mm. One hundred and twenty-eight SPECT projections of the NCAT phantom were simulated in a 360° clockwise rotation mode. The images were reconstructed and processed by Filtered Back Projection (FBP) reconstruction using a ramp combined with a Butterworth filter of order 5 and cut-off frequency 0.45 cycles/cm.
Image evaluation
The images were evaluated qualitatively by two nuclear medicine specialists and quantitatively by SSIM. The SSIM metric was calculated on various windows of an image. The measure between two windows x and y of common size N × N was:
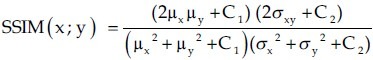
where
μx is the average of x;
μy is the average of y;
σx2 is the variance of x;
σy2 is the variance of y;
σxy is the covariance of x and y;
C1= (k1L)2, C2=(k2L)2 are two variables to stabilize the division with weak denominator;
L is the dynamic range of the pixel values (L = 255 for 8 bits/pixel gray scale images); and
k1 = 0.01 and k2 = 0.03 by default.
Approximately, μx and σx can be viewed as estimates of the luminance and contrast of x, and σxy measures the tendency of x and y to vary together, thus an indication of structural similarity.[16]
Results
SPECT system and LEHR collimator
We simulated a dual-headed variable angle scintillation gamma camera (Siemens Medical Solutions USA, Inc.) and related LEHR collimator with SIMIND Monte Carlo program.[22] The effects of 11 different collimator thicknesses, 2.400–2.410 cm, on calculated detector parameters for a 99mTc point source, a Jaszczak phantom, and a 4D-NCAT phantom scanning with an SIMIND-simulated SPECT are shown in Figure 1.
Figure 1.
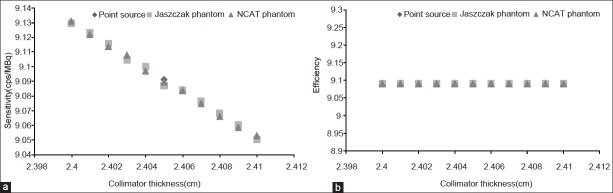
Scatter curves for the effect of LEHR collimator thickness on (a) sensitivity and (b) efficiency of the gamma camera. The results from scanning of a 99mTc point source, a Jaszczak phantom, and an NCAT phantom in an SIMIND-simulated SPECT
The results of the image quality obtained by visual inspection and on using the SSIM index of reconstructed images of the NCAT phantom SPECTs, respectively, showed improved quality, >0.75%, in lesion detectability for scanning with a 2.405 cm LEHR collimator thickness. For the reconstructed SPECT images of the Jaszczak phantom, the smallest cold sphere and rods observed were 12.7 and 9.5 mm in diameter, respectively.
Discussion
The effects of collimator thickness modifications on performance parameters like sensitivity and efficiency [Figure 1a and b], as well as the results of quantitative and qualitative analysis of the reconstructed SPECT images of the phantoms revealed that an LEHR collimator with a thickness of 2.405 cm can optimally diminish scattered radiation and collimate the rays carrying information about radioisotope distribution in the organ of target for detecting the lesions. Whereas there were no significant differences on the SSIM indexes for the images, few scan readers reported small improvement, >0.75%, on image quality with the collimator thickness of 2.405 cm.
Some studies on collimators and related hole diameters have shown that properties of a collimator play an essential role for constructing a detailed image from an organ,[34,35] and also improved the quality of pediatric 123I-MIBG images obtained with medium- energy collimator as compared with LEHR and Low-Energy Ultra-High-Resolution collimators for both SPECT and planar 123 I images.[36] However, it is difficult to evaluate simultaneously the effect of the three parameters of the collimator, including the diameter of a hole, the length of the collimator, and the number of holes of the collimator.
Ishikava et al. proposed a statistically derived optimization criterion. They also suggested a role for the size of photomultiplier tubes.[37] Our results also showed in addition to the decreased frequency of photon interactions from different thicknesses of the collimator, decreased related photon frequencies, and decreased sensitivity and efficiency [Figure 1]. These are indeed needed to compromise between the parameters according to the energy of radioisotope and also the type of organs to be imaged; otherwise, the improvement of spatial resolution with increasing collimator thickness affects the sensitivity and ability for detection and also increases the radiation absorbed dose in the patients. Moore et al. have compared imaging performance parameters of a parallel hole collimator with those of a fan beam collimator for a suitable design of collimator, and proposed task-dependent treatments.[38] Our results confirm suitability of thickness of the Siemens produced SI-LEHR collimator.[39] On the other hand, the role for some scatter- and attenuation-correction methods must be considered for implementation of a collimator effect. There are some correction techniques that are commonly used for performing corrections. Collimator correction has been shown to improve the reconstructed resolution, but the correction can generate ringing artifacts, which lower image quality. Bayesian reconstruction methods, as an approach, could reduce these artifacts.[40]
Conclusion
Poor resolution of SPECT has impaired its use in clinical practice. It is well known that collimators with a high resolution improve the ability to visualize small lesions and structures in a lesion, and the detection ability of the SPECT system, provided that the count density is satisfactory. The results of our current study show that the LEHR collimator with a thickness of 2.405 cm offers the best resolution when compared with other thicknesses. These data may encourage other investigators and allow the users to have a better evaluation in comparison to other equipments commonly used in nuclear medicine.
Acknowledgments
We express our gratitude to the head and all staff members of the Nuclear Medicine Department of Imam Reza Hospital of Mashhad University of Medical Sciences for their sincere cooperation. A part of the simulation study was completed in the Medical Radiation Physics Department of Clinical Sciences - Lund, Lund University, Lund, Sweden. We wish to have a special thanks to Professor Sven-Erik Strand, head of the department, for providing a proper study condition. This article originated from a PhD thesis, no. A-253, financially supported by the office of vice-president for research in Mashhad University of Medical Sciences, Iran.
Footnotes
Source of Support: Office of vice-president for research in Mashhad University of Medical Sciences, Mashhad, Iran
Conflict of Interest: None declared
References
- 1.Zeng GL. A skew-slit collimator for small-animal SPECT. J Nucl Med Technol. 2008;36:207–12. doi: 10.2967/jnmt.108.055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beekman FJ, van der Have F, Vastenhouw B, van der Linden AJ, van Rijk PP, Burbach JP, et al. U-SPECT-I: A novel system for submillimeter-resolution tomography with radiolabeled molecules in mice. J Nucl Med. 2005;46:1194–200. [PubMed] [Google Scholar]
- 3.McQuaid SJ, Southekal S, Kijewski MF, Moore SC. Joint optimization of collimator and reconstruction parameters in SPECT imaging for lesion quantification. Phys Med Biol. 2011;56:6983–7000. doi: 10.1088/0031-9155/56/21/014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyra M, Ploussi A. Filtering in SPECT image reconstruction. Int J Biomed Imaging. 2011;2011:1–14. doi: 10.1155/2011/693795. doi:10.1155/2011/693795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi H, Momose M, Kanaya S, Kondo C, Kusakabe K, Mitsuhashi N. Scatter correction by two-window method standardizes cardiac I-123 MIBG uptake in various gamma camera systems. Ann Nucl Med. 2003;17:309–13. doi: 10.1007/BF02988527. [DOI] [PubMed] [Google Scholar]
- 6.Seo Y, Wong KH, Sun M, Franc BL, Hawkins RA, Hasegawa BH. Correction of photon attenuation and collimator response for a body-contouring SPECT/CT imaging system. J Nucl Med. 2005;46:868–77. [PubMed] [Google Scholar]
- 7.Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011;38:S69–77. doi: 10.1007/s00259-011-1770-8. [DOI] [PubMed] [Google Scholar]
- 8.Ljungberg M, Sjögreen K, Liu X, Frey E, Dewaraja Y, Strand SE. A 3-dimensional absorbed dose calculation method based on quantitative SPECT for radionuclide therapy: Evaluation for (131)I using montecarlo simulation. J Nucl Med. 2002;43:1101–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Zanzonico PB, Bigler RE, Sgouros G, Strauss A. Quantitative SPECT in radiation dosimetry. Semin Nucl Med. 1989;19:47–61. doi: 10.1016/s0001-2998(89)80035-2. [DOI] [PubMed] [Google Scholar]
- 10.Beekman FJ, Kamphuis C, King MA, van Rijk PP, Viergever MA. Improvement of image resolution and quantitative accuracy in clinical Single Photon Emission Computed Tomography. Comput Med Imaging Graph. 2001;25:135–46. doi: 10.1016/s0895-6111(00)00064-1. [DOI] [PubMed] [Google Scholar]
- 11.Wilderman SJ, Dewaraja Y, Koral KF. Accurate modeling of nuclear-medicine collimators in monte carlo simulation of high-energy photons. Nucl Instrum Methods Phys Res A. 1999;422:745–50. [Google Scholar]
- 12.Stabin MG, Tagesson M, Thomas SR, Ljungberg M, Strand SE. Radiation dosimetry in nuclear medicine. Appl Radiat Isot. 1999;50:73–87. doi: 10.1016/s0969-8043(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 13.Lanconelli N. The importance of Monte Carlo simulations in modeling detectors for nuclear medicine. Math Comput Simul. 2010;80:2109–114. [Google Scholar]
- 14.Dewaraja YK, Ljungberg M, Koral KF. Characterization of scatter and penetration using montecarlo simulation in 131I imaging. J Nucl Med. 2000;41:123–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Rouse DM, Hemami Sh S. Proceedings of SPIE Human Vision and Electronic Imaging (HVEI) XIII. San Jose, California, United States: 2008. Analyzing the role of visual structure in the recognition of natural image content with multi-scale SSIM; pp. 1–14. [Google Scholar]
- 16.Wang Z, Simoncelli EP, Bovik AC. IEEE Asilomar Conf. on Signals, Systems, and Computers. Pacific Grove, California, United States: 2003. Multi-scale structural similarity for image quality assessment; pp. 9–12. [Google Scholar]
- 17.Motta D, Schnert S. Optical properties of bialkali photocathodes. Nucl Instr Meth Phys Res A. 2005;539:217–35. [Google Scholar]
- 18.Data sheet of Siemens E.CAM dual head gamma camera. Siemens Medical Solutions USA, Inc. Illinois, USA. 1992:1–24. [Google Scholar]
- 19.Vittori F, Notaristefani F, Malatesta T. Crystals and light collection in nuclear medicine. Nucl Phys B (Proc Suppl) 1999;78:616–21. [Google Scholar]
- 20.The SIMIND Monte Carlo Program (2010) [Last cited 1 Dec 2010]. Available from: http://www.radfys.lu.se/simind .
- 21.De Vries DJ, Moore SC, Zimmerman RE, Mueller SP, Friedland B, Lanza RC. Development and validation of a Monte Carlo simulation of photon transport in an Anger camera. IEEE Trans Med Imaging. 1990;9:430–8. doi: 10.1109/42.61758. [DOI] [PubMed] [Google Scholar]
- 22.Bahreyni Toossi MT, Islamian JP, Momennezhad M, Ljungberg M, Naseri SH. SIMIND Monte Carlo simulation of a single photon emission CT. J Med Phys. 2010;35:42–7. doi: 10.4103/0971-6203.55967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Data sheet of Flangeless Deluxe Jaszczak SPECT Phantom (1998) [Last cited 3 Dec 2010]. Available from: http://www.spect.com/pub/Flangeless_Jaszczak_Phantom.doc.pdf .
- 24.Groch MW, Erwin WD. Single-photon emission computed tomography in the year 2001: Instrumentation and quality control. J Nucl Med Technol. 2001;29:9–15. [PubMed] [Google Scholar]
- 25.Segars WP. 4D NCAT Phantom for medical imaging research. [Last cited 3 Dec 2010]; Available from http://www.dmip.rad.jhmi.edu/people/faculty/Paul/Segars_research.htm#NCAT . [Google Scholar]
- 26.Amer Inst of Phys. New York, USA: 1987. AAPM Report No 22. Rotating scintillation camera SPECT acceptance testing and quality control. [Google Scholar]
- 27.Hines H, Kayayan R, Colsher J, Hashimoto D, Schubert R, Fernando J, et al. National electrical manufacturers association recommendations for implementing SPECT instrumentation quality control. J Nucl Med. 2000;41:383–9. [PubMed] [Google Scholar]
- 28.Performance measurements of scintillation cameras. 1300 North 17th Street, Suite 1752, Rosslyn, Virginia, USA: 2001. NEMA NU1-2001. National Electrical Manufacturers Association (NEMA) [Google Scholar]
- 29.Quality control of nuclear medicine instruments. Vienna, Austria: IAEA; 1991. International Atomic Energy Agency (IAEA) [Google Scholar]
- 30.Institute of Physics and Engineering in Medicine. New York, England: IPEM Pub; 2003. IPEM 86. Quality control of gamma camera systems. [Google Scholar]
- 31.International Atomic Energy Agency (IAEA) Nuclear Medicine Resources Manual. Vienna, Austria: IAEA Pub; 2006. Quality assurance and quality control protocols for radiopharmaceuticals. [Google Scholar]
- 32.Jarritt PH, Perkins AC, Woods SD British Nuclear Medicine Society. Audit of nuclear medicine scientific and technical standards. Nucl Med Commun. 2004;25:771–5. doi: 10.1097/01.mnm.0000135041.46837.ca. [DOI] [PubMed] [Google Scholar]
- 33.Groch MW, Erwin WD. Single-photon emission computed tomography in the year 2001: Instrumentation and quality control. J Nucl Med Technol. 2001;29:12–18. [PubMed] [Google Scholar]
- 34.Autret D, Bitar A, Ferrer L, Lisbona A, Bardiès M. Monte Carlo modeling of gamma cameras for I-131 imaging in targeted radiotherapy. Cancer Biother Radiopharm. 2005;20:77–84. doi: 10.1089/cbr.2005.20.77. [DOI] [PubMed] [Google Scholar]
- 35.Kimiaei S, Ljungberg M, Larsson SA. Evaluation of optimally designed planar-concave collimators in single-photon emission tomography. Eur J Nucl Med. 1997;24:1398–404. doi: 10.1007/s002590050166. [DOI] [PubMed] [Google Scholar]
- 36.Snay ER, Treves ST, Fahey FH. Improved quality of pediatric 123I-MIBG images with medium-energy collimators. J Nucl Med Technol. 2011;39:100–4. doi: 10.2967/jnmt.110.080309. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa M, Kobayashi T, Sakurai Y, Kanda K. Optimization technique for a Prompt Gamma-ray SPECT collimator system. J Radiat Res. 2001;42:387–400. doi: 10.1269/jrr.42.387. [DOI] [PubMed] [Google Scholar]
- 38.Moore SC, Kouris K, Cullum I. Collimator design for single photon emission tomography. Eur J Nucl Med. 1992;19:138–50. doi: 10.1007/BF00184130. [DOI] [PubMed] [Google Scholar]
- 39.Data sheet of Siemens E.CAM Dual Head gamma camera. E. cam signature series: All about quality, speed and comfort. Siemens Medical Solutions USA, Inc. Illinois, USA. 2006:14. [Google Scholar]
- 40.Kangasmaa T, Sohlberg A, Kuikka JT. Reduction of collimator correction artefacts with bayesian reconstruction in spect. Int J Mol Imaging. 2011;2011:1–6. doi: 10.1155/2011/630813. [DOI] [PMC free article] [PubMed] [Google Scholar]


